







































BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijt.arakmu.ac.ir/article-1-959-en.html


 1, Bouchra El khalfi2
1, Bouchra El khalfi2 

 , Mohamed Idiken2
, Mohamed Idiken2 

 , Souraya Sakoui2
, Souraya Sakoui2 

 , Reda Derdak2
, Reda Derdak2 

 , Ouafaa Aniq Filali2
, Ouafaa Aniq Filali2 

 , Abdelhakim Elmakssoudi3
, Abdelhakim Elmakssoudi3 

 , Abdelaziz Soukri2
, Abdelaziz Soukri2 


2- Department of Biology, Laboratory of Physiopathology, Genetics, Molecular and Biotechnology (PGMB), Faculty of Sciences Aïn Chock, Research Center of Health and Biotechnology, Hassan II University of Casablanca, Casablanca, Morocco.
3- Department of Chemistry, Laboratory of Organic Synthesis, Extraction, and Valorization, Faculty of Sciences Aïn Chock, Hassan II University of Casablanca, Casablanca, Morocco.
Introduction
Over the last decades, pharmaceutical industry began to consider the idea of treating several free radical-related diseases, such as cancer, diabetes, kidney failure, Alzheimer’s, and Parkinson’s by catalyzing the synthesis of certain bioactive compounds through Multicomponent Reactions (MCRs) [7]. This is an approach in which three or more reactants are combined into a single reaction that leads to the formation of a desired compound. The MCRs cover such topics as sustainability, atom economy, and eco-efficiency. Consequently, we can reduce the required time and energy, which is regarded as step efficiency. Among the compounds produced by using the one-pot reaction we cited, such as pyrano-[2,3-c]-pyrazoles class, these valuable synthetic products are aberrantly exploited as efficient antioxidant drugs and their mimetic effect have renewed much interests within the scientific community. They are considered the cornerstone of a new concept in drug discovery and the pharmaceutical attention has quickly turned towards the “drug-like molecules” subject [8].
The pyrano-[2,3-c]-pyrazoles are the subject of modern biochemistry that is growing rapidly and has stimulated extensive applications. Chief among these are multiple pharmacological and biological properties, such as antioxidant, antibacterial, anti-molluscicidal, analgesic, antiviral potential, and inhibitors of human checkpoint kinase-1 [9]. Upon an extensive literature review, it was confirmed that the wide-range of pyrano-[2,3-c]-pyrazoles activities are linked to the structural base core of the condensed pyrane and pyrazole [10]. In this regard, researchers expended efforts on the development of a single structural framework by associating both motifs (pyrane and pyrazole) in the hope of enhancing the biological activity and pharmacological properties of this compound [11].
The current study aimed to synthesize pyrano-pyrazoles derivatives and examine their antioxidant activities. We first synthesized three derivatives, i.e., 5a, b, c, via rapid, simple, and efficient “one-pot reaction”, catalyzed by the Na2CaP2O7. Next, we characterized these compounds via spectroscopic analysis (IR; 1H-NMR; 13C-NMR) and the melting point. As part of an ongoing work, the synthesized derivatives were subjected to in vivo antioxidant screening assays for the first time, using a eukaryotic unicellular freshwater ciliate “Tetrahymena”, a free-living unicellular eukaryote. This species appeared to be a suitable experimental model [12], which has been used widely in pharmacological and environmental studies, because it can easily be sub-cultured and maintained at optimal laboratory conditions [13]. The in vivo antioxidant activity of pyrano-pyrazoles derivatives is accompanied by the 1,1-diphenyl-2-picrylhydrazyl (DPPH) test, confirming their protective potential. Specifically, the protective effects of pantoprazole on Tetrahymena thermophila have not been evaluated to date. This fact encouraged us to examine and discuss their effects, for the first time, on the physiological and kinetic parameters of T. thermophila cells under oxidative and nitrative stress conditions.
Previously, studies have extensively investigated the physiological features of this free-living protozoan [14]. the current study was designed to investigate T. thermophila at the behavioral, biochemical and molecular levels, using cellular, enzymatic and protein expression assays, respectively [15]. Based on our current data, we have made exciting findings and progress on this subject; however, some challenges still remain regarding the identification of the cellular and molecular pathways that are activated in Tetrahymena cells after exposure to certain stressors in the long-run.
Materials and Methods
Preparation of pyrano-[2,3-c]-pyrazoles: The three pyrano-pyrazole derivatives were synthesized by a 4-part reaction between aromatic aldehyde 1, ethyl acetoacetate 2, hydrazine hydrate 3, and malononitrile 4. The synthesis occurred in the presence of the pyrophosphate Na2CaP2O7 as a catalyst according to a previously described experimental procedure (Figure 1S).
Please refer to the “Supplemental Materials” at the end of the article. The choice of this catalyst was made based on the satisfactory results from the application in certain organic syntheses [16]. The reactions and purification of pyrano-pyrazole 5 were monitored by Thin-Layer Chromatography (TLC) (Table 1).
.jpg)
Thereafter, the synthesized products were characterized by melting point and spectroscopic analyses (IR, 1H NMR, 13C NMR) (Figure S2, S3, S4, S5, S6, S7, S8, S9, S10) [17].






