Introduction
Heavy metals are dangerous to health of organisms and ecosystem. Environmentally, persistent and high toxicity of heavy metals is hazardous to the health of organisms [1]. Cadmium is considered as a major toxic, industrial and environmental pollutant. Cadmium is used in industries due to its physical and chemical properties [2]. As cadmium enters the body [3], it accumulates in various organs and systems, causing damage to such organs as brain, lungs, blood system, bone, liver, spleen, pancreas and testis [4-6].
In the kidneys, cadmium causes severe toxicity and defects to the glomeruli and proximal tubules, and leads to aminoaciduria, glycosuria, calciuria, phosphaturia, and Fanconi syndrome [7]. Cadmium can be absorbed back into the glomerular filtrate due to the interaction of Procalcitonin (PCT) receptors [8]. The biological life of cadmium is 15-30 years [9], which warrants the study of its toxic effects in various organ systems under chronic and acute cadmium exposure.
Millardia meltada (M. meltada), a stable animal belonging to the rodent family Muridae, is commonly found in Pakistan, Bangladesh, India, Nepal and Sri Lanka. The combined length of head and body is 13-16 cm with a 12-14cm tail [10]. It is yellowish to brownish gray dorsally and whitish ventrally. It lives in tunnels typically situated in embankments at the peripheries of fields. It is dispersed from Baluchistan to Khyber Pakhtunkhwa (formerly NWFP) and in some areas of southern Punjab [11]. The aim of this study was to investigate the effects of chronic and acute cadmium exposure on the morphology and histopathology of the blood, liver and kidneys of field rats (Millardia meltada), which may be considered as biomarkers of cadmium toxicity for other mammals.
Materials and Methods
Chemicals: All of the chemicals used were of analytical grades. Cadmium chloride (CdCl2; MERCK, CAS≠ 10108-64-2; Germany) was obtained from Bio-Control Lab, Department of Zoology, Wildlife and Fisheries, University of Agriculture, Faisalabad.
Animals and Maintenance: The rats (M. meltada) were captured from wheat fields, and the study was undertaken at Bio Control Lab, University of Agriculture, Faisalabad. Pathogen-free rats were selected and were assigned to this study. They were housed under conventional conditions, in suspended stainless-steel cages fitted with a wire-mesh floor and front. The room temperature was kept at 22±2° C with the relative humidity at 40%-70%. A 12 h light/dark cycle was maintained, and the number of circulated air changes was about once every 10 h. Prior to the experiment, all rats were fed the basic diet without any additions. Drinking water was supplied in glass bottles, which were cleaned once daily. Food and water were provided ad libitum. All of the prevalent ethical guidelines of the University of Agriculture at Faisalabad were followed with regard to animal care and experiments.
Clinical Observations: During the study, the animals were subjected to a clinical examination on daily basis. Abnormal signs and symptoms, such as loss of appetite, refusal to drink, characteristics of feces, development of abscesses and/or wounds, and loss of hair were taken into account and documented.
Experimental Design and Treatment: The rats were acclimatized to the laboratory environment for one week, and the 24 animals were divided into two groups of 12 rats each. Then they were subdivided into one control and two treatment subgroups with Cd in added to the feed or drinking water. The treatment subgroups received either 15mg/kg (low) or 30mg/kg per (high) Cd concentration in their feed.
Observations and Analyses: The rats were weighed daily and observed for their health condition and behaviors. Changes in the morphology such as the fur texture and color, and behavioral responses to tapping stimuli were documented. The food and water intakes were measured on a daily basis throughout the study. Also, few rats were reallocated among the groups in order to equalize the mean body weights at baseline.
Application of Bait: The pellet feed containing Cd was given to the rats in the experimental sub-groups only. The control rats were given Cd-free feed over the two months trial. Data representing food and water intakes were recorded on a daily basis.
Determination of Cd Concentration in Tissues: After Cd exposure, blood samples were taken, animals were sacrificed, the kidney and liver tissues (1g each) were removed and placed in polypropylene vials, containing 10% formalin. The tissues were grounded and homogenized in 5ml normal saline before digestion in a mixture of 60% hydrochloric acid and 70% nitric acid. The digest was allowed to cool and filtered through Whatman’s filter paper, leaving a whitish residue. The filtrate was made up to 50ml, using distilled water and refrigerated until further analyses. The concentration of Cd was analyzed, using an atomic absorption spectrophotometer.
Histopathological Evaluations: The Bancroft and Gamble method [12] was used for the histopathological examination of the tissue samples. The collected tissues were fixed in 10% buffered formalin for 3 days. To remove the fixative, tissues were washed over night in running tap water. The water in the tissue samples was removed by placing them in a series of ethanol as follows: 50% for 7 h, 70% and 100% for 4 h, absolute ethanol I and II for 1h each, , and ethanol plus xylene for 45min. The tissues were cleared from dehydrating agent by immersing them in xylene (xylene I for 30 min and xylene II for 15 min). The tissues were then embedded in melted paraffin I, II and III for 2 h, each.
After the paraffin infiltration process, the tissue samples were embedded in paraffin wax in a special plastic mold to make blocks for further microscopic examinations. Sections were then made into thin slices (5 µm) on a microtome, floated on surface of a warm water bath at 50-55° C, and were mounted directly on slides and numbered corresponding to the tissue specimens. Thin smears of albumin were coated on the slides, and were dried at 37-40° C for 2-3 hours. The slides were incubated in an oven at 45-50° C for 30-60 minutes and then stained with hematoxylin and eosin stains (H & E). Slides were subsequently examined by light microscopy at 200 X and 400 X magnification for histopathological studies.
