INTRODUCTION
Arsenic, a naturally
occurring element and by-product of copper, lead and other metals smelters, is
the top environmentally hazardous substances, which were demonstrated to be a
human carcinogen (1,2,3). Arsenic exist in several oxidative states but it's
pentavalent (arsenate, AS5+) and trivalent (arsenite, AS+3) forms are most
prominent in the environment which have toxicological significance (2). Base on
the American FDA recommendation, arsenic
trioxide (arsenite) has
been used for the treatment of relapsed or refractory of acute promyelocytic
leukemia in 2000 (4,5).
There
are investigations which report
the presence of <5µM of sodium arsenite in the serum of malignant patients (6,7). Trivalent salt of arsenic (arsenite) is considered more toxic than
pentavalent one and the report indicated that the sodium arsenite causes
genetic and epigenetic changes in mouse testicular leydig cells (8,9).
Sodium arsenite also induces apoptosis in different type of cells such as rat
midbrain neuroepithelial, CD4+ T
cells, human neutrophils, Gclm mouse embryo fibroblasts and human bone marrow mesenchymal stem cell
in micromolar concentration (10-13). Sodium arsenite readily react with thiol group of enzymes, receptors
or coenzymes which may inhibit important biochemical events that could alter
cellular redox status and eventually lead to cytotoxicity (14,15). Some other
mechanisms including genotoxicity, alteration in DNA repair and methylation,
oxidative stress, co-carcinogenesis, and tumor promotion also have been
reported for arsenite toxicity (9,16,17).
Multipotent rat bone marrow mesenchymal stem
cells (MSCs) representing <0.01-0.001% of the total nucleated bone marrow
and
posses two fundamental characteristics: the ability of extensive replication
and the capacity of multilineage differentiation among bone, cartilage and
adipose cell lineages (18-20). Yadev, et al. in their report showed that high
concentration (>5µM) of sodium arsenite affect viability, DNA synthesis,
morphology, cell cycle and apoptosis of human bone marrow mesenchymal stem
cells (hMSCs) (13). Result of our
previous studies showed that the sodium arsenite (<5µM) in 36hrs caused
significant reduction of rat bone marrow mesenchymal stem cells (rMSCs)
viability due to caspase dependent apoptosis in culture media (21). In
addition, we have shown that much lower concentration of sodium arsenite (25nM)
caused the significant reduction of BMCs viability after 21 days due to caspase dependent
apoptosis, but there is no data available on the effect of sodium arsenite on
differentiation property of MSCs (22). Therefore, in this report, we
investigated the effects of 1 and 25 nM of sodium arsenite on morphology,
viability, calcium concentration, alkaline phosphatase activity and
mineralization of rat bone marrow mesenchymal stem cells following its
differentiation to osteoblasts.
MATERIALS AND
METHODS
Marrow cell culture
In
the present study, Wistar rats (6-8 weeks old) were purchased from Pastor
Institute (Tehran, Iran)
and kept in the animal house of Arak
University under standard
condition of light and food. The animals were sacrificed by excessive
chloroform inhalation and then their tibia as well as femur were removed and
cleaned from the adherent soft tissue. Then the two ends of the bones were cut
off and bone marrow was flash out using
2 ml DMEM (Dulbeccos Modified Eagles Medium, Gibco, Germany) supplemented with
15% FBS (Fetal Bovine Serum, Gibco, Germany) and penicillin-streptomycin
(Gibco, Germany).
Bone
marrow content was centrifuged at 1200 rpm for 5 minutes and re-suspended in 5
ml DMEM containing 15% FBS and antibiotics then plated in 25-cm2
flasks and incubated at 37 °C with atmosphere of 5% CO2. Two days
after culture initiation, the first medium replacement was performed and then
medium was changed two times a week till the bottom of the flask was covered
with the cells (till confluency). The cells were trepsinized (trypsin-EDTA, Gibco, Germany)
and passed to another culture flask as the first passage and then the cultures
were expanded through two additional subcultures for more purification of the
mesenchymal stem cells which were used for further investigation.
Osteogenic induction
Mineralization
was induced on confluent monolayers of cells by addition of DMEM containing 15%
(v/v) FBS, streptomycin-penicillin
and osteogenic supplements [1mM sodium glycerophosphate, 50 µgmM L-ascorbate and 10-8 M
dexamethasone (all the chemicals were purchased from Sigma- Aldrich company)].