The purity of the products was confirmed, using High-Performance Liquid Chromatography (HPLC) method (Figure S11 in supplementary material).
Biological & experimental data
Strains and growth conditions: The toxicological assays were performed by T. thermophila strain “SB 1969”. The cells were cultured to exponential phase (1.5x105 cells/mL) in the PPYE medium and maintained at a room temperature of 32°C. This liquid medium consisted of 1.5% (w/v) of peptone and 0.25% (w/v) of yeast extract. Before each experiment, the viability of Tetrahymena cells were examined under light microscopy.
Toxicity test of the molecules: To evaluate the toxicity of pyranopyrazoles on the ciliates, the synthesized compounds (5a, b, c) were taken at a final concentration of 10mg/mL in dimethyl sulfoxide (0.1%, v/v). Next, a panel of nominal concentrations was performed (Table 2).
.jpg)
The cells were supplemented with 5µL of each dilution and maintained in continuous culture as described earlier, and samples were taken to check for the toxic effect of the specific compounds (pyranopyrazoles). The number of surviving protozoans was counted after 72hr of exposure to the toxin, and we used the non-lethal concentration of pyranopyrazoles that preserved the normal shape of T. thermophila.
Treatment with pyranopyrazoles derivatives: To examine the protective effect of each derivative (5a, b, c), we conducted our experiments under a series of time-course conditions. The experimental and control samples were kept in continuous culture for 96hr at 32°C. To measure the protozoan density, we used 1mL of the culture media hourly for 3hr, and the optical density was determined at 600nm in triplicate, using a Jenway 7315 UV-visible spectrophotometer. Simultaneously, the shape and motility of Tetrahymena cells were examined, using a KRÜSS Optronic light microscope.
Counting cells: The Tetrahymena cells were fixed in 2% formaldehyde and Phosphate Buffered Saline (PBS). Then 5μL of each sample (treated and control) was placed on the hemocytometer plate (Malassez) to count the cells. The cell counting was repeated three times.
In vitro antioxidant activity: We used DPPH, a stable radical, to measure the free radical scavenging activity in vitro. At this experimental condition, the derivatives 5a, b, c served as the antioxidants and inhibited DPPH, by causing a color change, from deep violet to yellow. The degree of discoloration that occurred represented the radical-scavenging potential for each of the designated compounds. The scavenging activities of 5a, b, c and ascorbic acid were determined at concentrations ranging from 2–10 mg/mL with the DPPH solution 0.004 %.These were left for 30 minutes at room temperature and the absorbance was read again in a UV-spectrophotometer at 517nm [18, 19].
Results
Characterization of the synthesized pyranopyrazoles: The analyses of the results shown in Table 1 demonstrated that the pyrano-[2,3-c]-pyrazoles were produced in good yields (93-95%), influenced by the nature of the substituent at the para position of the phenyl group. The yield decreased when the aromatic aldehyde rich in electron, substituted by a methyl (CH3) group in the para position, was replaced by an aromatic aldehyde, which was poor in electrons and substituted by a nitro group (-NO2). This result is in agreement with the experimental results obtained previously by Maddila et al. [20]. This indicated that the reactivity of aromatic aldehydes can be accentuated by the introduction of an electron donor group on the benzene nucleus.
Screening of biological activities
Stress effect on tetrahymena cells: As shown in Figure 1A, the oxidative and nitrative stress induced a change in the growth of T. thermophila. Notably, the stress caused a lag phase elongation compared to the normal growth conditions. Furthermore, the toxic effect of stress was more pronounced when we used the hydrogen peroxide (H2O2) compared to Sodium Nitroprusside (SNP) as a stressor. Under this experimental condition, several cellular parameters were affected, including the morphology; growth rate and ciliary motility.
.jpg)
Anti-oxidative and anti-nitrative stress of 5a compound
Effect on the growth of the protozoan: As seen in Figure 1B, the obvious finding confirmed that the compounds (5a) had an impressive effect on the oxidative and nitrative stresses. Also, we noticed that this derivative stimulated the proliferation of Tetrahymena (TT+5a), thus exceeded the normal growth rate of T. thermophila.
Effect on the shape and motility of the protozoan: The analysis of the shape of T. thermophila cells under stress conditions showed an alteration in their shape, by getting elongated and round (Figure 2).
.jpg)
We also observed a decrease in the cell number (Figure 3A). Conversely, the results in Table S2 confirmed that the supplementation with the 5a compound improved the main swimming speed of the ciliated protozoans (Table S3 in ESI).