Blood Analyses: Blood samples were collected in EDTA tubes after dissection of the rats for the weekly evaluation of following parameters:
Hemoglobin (Hb; g/dL): Sahli’s method [13] was used for the estimation of hemoglobin concentration.
Hematocrit (Hct) / Packed Cell Value (PCV) (Hct or PCV; %): Microhematocrit method [14] was used for the determination of packed cell value.
Mean Corpuscular Volume (MCV; fL): MCV was worked out from RBC counts and PCV, using the following formula:
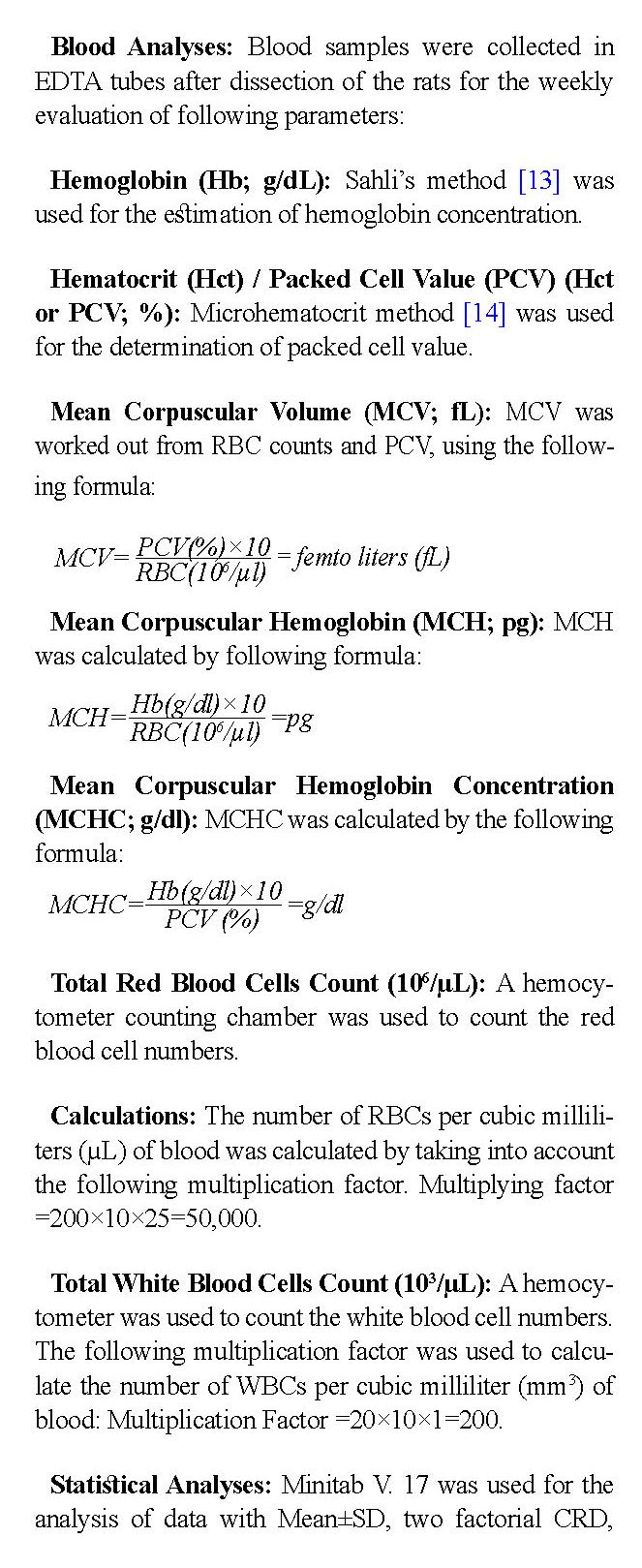
Statistical Analyses: Minitab V. 17 was used for the analysis of data with Mean±SD, two factorial CRD, ANOVA, and Tukey’s tests to measure the variation and correlation among the groups and subgroups. The statistical differences were considered significant at P<0.05.
Results
Morphological and Behavioral Changes: During the 1st month of the study, after the administration of Cd at low dose (15 mg/kg/D), the rats’ fur texture remained smooth and soft, the color remained blackish, and all rats actively responded to sound stimuli (knocking on cages). During the 2nd month, fur texture became somewhat hard and coarse but there was no change in the color. During the two months, rats showed fast to slow responses to the stimulus. At a daily high Cd dose (30 mg/kg), the fur color turned light blackish and the texture became coarse. No such changes were observed in the control rats.