Culture flasks were incubated for 21 days at 37°C with 5% CO2 and
their medium was changed every 3 days (23).
Exposure to
sodium arsenite
To perform the assays, cells were
cultured in separate culture dishes in presence of DMEM supplemented with
osteogenic media for periods (days) according to the design of the test, which
represented control and sodium arsenite treated (exposed to 1 and 25 nM of
sodium arsenite) groups.
Cell viability
assays
The viability test on control and
treated cells was carried out in an ELISA plate using MTT (4,
5dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), where after 4 hours of
incubation the mitochondrial succinate dehydrogenase in the live cells convert
yellow color tetrazolium into violet
crystal of formazan. Then 100µL of DMSO was added to each well of the plate and
formazan crystals were extracted in that following incubation for 30 min (1/2
hrs) in room temperature. The extracted solutions were transfer in another well
and absorbance was measured on an automated microplate reader (SCO diagnostic, Germany) at 505
nm.
Analysis of morphological changes
Following sodium arsenite treatment in an osteogenic
media for 21 days, the nuclear morphology of the cells was studied using
Hoechst 33342 at room temperature after 5 minutes of incubation in the dark.
The diameter of the cells was also measured in µm with the help of Motic Image
software (Micro optical group company version 1.2). after 21 days Hoechst is a
fluorescent dye which penetrate the
cells through the intact plasma membrane and stain the DNA , and where the
changes in nuclear morphology such as chromatin condensation and fragmentation
can be investigated (24).
The morphology of the cell cytoplasm was investigated
using another fluorescent dye (acridine orange) which stains the nuclei green
and the cytoplasm orange. The cells after staining were washed twice with PBS,
examined and immediately photographed under an inverted fluorescence microscope
(Olympus,
IX70) equipped with camera using 40X magnification.
Detection and quantification of mineralization
Cells in 6-well plates were washed with PBS and fixed in
10% (v/v) formaldehyde (Sigma-Aldrich) at room temperature for 15 minutes. The
cells were then washed twice with excess of dH2O and 1mL of 40mM
alizarin red solution (ARS) (pH 4.1) was added per well. The plates were then
incubated at room temperature for 20 minutes with gentle shaking. After which
the excess of dye was poured off and the plates were washed four times with dH2O.
Stained cells were investigated under light microscopy
using an inverted microscope. To quantify the level of absorbed alizarin red,
800µL of 10% acetic acid (v/v) was added to each well, and the plate was
incubated at room temperature for 30 minutes with gentle
shaking.
Then the loosely
attached cells were scraped from the plate with a cell scraper and transferred
to a 1.5-mL microcentrifuge tube. After vortexing for 30s, the slurry was
overlaid with 500µL mineral oil (Sigma-Aldrich), heated at 85°C for 10 minutes,
and then kept on ice for 5 minutes. The slurry was then centrifuged at 20,000g
for 15minutes and 500µL of the supernatant was transferred to a new microcentrifuge tube and 200µL of
10% ammonium hydroxide (v/v) was added to neutralize the acid. An aliquots of
the supernatant (100µL) was read in triplicate at 405 nm in a microplate
reader (SCO diagnostic, Germany) and quantified against standard graph (23).
In
order to prepare Alizarin Red standards graph, working ARS (40 mM) was diluted
20 times with a mixture of 5:2 of 10% acetic acid and 10% ammonium to give a
concentration of 2000µM. Then using serial dilution, standard solution of 2000
to 31.3µM was prepared and the absorption was taken at 405nm using a microplate
reader. The concentration of the unknown samples was calculated
using linear formula Y=0.179X+0.094 with R2=0.997 where Y is the
absorbance and X is the concentration (mM) of alizarin red.
Alkaline phosphatase activity
Alkaline
phosphatase (ALP) activity of control and treated cells in 6 well dishes was
determined by p-nitrophenyl-phosphate (pNPP) hydrolysis method, using the ALP
assay kit (Darman Kave, Iran). Cells were washed three
times with PBS and homogenized in lysis buffer (0.25 M Tris-HCl, Triton X-100,
PH:7.5) and the samples were centrifuged
at 12000 rpm for 10 minutes at 4˚c (25).
The supernatant was kept in -20˚C for the
analysis of ALP activity and protein content. The total protein content of each
sample was determined according to Bradford,
using bovine serum albumin (BSA) as standard. ALP activity was determined in
protein lysate base on equal amount of protein using p-nitrophenylphosphate (pNPP) as
substrate according to the kit instruction (Darman
Kave, Iran).