Effect on cell density: During the growth of Tetrahymena, we counted the cells at several time points up to 96hr. As seen in Figure 3A, the results illustrate that H2O2 and SNP significantly reduced the growth rate of the T. thermophila population by 50%, while the addition of the 5a derivative to the culture media protected a large number of cells against the stress. Therefore, this derivative increasingly enhanced the cell densities to levels that exceeded the normal rate.

Protective effect of 5b compound
Effect on the growth of the protozoan: The above results (Figure 1C) indicated the protective effect of 5b against oxidative stress (S). Under these experimental conditions, the protozoan growth (TT+5b) increased to a level that approached the normal rate for T. thermophila (TT).
Effect on the shape of the protozoans: The investigation of the stress effect on T. thermophila kinetics led to an alteration in their pear-shaped form, the cells transformed into elongated and spherical shapes. While the supplementation with the 5b compound caused the cells to maintain their normal shape despite the toxicity of both stressors (Figure 2).
The motility of Tetrahymena cells: The results shown in Table S3 (see supplementary material ESI) confirmed that the cells exposed to H2O2 and SNP had low motility subsequent to the shape transformation to spherical shape. Nonetheless, the addition of the 5b compound to the culture media led to a relative improvement of the ciliary motility.
Effect on cell density: As shown in Figure 3B, the addition of 5b derivative to the culture media reduced the toxic effects of the stressor. We also documented a substantial effect against oxidative stress compared to a slight effect after the nitrative stress.
Protective effect of 5c derivative
The cell growth: The induced stress led to perturbation of the T. thermophila growth rate (Figure 1D), hence after the supplementation with the 5c compound, the protozoans regained their normal growth rate, consistent with the expected antioxidant property of this molecule.
The shape and motility of Tetrahymena: Prompted by our finding, we confirmed once again that cells exposed to H2O2 and SNP were less motile with the acquisition of a peculiar shape (Figure 2). Nevertheless, the supplementation with the 5c molecule caused an improvement in the swimming speed of this species (Table S4).

The cell number of Tetrahymena: The results in Figure 3C illustrate that the stress restricted the growth rate of Tetrahymena. The harmful effects of stress were less pronounced when we supplemented the culture media with the 5c molecule, which protected against the stress caused under nitrative conditions. The results correlated with our previous data, confirming the property of this product in preserving the normal shape of the protozoans.
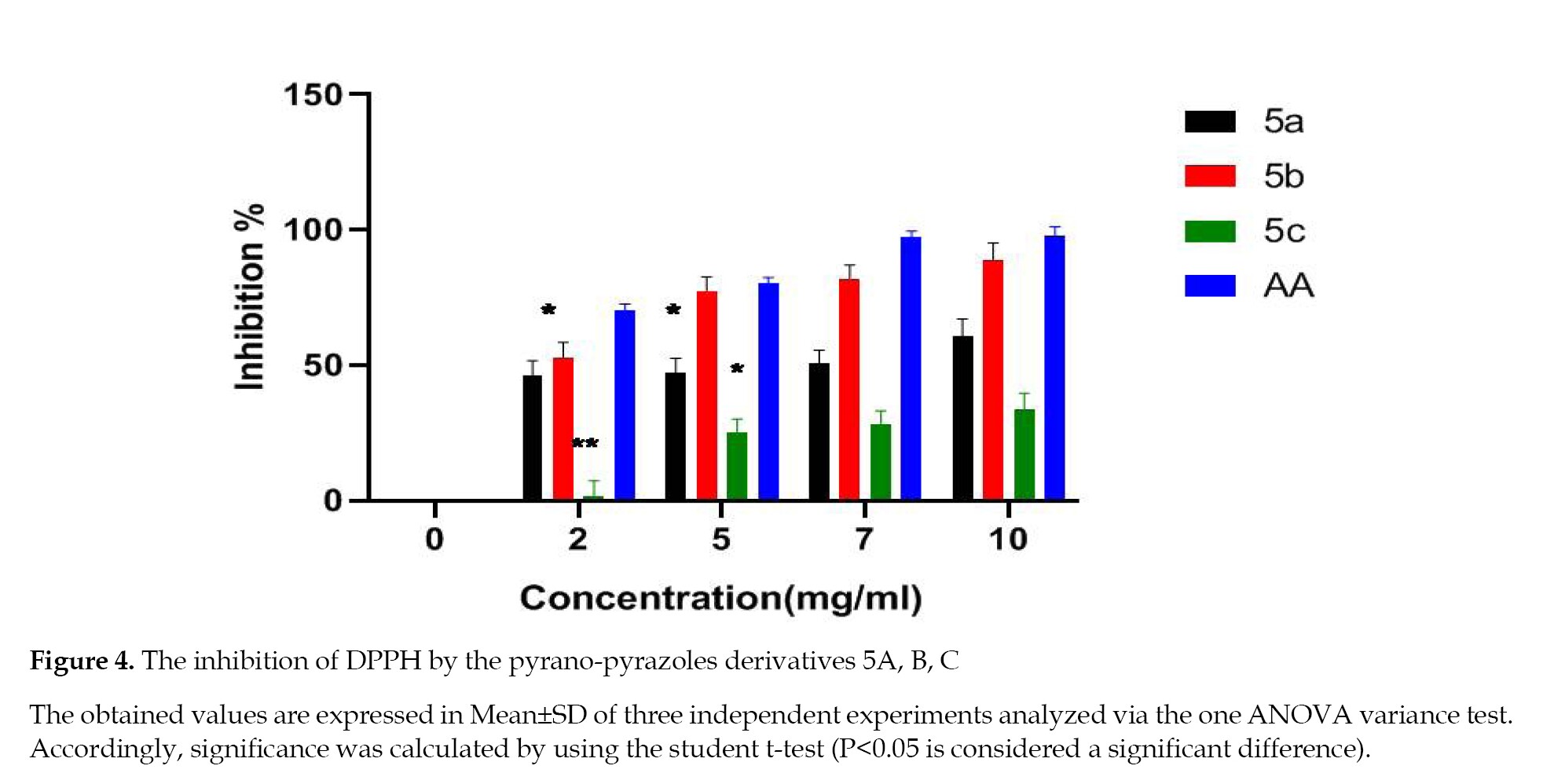
Antioxidant activity in vitro: Inhibition of DPPH Radical: The scavenging activity of 5a,b,c derivatives compared to ascorbic acid is presented in Figure S15, where the Ascorbic Acid (AA) represents the absorbance of the control (in a methanolic solution of DPPH) and a sample represents the absorbance of the tested compound in a methanolic solution of DPPH.