Physiological Changes
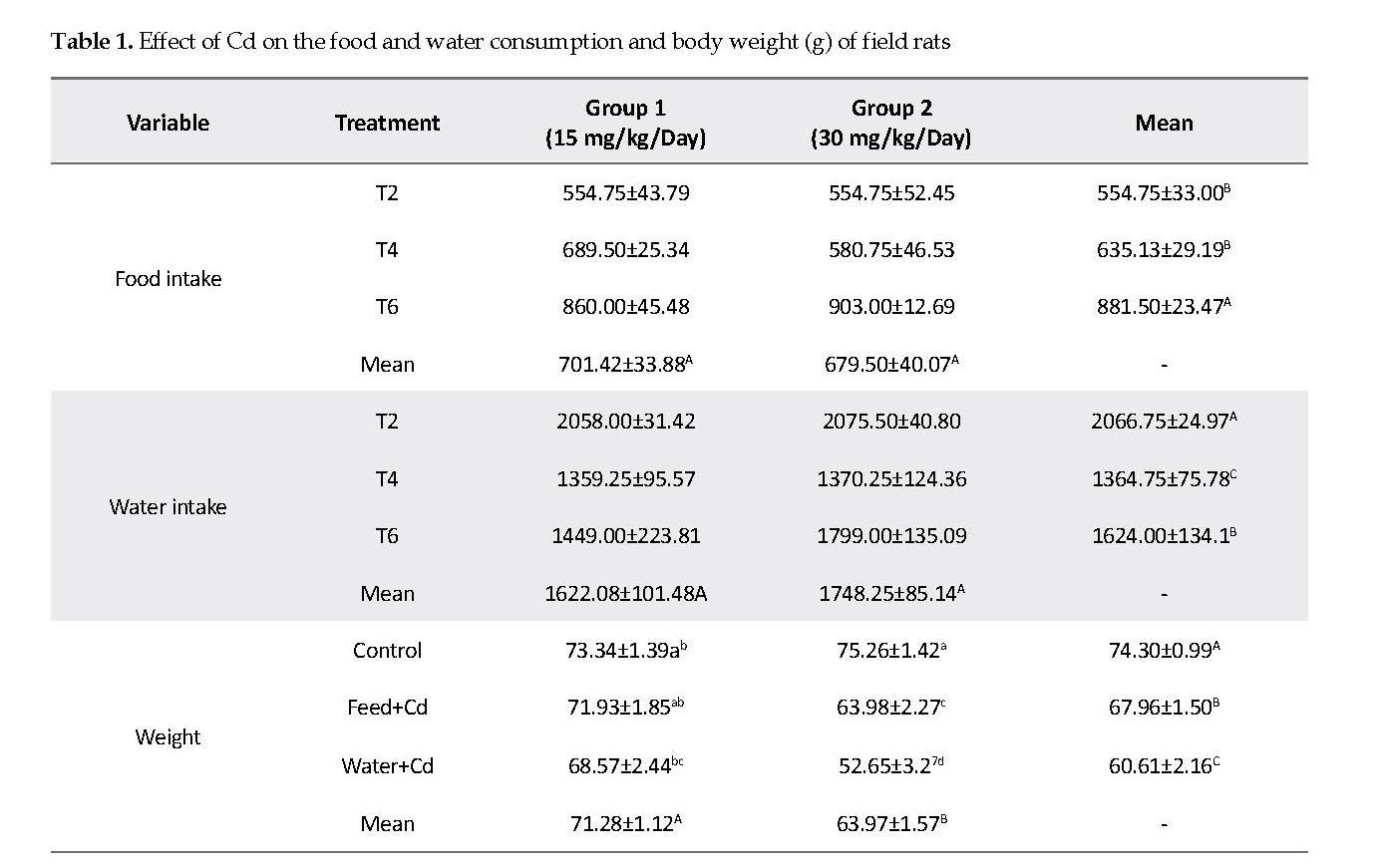
Effect of Cd on Feed, Water Intake and Body Weight: The feed consumption of rats exposed to low and high Cd decreased significantly as compared to that in the controls. Water intake was significantly increased (P<0.05) in feed+Cd (Group T2) as compared to water+Cd (Group T4) and the controls (Group T6). The control group had significantly higher body weight compared to those in the feed+Cd (Group T2) and water+Cd (Group T4). The rats fed with 15mg/kg Cd had significantly higher (P<0.05) body weight compared to those that received 30mg/kg Cd (Table 1).