Absorbance at 410 nm was measured using spectrophotometer (T80+ PG instrument ltd, England) and then ALP activity was
determined from a pNPP standard curve.
Calcium concentration
Cells in 6-well plates including control and treated ones
were first washed twice with PBS and then their calcium content was extracted
in 50 µl of 0.5 N HCl for 24 hours (26).
The amount of
calcium was determined using commercial kit (Darman Kave,
Iran) and the developed
color was measured at 575 nm using spectrophotometer (T80+ PG instrument ltd, England).
Statisticla analysis
Statistical evaluation of the data was performed using
one and two-way analysis of variance (ANOVA) Tukey test, with the help of
SPSS. Results were shown as mean±S.D and
P<0.05 was accepted as the minimum level of significance.
RESULTS
Effect of sodium arsenite on cell viability
Cell
viability assay (Table 1) showed that the 25 nM of sodium arsenite significantly decreased the
viability (p<0.001) of bone marrow
mesenchymal stem cell under osteogenic differentiation at 5th, 10th, 15th and
21th days as compared with control. Lower dose of sodium arsenite (1nM) showed
no significant effect (p>0.05) on the viability of the cells at 5th and 10th
days whereas at 15th and 21th days significant decrease of viability
(p<0.05) was observed. Analysis of data using two-way ANOVA showed that the
significant reduction (P<0.001) of viability depends on both dose and time
of exposure (Table 2).
Sodium arsenite induced morphological changes of MSCs differentiated
cells
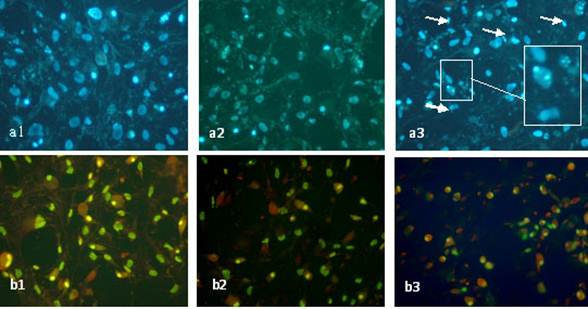
Morphological study of the nuclei of differentiated mesenchymal stem cells
treated with 1 and 25 nM
of sodium arsenite after 21 days showed significant reduction (p<0.05) in nuclei
diameter (Table 3) and chromatin condensation as well as nuclear breakage
(Figure1-a2and3). It can be also noticed that sodium arsenite at these
concentrations caused remarkable changes in the morphology of cytoplasm
(Figure1-c3) such as shrinkage and in some cells complete disappearance of
cytoplasm as compared to control cells.
DISCUSSION
The present study was designed to investigate the effect of low dose (nM)
of sodium arsenite on differentiation of MSCs to osteoblasts and attempt was to
characterize the cellular and molecular nature of differentiated MSCs in
response to this toxicant. Previous studies have shown that sodium arsenite
enhance apoptosis in cell types such as rat midbrain neuroepithelial , CD4+ T
cells , human neutrophils , Gclm mouse embryo fibroblasts and human bone marrow
mesenchymal stem cell (10-13). In this study, the viability of differentiated
MSCs in response to 1 and 25 nM of sodium arsenite have reduced significantly
at 15th day onwards but no effect was observed at 5th day, though the 25 nM
reduced the viability even at 5th day. As the low and high dose (1 and 25 nM)
of sodium arsenite showed significant effect on viability, therefore we may say
that there is only time limitation for sodium arsenite toxic effect. Since bone
matrix is in direct contact with peripheral blood, therefore the presence of
even low dose up to 1 nM might be of a great concern. In previous studies it
was shown that the concentration of sodium arsenite was less than 5µM in the
blood of malignant patients under therapy with this chemical (6,7). Since the
MSCs are able
to differentiate to osteoblasts, chondrocytes and adipocyte, therefore it
is considered as the main source of bone regeneration and remodeling during its
homeostasis (27-30). Thus it should be taken in consideration that, in presence of sodium
arsenite the health of the bone might be in great danger.