The calculated inhibition levels are summarized in Figure 4, indicating that 5a and 5b compounds showed significant scavenging effects while the 5c caused a very low effect, spanning from 1.83 to 33.5%.
Discussion
This paper reports the toxic effects of oxidative and nitrative stresses on this species under the selected experimental conditions, which affected the cellular morphology, growth and ciliary motility after treatment of the protozoans with H2O2. The oxidative stress inhibited or slowed down the growth rate of T. thermophila in a dose-dependent manner. The lethal effect of hydrogen peroxide has been well studied by Errafiy et al. [21]. This stressor has also inhibited the growth of other organisms, such as yeasts (Saccharomyces cervicea)-protozoa (amoeba) and several other pathogenic bacteria (Vibrio harveyi) [21].
The SNP-induced stress had a harmful effect on this species similar to that of H2O2. This might be due to the fact that SNP is a source of NO gas, the overproduction of which can modify certain cellular and molecular mechanisms. For example, the expression profile of some proteins, such as Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) plays a key role in the carbon metabolic route in Tetrahymena cells [15, 18]. This is in agreement with the previous work, demonstrating that both stressors affected the target protein directly and reduced its activity; which in turn affected the Tetrahymena’s growth rate [21].
The current study was devoted to the assessment of the protective effect of the synthesized products 5a,b,c. The results of our experiments demonstrated that the 5a and 5b compounds exhibited a prominent antioxidant effect as compared to 5c. These derivatives are likely to protect the cells’ morphology and shape, while increasing the cell number and improving the motility. Consequently, we corrected the retarded growth rate that was likely related to the overexpression of ROS and RNS. Previous studies on Tetrahymena used such natural products, as sage essential oils as an antioxidant supplement, which improved the effect against stressors through activating such enzymes as catalase, SOD and GAPDH [22].
The protective effect of the compounds 5a,b,c may be explained by such hypotheses as their potential to scavenge RONS, which is consistent with the activation of the antioxidant defence. Meanwhile, we believe that their activity can also be linked to the structure of the pyrazoles scaffold. Previous studies have shown that H2O2 and SNP significantly decrease the activities of CAT, SOD, and GPx in Tetrahymena cells [22]. Nevertheless, the treatment with the pyrano-pyrazoles may preserve the activities of these enzymes through the restoration of SOD level and activation of GPx that block the glutathione oxidation, mediated by H2O2 [22]. These compounds also induce the activity of CAT, inhibit sodium nitroprusside [23] and H2O2-stimulated lipid peroxidation. Consequently, these cellular activities tend to maintain the oxidative balance [2] (Figure S18).