Histopathology
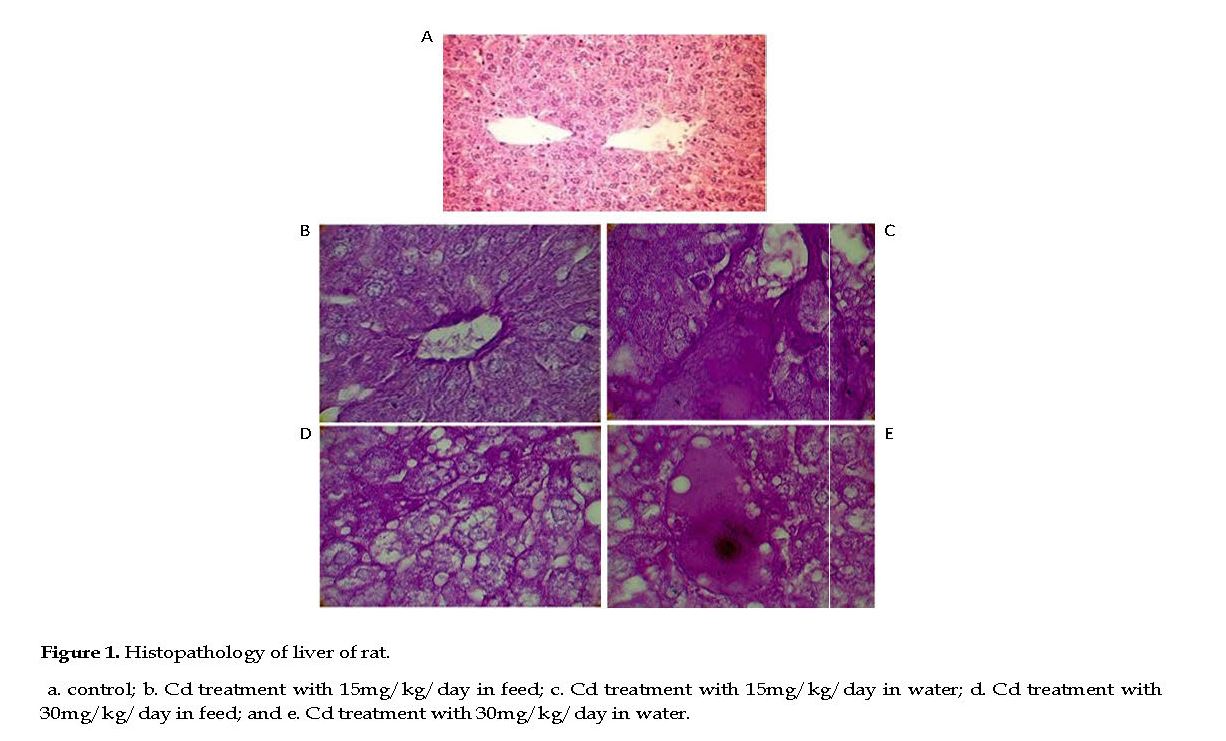
Effect of Cd on Rat Liver: In the control group, the liver lobules contained normal hepatocytes, parenchyma, fine hepatic cords and sinusoidal spaces. The hepatocyte nuclei were normal with fine chromatin observed in the nucleoli (Figure 1A). In the treated rats, the hepatic tissues developed many pathological changes, such as scattered chromatin and small nuclei compared to those seen in the controls. The cytoplasmic vacuolization of hepatocytes was prominent in rats treated with high Cd dose, with nuclear pyknosis, cell membrane ruptures, indistinct nuclei, nucleoli, and chromatin strands. Also, clear vacuoles were observed around the nuclei plus hemorrhagic spots, widespread fibrosis, interstitial mononuclear cellular infiltrations in the hepatocytes.
In low Cd group, mild degrees of vacuolar degenerations were observed in the cytoplasms. The hepatocytes nuclei appeared pyknotic sporadically, indicative of cell necrosis. A mild degree of cellular infiltration was noted throughout the hepatocytes, with mild to moderate congestion present in the parenchyma (Figures 1B & 1C). Intercellular necrotic gaps in the hepatic lobules appeared distinctly in high Cd dose group compared to those observed in the low Cd group. Also, moderate degrees of vacuolar degenerations were seen and the sinusoidal spaces were diminished due to cellular swelling in high Cd group. Also, there were numerous cell infiltrations throughout the parenchyma with sporadic pyknotic nucleoli in the hepatocytes, indicative of necrosis. Congestion in the hepatic parenchyma was also found and the hepatic portal showed atrophy with sinusoidal dilations (Figurs 1D & 1E).
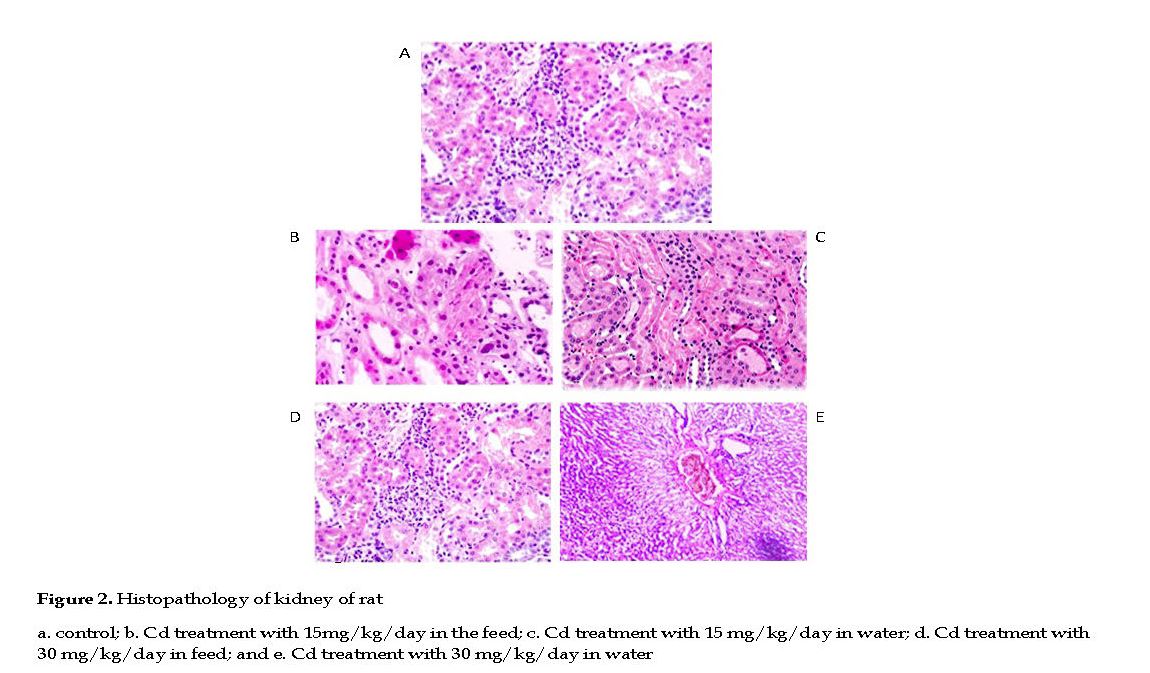
Effect of Cd on Rat Kidneys: No histological changes occurred in kidneys of the control group with normal arrangement of proximal and distal convoluted tubules, renal corpuscles, collecting duct and Henle’s loop. The renal parenchyma tubular epithelial cell, nuclei and glomeruli had normal appearances. The glomerular capillary loops were thin and delicate, and the endothelial and mesangial cells were normal in morphology and number, with the urinary spaces appeared clear (Figure 2A).