We found also, 1 and 25 nM of sodium arsentie after 21 days of treatment
caused chromatin condensation, nuclear diameter reduction and nuclear breakage
as well as cytoplasm shrinkage which all together might be considered as sign
of apoptosis and a reason for significant viability reduction (31). Many investigators have shown that the sodium arsenite cause activation
of caspases through internal and external pathway thus the viability reduction of
MSCs under osteogenic differentiation might be due to apoptosis (8,32-34). Also,
sodium arsenite induces oxygen free radicals production which might be another
reason of nuclear breakage (17,35). In addition, differentiation of MSCs to
osteoblasts is followed by changes in cytoskeleton content like actin, where it
is well documented that the sodium arsenite can affect the cytoskeleton, which can be another reason for cytoplasm shrinkage (36-38).
Our finding showed that the level of mineralization in term of quantitative
alizarin red, calcium concentration and alkaline phosphatase activity reduced
significantly (p<0.05) from the 10th day in the 1 and 25 nM group as
compared to the control group. After a certain period of time, in vitro
osteogenic mineralization starts, with respect to alkaline phosphatase activity
resulting in the release of phosphate ion which bring about large influx of
calcium ion into the cells (23,39). The influx of calcium is a necessary step in formation of
hydroxyapatite crystal which is the prompt step of bone formation (40). At this point, with respect to viability and mineralization data it may
be concluded that the effect of the sodium arsenite began as the osteogenic
changes occurred in the cell somehow after the 5th day.
As mentioned earlier, researches has shown
that sodium arsenite induces oxidative stress and the oxidative stress induced by oxygen free
radicals inhibit osteogenic differentiation processes thus it might be another reason of why sodium
arsenite caused impairment in osteogenic differentiation processes (17,35,41). Furthermore, osteogenic
differentiation depend on Wnt signaling, where in this pathway in the presence
of the β-catenin and ICF/LEF factor activation of alkaline phosphatase
genes takes place (42, 43). Researches has shown that
oxygen free radical can inhibit expression of alkaline phosphatase gene by
disrupting Wnt signaling which might be
a reason for significant reduction of enzyme activity in this study (41). In addition, investigators
showed that the free radicals can cause inhibition of calcium channel and
disruption of calcium homeostasis which itself might be a reason for
significant reduction of calcium influx due to sodium arsenite toxicity (44,45).
CONCLUSION
All together, it
is to be mentioned that the MSCs are pluripotent stem cells
that can differentiate to osteoblast and are also considered to be a major
source of bone formation and remodeling, thus there health should be under a
great consideration and attention (30,46). As sodium arsenite has been recommended to be used in therapy as well
as it was reported that this chemical is presented in some food material such
as rice and water therefore their consumption would increase the sodium
arsenite concentration in human blood, thus it might have profound effect on
bone homeostasis and remodeling (4,5,47,48). Therefore we strongly suggest more
investigation to be carried out regarding the relationship between bone
diseases such as osteoporosis and sodium arsenite toxicity in general
population.
REFRENCES
1.
Tchounwou PB, Centeno JA, Patlolla AK.
Arsenic toxicity, mutagenesis, and carcinogenesisa health risk assessment and
management approach. Molecular and cellular biochemistry. 2004;255(1):47-55.
2.
Kann S, Estes C, Reichard JF, Huang M,
Sartor MA, Schwemberger S, et al. Butylhydroquinone protects cells genetically
deficient in glutathione biosynthesis from arsenite-induced apoptosis without
significantly changing their prooxidant status. Toxicological sciences.
2005;87(2):365-84.
3.
Okoji R, Yu R, Maronpot R, Froines J.
Sodium arsenite administration via drinking water increases genome-wide and
Ha-ras DNA hypomethylation in methyl-deficient C57BL/6J mice. Carcinogenesis.
2002;23(5):777-85.
4.
Antman KH. Introduction: the history of
arsenic trioxide in cancer therapy. The oncologist. 2001;6(2):1-2.
5.
Zhang TD, Chen GQ, Wang ZG, Wang ZY, Chen
SJ, Chen Z. Arsenic trioxide, a therapeutic agent for APL. Oncogene.
2001;20(49):7146-53.
6.
McCollum G, Keng PC, McCabe MJ. Arsenite
delays progression through each cell cycle phase and induces apoptosis
following G2/M arrest in U937 myeloid leukemia cells. Journal of Pharmacology
and Experimental Therapeutics. 2005;313(2):877-87.
7.
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong
SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute
promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in
relapsed patients. Blood. 1997;89(9):3354-60.
8.