Pyrano-pyrazoles provide valuable tools for chemists and have a significant importance in the pharmaceutical field due to their multiple activities. These span their drug-like and mimetic proprieties, expanding the use of plants and the derivatives by designing the analog of these natural products [11]. We successfully designed useful heterocyclic compounds through the use of greener protocols, including Na2CaP2O7 as an efficient heterogeneous catalyst [24, 25]. The main advantage of this procedure is obtaining the desired products at high purity, short reaction time, ample yields, and simple procedures, compared to the classic methods described in the literature [7].
In this context, the derivatives have considerable antioxidant potentials that are justified based on the structure and function studies. Our literature review confirmed that the substitution at N or C-3 position of 1H,4H-dihydro-pyrano-(2,3-c)-pyrazoles could potentially improve their antioxidant activities. These properties increased when the aromatic ring contained an electron-donating methoxy group (OCH3). This finding is in agreement with our experimental results observed in the current study. The methyl group on the phenyl ring had an effective impact on the antioxidant activity of the pyrano-pyrazoles 5b compared to that of 5c, containing the electron-withdrawing nitro group [20] (Figure 4).
We can conclude that a single administration of this compound controlled the circulation of RONS in Tetrahymena cells, likely by suppressing the lipid peroxidase pathway and restoration of the enzymatic activities. Our observations are in agreement with those reported by a previous in vitro study on the bioactivity of pyrano-pyrazoles [26, 27].
In summary, this study demonstrated that the use of the pyrano-pyrazole derivatives had cytoprotective properties against the harmful effects of stressors, such as H2O2 and SNP, by quenching free radicals while improving the activities of antioxidant enzymes.
Conclusions
Limitations of the study: Given the above facts, a single bioassay is not able to reflect the full picture of the derivatives’ effects on an organism.
Recommendations for future studies: We recommend using a battery of representative in vitro and in vivo experiments. Morphological, structural, biochemical, and especially molecular assays provide broad information about the capacity of these compounds to counter the toxic effect of stressors.
Ethical Considerations
Compliance with ethical guidelines
Funding
Author's contributions
Conflict of interest
Acknowledgements
Supplemental Materials
1. General information
The crystalline structure of the Na2CaP2O7 was identified by using X-Ray Diffraction (XRD) analysis (Bruker D8 Advance X-ray diffractometer with Cu-Kα radiation: λ= 1.5406 A°). The melting point of the pyrano-pyrazoles derivatives was determined using a Buchi 510 apparatus. The NMR spectra of 1H and 13C were recorded on a Bruker 300 MHz in DMSO-d6. The chemical shifts (δ) are expressed in ppm. The IR spectra of the samples were acquired as KBr pellets on FTIR (IR Affinity - 1S, Fourier Transform Infrared Spectrophotometer, Shimadzu). Scanning Electron Microscope (SEM) studies were performed on a HIROX SH-4000M. Analytical thin-layer chromatography was performed with Silica on TLC Alu foils purchased from Sigma Aldrich. Visualization of the developed chromatogram was performed by UV light (254nm). HPLC analyses were carried out on a Shimadzu LC-20AT equipped with a C18, 5μm x 250 mm column, detection at λ=254nm.
Analysis of the pyrano-pyrazoles 5a, 5b, and 5c were carried out using an isocratic mobile phase of 2% acetonitrile-98% water (The flow rate was 1 mL.min-1). The mobile phase was filtered through a 0.45μm nylon membrane filter before use. All reactions were carried out under air. Solvents and starting materials (Aldrich) were used without further purification.
2. General procedure for preparation of pyranopyrazoles 5a-c
The catalyst Na2CaP2O7 (20mol %) was added to a mixture of the aldehyde 1 (1 mmol), ethyl acetoacetate 2 (1mmol), hydrazine hydrate 3 (2 mmol), malononitrile 4 (1.2 mmol), and 1 ml of water in a 5 ml flask fitted with a reflux condenser. The resulting mixture was heated to reflux (an oil bath) with stirring for 60 min. Acetone (2 ml) was added and the mixture stirred for 2 min. The products and catalyst were isolated as described above and recrystallized from 96% ethanol (20 ml) to afford pyrano[2,3-c] pyrazoles 5a-c (Figure S1).
.jpg)
3. NMR and infrared spectral analysis
See Figures S2, S3, S4, S5, S6, S7, S8, S9, S10.
4. The HPLC analysis of pyranopyrazoles 5a,b,c
See Figure S11.

6. Method for the preparation of catalyst
The synthesis of the nano-structured diphosphate Na2CaP2O7 has been carried by using the Na2CO3, CaCO3, and NH4H2PO4 in 1: 1: 2 proportions, respectively (purity of starting materials greater than 99%). These chemicals were mixed in an agate mortar and progressively heated in a porcelain crucible. The synthesis of Na2CaP2O7 particles was confirmed by powder XRD, IR, SEM, and TEM studies. The steps of Na2CaP2O7 synthesis are summarized in Figure S12.