In Cd treated groups, the kidneys appeared pale, tissue lesions were observed in the medulla and cortex, with necrosis, dilation and calcinosis seen in the tubules. Massive local hemorrhage and destruction of the basement membrane occurred in the renal tissue. There were abnormal renal structures, thin epithelial layer and lumen, and curly microvillus brush borders. In low Cd group, the renal parenchyma showed cell infiltrations and few pyknotic nuclei in the tubular epithelial cells, indicative of cell necrosis.
In few slides, fluid was present in the lumen of the renal tubules with mild to moderate degrees of congestion in the renal parenchyma (Figures 2B & 2C). In high Cd group, severe necrosis and degeneration in tubules were observed, and the glomeruli were muddled with significant distortions in the Bowman’s capsules. The cellular integrity was compromised significantly in the proximal and distal convoluted tubules with disrupted nuclei, pale cytoplasm and scanty chromatin observed (Figures 2D & 2E).
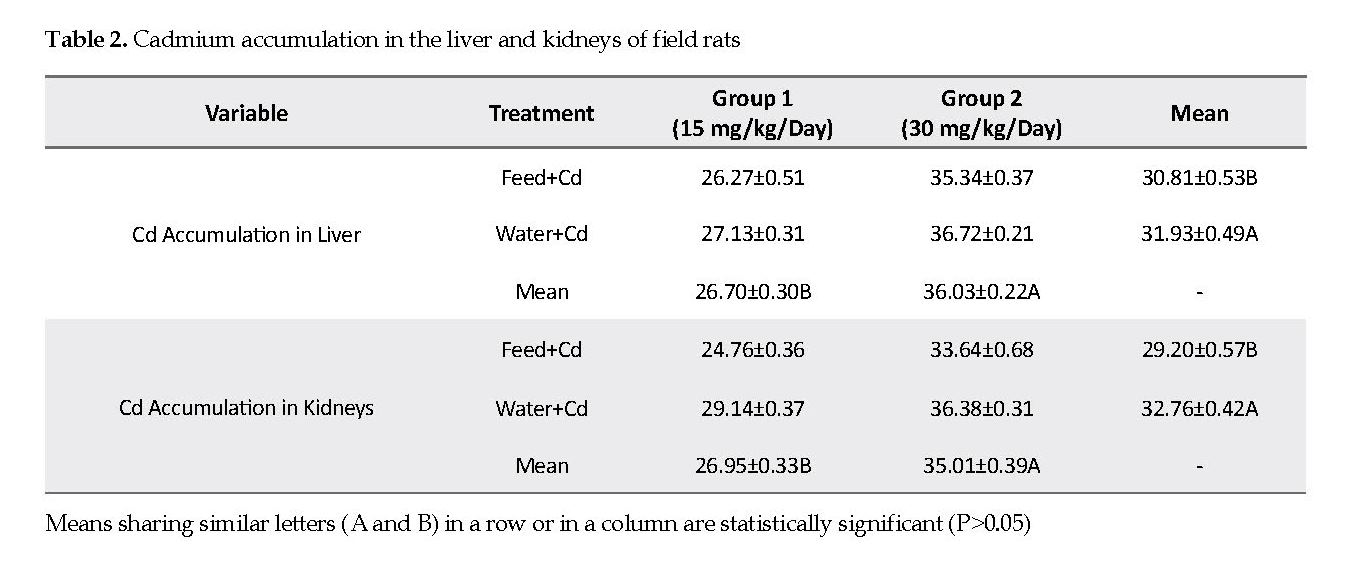
Cadmium Concentration in Liver and Kidney Tissues: The mean values of Cd accumulation in the liver and kidneys were significantly lower in low Cd group (15 mg/kg) as compared to that in high Cd group (30 mg/kg). The mean Cd accumulation in the liver and kidneys were significantly higher in water+Cd group (P<0.05) than that in the feed+Cd group (Table 2).
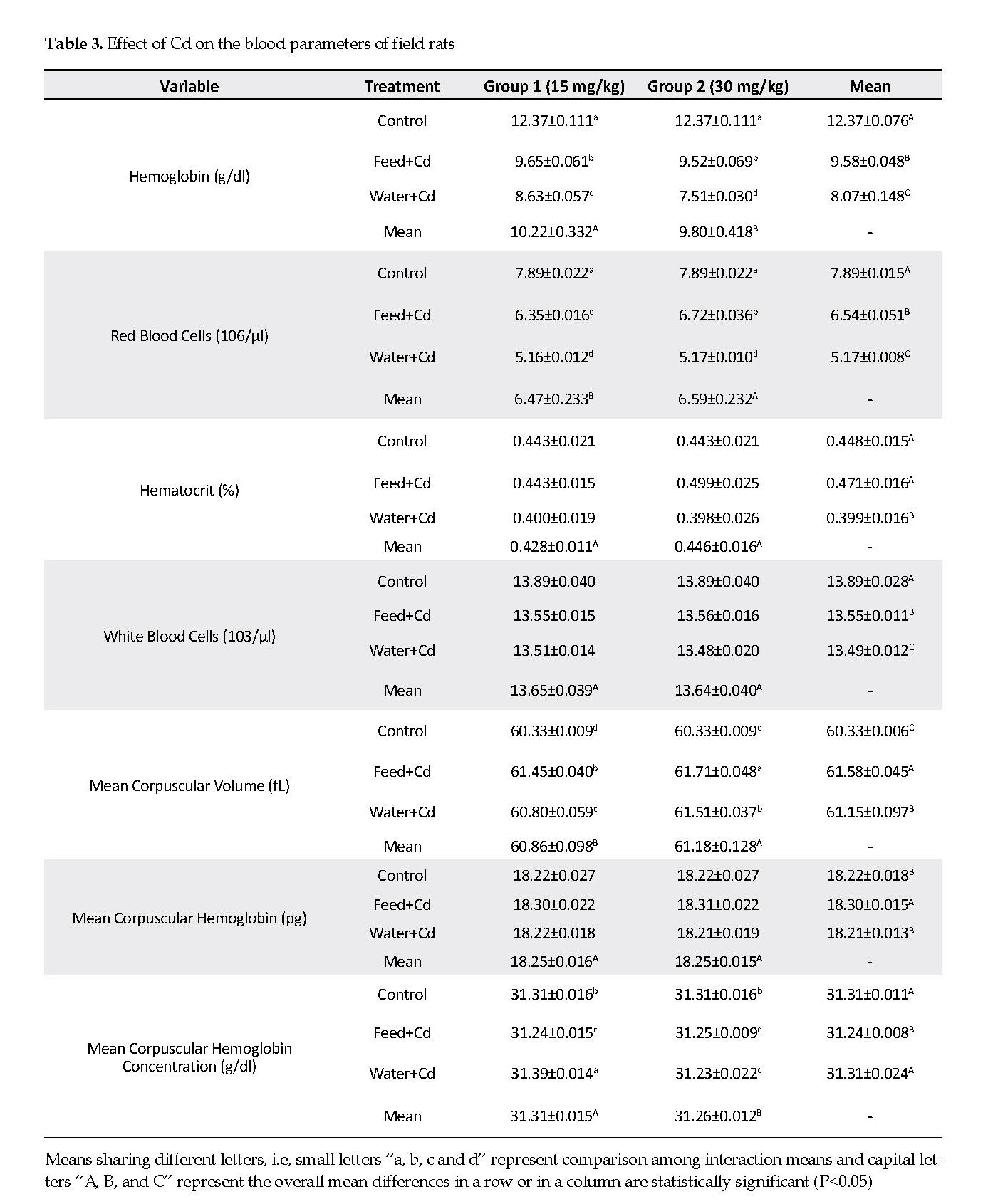
Effect on Blood Parameters: Hemoglobin was significantly higher (P<0.05) in low Cd group (15 mg/kg) compared to that in the high Cd group (30 mg/kg). Hemoglobin was significantly higher (P<0.05) rats receiving water plus low Cd compared to that in the high Cd group. The control group had significantly higher (P<0.05) hemoglobin compared to either group that received Cd in their feed or water (Table 3).
Red Blood Cell (RBC) numbers were significantly higher (P<0.05) in the high Cd group than in the low Cd group. The control group had significantly higher (P<0.05) red RBC compared to those receiving Cd in their feed or water. The mean hematocrit value was significantly lower (P<0.05) in rats receiving Cd in water than those noted for Cd in the feed, and in the control groups. The mean White Blood Cells (WBC) value was significantly lower (P<0.05) in rats receiving Cd in either feed or water, than in the controls (Table 3).
The Mean Corpuscular Volume (MCV) significantly decreased (P<0.05) in low Cd group compared to that in high Cd group. Treatment with Cd in the feed significantly increased (P<0.05) the MCV compared to Cd present in the water, and the levels observed in the controls.
A similar relationship was observed for the Mean Corpuscular Hemoglobin (MCH), i.e. the MCH was significantly higher when Cd was added to the feed than to the water. The Mean Corpuscular Heamoglobin Concentration (MCHC) was significantly higher (P<0.05) in low Cd group than in high Cd group. Also, treatment of rats with Cd in the water stimulated a significantly higher MCHC that Cd in the feed in both low and high Cd, compared to the controls (Table 3).