7. XDR of the catalyst
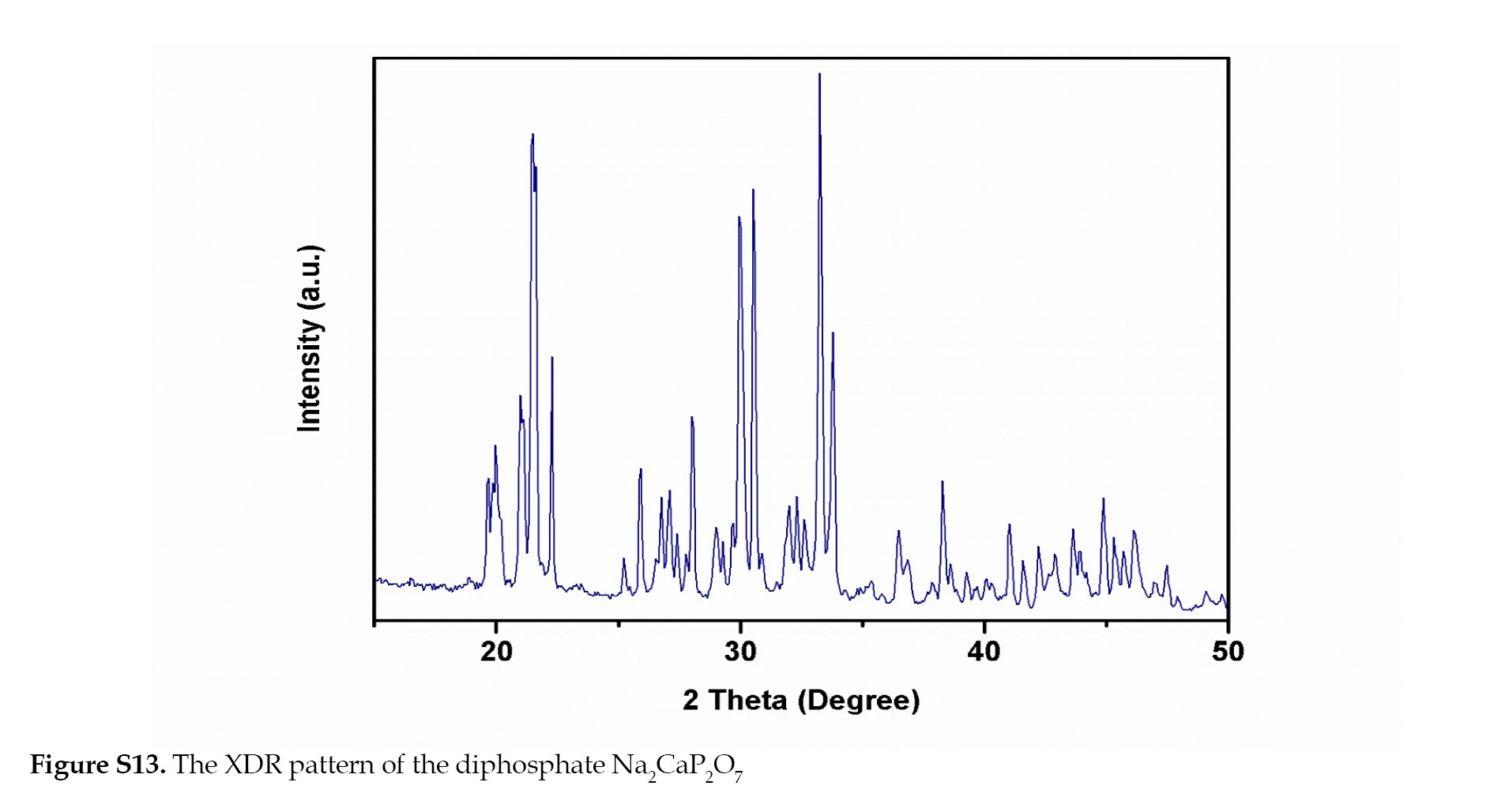
The powder obtained was analyzed by X-ray diffractometer, the diffractogram was recorded using a Bruker D8 Advance diffractogram. The spectrum of the Na2CaP2O7 diphosphate is reproduced in Figure S13 the crystallographic data obtained are gathered in Table S1.

The diffractometer uses copper anticathode radiation Cu-Kα radiation: λ= 1.5406 A°. The crystalline parameters were refined on a computer using the least-squares method via the AFPAR program. The acquisition is carried out at ambient temperature with a scanning mode θ/2 (and a Bragg angle θspanning from10° to 50°.
8. The infrared spectrum of the catalyst
The infrared spectrum of the diphosphate Na2CaP2O7 in powder form is shown in Figure S14. The appearance of symmetrical vibration bands of P-O-P at 720 cm-1 and anti-symmetric vibration bands at 893 cm-1 confirm the existence of P2O7 (Figure S14).

Two vibration fields have proved the presence of the PO4 group, the first at 996 cm−1 and 1031 cm−1 and the second going from 1130 to 1278 cm−1 (Figure S15).
9. Transmission and scanning electron microscopy
The morphological studies of the surface of Na2CaP2O7 are performed by using the scanning electron microscope HIROX SH-4000M. To visualize the microstructure of the Na2CaP2O7, Transmission Electronic Microscopy (TEM) was used on an FEI microscope operated at 120 kV (Figures S16 and S17).