Discussion
Heavy metals are known to be hazardous to the health of organisms and ecosystem. Cadmium (Cd) is an environmental toxin of prevalent exposure and persistent toxicity [15]. The present study was conducted to investigate the effects of acute and chronic Cd exposure on the morphology and histopathology of field rats. We observed that the fur texture changed to some extent as hard and coarse and its color remained blackish (15 mg/kg body weight/day). A light blackish fur color and hard and coarse fur texture were observed with the high dose of Cd (30 mg/kg body weight/day). The mean values for weight were also significantly declined with respect to the time and mode of exposure. The weight decline in rats was greater with high Cd than in low Cd treatment.
Effects on Food and Water Consumption: This study found that the feed and water intakes were decreased in rats treated with Cd. Similarly, it has been reported that Cd accumulates in liver, resulting in reduced Cd concentrations in other organs [16] The intake ratio declined less in low Cd treated rats compared to those treated with high Cd. The intake ratio decreased significantly in rats which were given Cd in water as compared to those that were given Cd in th feed. Control group had significantly higher body weight compared to both feed+Cd and water+Cd groups. Our findings suggest that the addition of Cd to the feed and water adversely affects the animals’ appetite, digestion and ingestion. Our findings are consistent with those reported by other studies previously [16, 17].
Effects on the Liver: Liver is known to be a target organ and exposure of liver to cadmium is critical for the induction of toxicity. Histopathology of tissues revealed that cellular lesions occurred in treated rats. In the low Cd dose group, mild degrees of vacuolar degenerations in the cells’ cytoplasm were observed. The hepatocytes showed pyknotic nuclei sporadically suggestive of cell necrosis. A mild degree of cellular infiltration was seen throughout the liver parenchyma. Mild to moderate congestion was present in the parenchyma.
In high Cd dose group, moderate degrees of vacuolar degenerations were seen and sinusoidal spaces were diminished due to cell swelling. Hepatic portals showed atrophy and sinusoidal dilation. These histological alterations may have occurred due to the formation of free radicals and per-oxidation of lipids due to Cd toxicity [18]. In this study, Cd was accumulated largely in the liver and kidneys in rats. This heavy metal was deposited in these tissues at higher concentrations if it was dissolved in the drinking water compared to being added to their feed [19].
Effects on the Kidneys: Histopathological observations revealed that Cd caused edema [20] apoptosis, necrosis and tissue degeneration in the kidneys [21]. In the present study, in low Cd dose group, renal parenchyma showed sporadic cell infiltration and pyknotic nuclei of tubular epithelial cells, indicative of necrosis. Fluid was also present in lumen of renal tubules occasionally. Mild to moderate degrees of congestion were seen in the renal parenchyma.
In high Cd dose group, severe tubular necrosis and degenerations were observed. Glomeruli were muddled and significant distortion in Bowman’s capsules was noted. Cellular integrity was compromised in proximal and distal convoluted tubules. Disrupted nuclei, paler cytoplasm and scanty chromatin were observed. It has been reported that Cd exposure affected the glomeruli similar to those observed in our study [22].
Hematological Effects: This study observed reductions in the Hb, RBC, WBC, Hct, and MCH parameters while increases in the values of MCV and MCHC. The high Cd dose decreased the number of RBC’s, leading to anemia. Similar results have also been reported previously [23]. The number of RBC’s was also reduced due to hemolysis, secondary to the Cd toxicity [24]. A reduction in the hemoglobin concentration has also been detected previously in rats treated with paracetamol [25] and cadmium chloride [26].
Conclusions
This study concludes that Cd causes morphological and physiological abnormalities as well as pathological effects on rats’ organ systems. Cadmium affects the blood by lowering the cell counts and the volume, leading to anemia. The morphology, histopathology and hematology outcomes of this study can potentially be considered as the biomarkers of Cd toxicity in other laboratory animals.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted after formal approval of Ethical Committee and it was according to the existing ethical codes of animal handling and experimentation of the Institute.
Funding
This study extracted from the master thesis of the first author Javeria Wakeel in Department of Zoology, Faculty of Wildlife and Fisheries, University of Agriculture, Faisalabad, Pakistan.
Author's contributions
Conceptualization: Nazia Ehsan; Methodology: Javeria Wakeel and Nazia Ehsan; Investigation: Javeria Wakeel; Writing-original draft: Rana Waseem Akhtar and Syed Aftab Hussain Shah; Writing-review & editing: Syed Aftab Hussain Shah; Resources: All authors; Supervision: Nazia Ehsan.
Conflict of interest
The authors declared no conflict of interest
References
Satarug S, Vesey DA, Gobe GC. Kidney cadmium toxicity, diabetes and high blood pressure: The perfect storm. Tohoku J Experim Med. 2017; 241(1):65-87. [DOI:10.1620/tjem.241.65] [PMID]
Krichah R, Rhouma B, Hallegue D, Tebourbi O, Joulin V, Couton D, et al. Acute cadmium administration induces apoptosis in rat thymus and testicle, but not liver. Polish J Envir Stud. 2003;1: 589–94.