10. Antioxidant activity in vitro: The DPPH test
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical method is an antioxidant assay based on electron-transfer that produces a purple solution in ethanol. This free radical, stable at room temperature, is reduced in the presence of an antioxidant which induces the coloration of ethanol solution. The use of the DPPH assay provides an easy and rapid way to evaluate antioxidants by spectrophotometry (Tables S2, S3, S4, S5).

11. Cellular mechanism of pyrazoles scaffolds
The main role of these synthetic compounds is the activation of the cellular antioxidants’ enzymes. These enzymes controlled the Reactive Oxygen and Nitrogen Species (RNOS) via several reactions. Firstly the SOD converted O2− to H2O2, this hydrogen peroxide is also degraded to H2O and O2 by the catalase, thereafter the peroxide is neutralized by the glutathione peroxidase (Figure S18).
References
- Huang AG, Tu X, Liu L, Wang GX, Ling F. The oxidative stress response of myclobutanil and cyproconazole on Tetrahymena thermophila. Environ Toxicol Pharmacol. 2016; 41:211-8. [DOI:10.1016/j.etap.2015.12.008]
- Baudin B. Stress oxydant et protections antioxydantes. Rev Francoph des Lab . 2020; 2020(522):22–30. [DOI:10.1016/S1773-035X(20)30159-3]
- Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015; 97:55-74. [DOI:10.1016/j.ejmech.2015.04.040] [PMID]
- Xu D, Hu M, Wang Y, Cui Y. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. 2019; 24(6):1123. [DOI:10.3390/molecules24061123]
- Nabi S, Tanveer S, Ganie SA. Glutathione-S-transferase , superoxide dismutase (gst , sod) levels , protein content and lipid peroxidation in schizothorax plagiostomus under the infection of pomphorhynchus in Nallah Sukhnag of Kashmir Valley. Pak J Biol Sci. 2017; 20(9):442-6. [DOI:10.3923/pjbs.2017.442.446]
- Khairujjaman M, Choudhury S, Borah A. An in silico investigation on the inhibitory potential of the constituents of Pomegranate juice on antioxidant defense mechanism : Relevance to neurodegenerative diseases. IBRO Rep. 2019; 6:153-9. [DOI:10.1016/j.ibror.2019.05.003]
- Reddy GM, Garcia JR, Reddy VH, Kumari AK, Zyryanov GV, Yuvaraja G. An efficient and green approach: One pot, multi component, reusable catalyzed synthesis of pyranopyrazoles investigation of biological assays. J Saudi Chem Soc. 2019; 23(3):263-73. [DOI:10.1016/j.jscs.2018.07.003]
- Ismail MMF, Khalifa NM, Fahmy HH, Nossier ES, Abdulla MM. Design, docking, and synthesis of some new pyrazoline and pyranopyrazole derivatives as anti-inflammatory agents. J Heterocycl Chem. 2014; 51(2):450-8. [DOI: 10.1002/jhet.1757]
- Tok F, Koçyiğit-Kaymakçıoğlu B. Design, synthesis and biological screening of novel 1, 5-Diphenyl-3-(4-(trifluoromethyl) phenyl)-2-pyrazoline derivatives. Acta Chim Slov. 2020; 67(4):1139-47. [DOI:10.17344/acsi.2020.6028]
- Mamaghani M, Hossein Nia R. A Review on the recent multicomponent synthesis of pyranopyrazoles. Polycycl Aromat Compd. 2019; 41(2):223–91. [DOI:10.1080/10406638.2019.1584576]
- Das D, Banerjee R, Mitra A. Bioactive and pharmacologically important pyrano [2,3-c] pyrazoles. J Chem Pharm Res. 2014; 6(11):108-16. https://www.jocpr.com/abstract/bioactive-and-pharmacologically-important-pyrano23cpyrazoles-4828.html
- Mar PD, Khalfi B El, Perez-Castiñeira JR, Serrano A, Soukri A. Cell stress by phosphate of two protozoa tetrahymena thermophile and tetrahymena pyriformis. Adv Biosci Biotechnol. 2017; 8(12):451. [DOI:10.4236/abb.2017.812033]
- An TN, Alam M, Son NV, Cuong NV, Quang NM, Tri MD, et al. Synthesis, physical chemistry, molecular docking, bioactivities and antioxidant activity of α–amino phosphonates based on phenothiazine using PEG–400 as green catalyst. ChemistrySelect. 2019; 4(31):8915-20. [DOI:10.1002/slct.201901560]
- Ye Q, Zhang C, Wang Z, Feng Y, Zhou A, Xie S, et al. Induction of oxidative stress, apoptosis and DNA damage by koumine in Tetrahymena thermophila. PLoS One. 2019; 14(2):e0212231. [DOI:10.1371/journal.pone.0212231
- Fourrat L, Iddar A, Valverde F, Serrani A, Soukri A. Effects of oxidative and nitrosative stress on tetrahymena pyriformis glyceraldehyde-3-phosphate dehydrogenase. J Eukaryot Microbiol. 2007; 54(4):338-46. [DOI:10.1111/j.1550-7408.2007.00275.x]
- Zahouily M, Elmakssoudi A, Mezdar A, Rayadh A, Sebti S, Lazrek HB. Three components coupling catalysed by Na2CaP2O7: Synthesis of α-amino phosphonates under solvent-free conditions at room temperature. Letters Organic Chem. 2005; 2(5):428-32. [DOI:10.2174/1570178054405887
- Maleki B, Nasiri N, Tayebee R, Khojastehnezhad A, Akhlaghi HA. Green synthesis of tetrahydrobenzo [b] pyrans, pyrano [2,3-c] pyrazoles and spiro [indoline-3,4′-pyrano [2,3- c] pyrazoles catalyzed by nano-structured diphosphate in water. RSC Adv. 2016; 6(82):79128-34. [DOI:10.1039/C6RA15800E
- Mar PD, Khalfi B El, Soukri A. Protective effect of oregano and sage essentials oils against oxidative stress in Tetrahymena thermophila and Tetrahymena pyriformis. J King Saud Univ. 2018; 32(1):279-87. [DOI: 10.1016/j.jksus.2018.05.005]
- Azaam MM, Kenawy ER, El-din ASB, Khamis AA, El-Magd MA. Antioxidant and anticancer activities of α-aminophosphonates containing thiadiazole moiety. J Saudi Chem Soc. 2018; 22(1):34-41. [DOI: 10.1016/j.jscs.2017.06.002]
- Maddila S, Gorle S, Shabalala S, Oyetade O, Narayana S, Lavanya P, et al. Ultrasound mediated green synthesis of pyrano-[2,3-c]-pyrazoles by using Mn doped ZrO 2. Arab J Chem. 2019; 12(5):671-9. [DOI:10.1016/j.arabjc.2016.04.016]
- Errafiy N, Soukri A. Purification and partial characterization of glyceraldehyde-3-phosphate dehydrogenase from the ciliate Tetrahymena thermophila. Acta Biochim Biophys Sin. 2012; 44(6):527-34. [DOI:10.1093/abbs/gms028] [PMID]
- Mar Pape D, Otto H, EL khalfi B, Soukri A. Biochemical study of the protective effect of salvia officinalis essential oil against the oxidative stress induced by Hydrogen Peroxide in Tetrahymena thermophila. Der Pharma Chem J. 2019; 11(3):8-12. https://www.derpharmachemica.com/pharma-chemica/biochemical-study-of-the-protective-effect-of-emsalvia-officinalisem-essential-oil-against-the-oxidative-stress-induced-by-hydroge-18354.html
- Abhinav B, Dinesh C, Alpana A, Pratyush K, Shrikant M. Green synthesis of pyranopyrazole using microwave assisted techniques. GSC Biol Pharm Sci. 2020; 10(02):111-9. [DOI:10.30574/gscbps.2020.10.2.0026]
- BBennazha J, Zahouily M, Sebti S, Boukhari A, Holt EM. Na2CaP2O7, a new catalyst for Knoevenagel reaction. Catal Commun. 2001; 2(3–4):101–4. [DOI:10.1016/S1566-7367(01)00015-2]
- Solhy A, Elmakssoudi A, Tahir R, Larzek M, Bousmina M, Zahouily M. Clean chemical synthesis of 2-amino-chromenes in water catalyzed by nanostructured Diphosphate Na2CaP2O7. Green Chem. 2010; 12:2261-7. [DOI:10.1039/c0gc00387e]
- Addoum B, El Khalfi B, Derdak R, Sakoui S, Elmakssoudi A, Soukri A. The one-pot synthesis of some bioactive pyranopyrazoles and evaluation of their protective behavior against extracellular H2O2 and SNP in T. Thermophila. Jordan J Biol Sci. 2021; 14(1):31-9. http://jjbs.hu.edu.jo/files/vol14/n1/Paper%20Number%206.pdf
- Chougala BM, Samundeeswari S, Holiyachi M, Shastri LA, Dodamani S, Jalalpure S, et al. Synthesis, characterization and molecular docking studies of substituted 4-coumarinylpyrano-[2,3-c]-pyrazole derivatives as potent antibacterial and anti-inflammatory agents. Eur J Med Chem. 2017; 125:101-16. [DOI:10.1057/s41305-017-0056-9
- Khurana JM, Chaudhary A. Efficient and green synthesis of 4H-pyrans and 4H-pyrano[2,3-c] pyrazoles catalyzed by task-specific ionic liquid [bmim] OH under solvent-free conditions. Green Chem Lett Rev. 2012; 5(4):633-8. [DOI:10.1080/17518253.2012.691183]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