Andujar PL, Bensefa-Colas, Descatha A. [Intoxication aiguë et chronique au cadmium (French)]. La Revue de Médecine Interne. 2010; 31(2):107-15. [DOI:10.1016/j.revmed.2009.02.029] [PMID]
Cai Y, Aoshima K, Katoh T, Teranishi H, Kasuya M. Renal tubular dysfunction in male inhabitants of a cadmium-polluted area in Toyama, Japan, eleven-year follow-up study. J Epidemiol. 2001; 11:180-9. [DOI:10.2188/jea.11.180] [PMID]
Emmanuel E, Allison K, Margaret M, Carmen A, Elizabeth A, Kanesha L, et al. Cadmium pathways during gestation and lactation in control versus metallothoinein 1,2-knockout mice. Soc Toxicol. 2003; 71(2):154-63. [DOI:10.1093/toxsci/71.2.154] [PMID]
Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress. Part 1: Mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001(1):529-39. [DOI:10.2174/1568026013394831] [PMID]
Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009; 238(3):201-8. [DOI:10.1016/j.taap.2009.04.020] [PMID]
Barbier O, Jacquillet G, Tauc M, Cougnon M, Poujeol P. Effect of heavy metals on, and handling by, the kidney. Nephron Physiol. 2005; 99(4):105-10. [DOI:10.1159/000083981] [PMID]
Henson M, Chedrese P. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Experim Biol Med. 2004; 229(5):384-92. [DOI:10.1177/153537020422900506] [PMID]
Molur S, Nameer PO. Millardia meltada. The IUCN Red List of Threatened Species. 2016; e.T13525A115115706. [DOI:10.2305/IUCN.UK.2016-3.RLTS.T13525A22461465.en]
Musser GG, Carleton MD. Superfamily muroidea. In: Wilson DE, Reeder DM, editors. Mammal species of the world, third ed. Baltimore: The Johns Hopkins University Press; 2005.
Bancroft JD, Gamble M. Theory and practice of histological techniques. Sixth ed. London: Churchill Livingstone; 2008.
Sahli H. Lehrbuch der klinischen untersuchungs-Methoden. Franz Deuticke; 1905.
Bull BS, Koepke JA, Simson E, van Assendelft OW. Procedure for determining packed cell volume by the microhematocrit method; Approved standard, third ed. Wayne. Pennsylvania: NCCLS; 2000.
Abbas HH, Zaghloul KH, Mousa MA. Effect of some heavy metal pollutants on some biochemical and histopathological changes in Blue tilapia, Oreochromis aureus. Egypt J of Agricul Res. 2002; 80:1395-411.
DelRaso NJ, Foy BD, Gearhart JM, Frazier JM. Cadmium uptake kinetics in rat hepatocytes: Correction for albumin binding. Toxicol Sci. 2003; 72(1):19-30. [DOI:10.1093/toxsci/kfg009] [PMID]
Elsenhans B, Strugala G, Schmann K. Longitudinal pattern of enzymatic and absorptive functions in the small intestine of rats after short-term exposure to dietary cadmium chloride. Arch Envir Contam Toxicol. 1999; 36:34-6. [DOI:10.1007/s002449900480] [PMID]
Eriyamremu GE, Asagba SO, Onyeneke EC, Adaikpo MA. Changes in carboxypeptidase A, dipeptidase and Na+/K+ ATPase activities in the intestine of rats orally exposed to different doses of cadmium. Bio Metals. 2005; 18(1):1-6. [DOI:10.1007/s10534-004-1202-3] [PMID]
Renugadevi J, Prabu SM. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Experim Toxicol Pathol. 2010; 62(2):171-81. [DOI:10.1016/j.etp.2009.03.010] [PMID]
Choi JH, Rhee SJ. Effects of vitamin E on renal dysfunction in chronic cadmium-poisoned rats. J Med Food. 2003; 6(3):209-15. [DOI:10.1089/10966200360716625] [PMID]
Damek-Poprawa M, Sawicka-Kapusta K. Histopathological changes in the liver, kidney and testes of bank voles environmentally exposed to heavy metal emissions from the steelworks and zinc smelter in Poland. Envir Res. 2004; 96(1):72-8. [DOI:10.1016/j.envres.2004.02.003] [PMID]
Jemai H, Lachkar HA, Messaoudi I, Kerkeni A. Effect of zinc pre-treatment on blood glutathione, serum zinc and kidney histological organization in male rats exposed to cadmium. J Trace Elements Med Biol. 2010; 24(4):277-88. [DOI:10.1016/j.jtemb.2010.07.001] [PMID]
Guedenon P, Edorh PA, Hounkpatin ASY, Alimba CG, Ogunkanmi A, Nwokejiegbe EG, et al. Haematological study of Clarias gariepinus exposed to chronic and subchronic doses of cadmium, mercury and combined cadmium and mercury. Sci Nat. 2012; 4(2):2-19.
Lavicoli I, Carelli G, Stanek E J, Castellino N, Calabrese EJ. Effects of low doses of dietary lead on red blood cell production in male and female mice. Toxicol Letter. 2003; 137(3):193-9. [DOI:10.1016/S0378-4274(02)00404-6]
Lahouel M, Boulkour S, Segueni N, Fillastre JP. [Effet protecteur des flavonoïdes contre la toxicité de la vinblastine, cyclophosphamide et du paracétamol par inhibition de la peroxydation lipidique et augmentation du glutathion hépatique (French)]. Pathologie Biologie. 2004; 52(6):314-22. [DOI:10.1016/j.patbio.2004.01.001] [PMID]
Ognjanović BI, Marković SD, Pavlović SZ, Žikić RV, Štajn AŠ, Saičić ZS. Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: Protective effect of selenium. Physiol Res. 2008; 57(3):403-11.








