Lewis AS. Organic versus inorganic
arsenic in herbal kelp supplements. Environmental health perspectives.
2007;115(12):A575-6.
9.
Singh K, DuMond J. Genetic and
epigenetic changes induced by chronic low dose exposure to arsenic of mouse
testicular Leydig cells. International Journal of Oncology. 2007;30(1):253-60.
10.
Sidhu JS, Ponce RA, Vredevoogd MA, Yu X,
Gribble E, Hong SW, et al. Cell cycle inhibition by sodium arsenite in primary
embryonic rat midbrain neuroepithelial cells. Toxicological sciences.
2006;89(2):475-84.
11.
Tenorio EP, Saavedra R. Differential
effect of sodium arsenite during the activation of human CD4+ and CD8+ T
lymphocytes. International immunopharmacology. 2005;5(13-14):1853-69.
12.
Watson R, Redmond HP, Wang JH,
Bouchier-Hayes D. Mechanisms involved in sodium arsenite-induced apoptosis of
human neutrophils. Journal of leukocyte biology. 1996;60(5):625-32.
13.
Yadav S, Shi Y, Wang F, Wang H. Arsenite
induces apoptosis in human mesenchymal stem cells by altering Bcl-2 family
proteins and by activating intrinsic pathway. Toxicology and applied
pharmacology. 2010;244(3):263-72.
14.
Jin Y, Sun G, Li X, Li G, Lu C, Qu L.
Study on the toxic effects induced by different arsenicals in primary cultured
rat astroglia. Toxicology and applied pharmacology. 2004;196(3):396-403.
15.
Liao WT, Lin P, Cheng TS, Yu HS, Chang
LW. Arsenic promotes centrosome abnormalities and cell colony formation in p53
compromised human lung cells. Toxicology and applied pharmacology.
2007;225(2):162-70.
16.
Chan PC, Huff J. Arsenic carcinogenesis
in animals and in humans: mechanistic, experimental, and epidemiological
evidence. Journal of Environmental Science & Health Part C.
1997;15(2):83-122.
17.
Lee PC, Ho IC, Lee TC. Oxidative stress
mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte
chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells.
Toxicological sciences. 2005;85(1):541-50.
18.
Wollert KC, Drexler H. Mesenchymal Stem
Cells for Myocardial Infarction. Circulation. 2005;112(2):151-3.
19.
Eslaminejad MB, Nazarian H, Falahi F,
Taghiyar L, Daneshzadeh MT. Ex vivo Expansion and Differentiation of
Mesenchymal Stem Cells from Goat Bone Marrow. Iranian Journal of Basic Medical
Sciences. 2009;12(2),70-9.
20.
Eslaminejad MB, Talkhabi M, Zeynali B.
Effect of lithium Chloride on Prolioferation and Bone Differentiation of Rat
MarrowDerived Mesenchymal Stem Cellsin Culture. Iranian Journal of Basic
Medical Sciences2008;11(3),143-51.
21.
Abnosi MH, Soleimani Mehranjani M,
Momeni HR, Mahdieh Najafabadi M, Shojafar E, Barati M. Effect of sodium
arsenite on rat bone marrow mesenchymal stem cells: cells viability and
morphological study. Scientific Journal of Hamedan University
of Medical Sciences.2010;17(2): 10.
22.
Abnosi MH, Jafari Yazdi Z. Low dose and
long term toxicity of sodium arsenite caused caspase dependent apoptosis base
on morphology and biochemical character. Cell Journal (Yakhteh). 2012; 55(3):
inpress.
23.
Gregory CA, Grady Gunn W, Peister A,
Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in
culture: comparison with cetylpyridinium chloride extraction. Analytical
biochemistry. 2004;329(1):77-84.
24.
Yao G, Ling L, Luan J, Ye D, Zhu P.
Nonylphenol induces apoptosis of Jurkat cells by a caspase-8 dependent
mechanism. International immunopharmacology. 2007;7(4):444-53.
25.
Na K, Kim SW, Sun BK, Woo DG,
Yang HN, Chung HM, Park KH. Osteogenic differentiation of rabbit mesenchymal
stem cells in thermo-reversible hydrogel constructs containing hydroxyapatite
and bone morphogenic protein-2 (BMP-2). Biomaterials. 2007;28(16):2631-7.
26.
Ogura N, Kawada M, Chang WJ, Zhang Q,
Lee SY, Kondoh T, et al. Differentiation of the human mesenchymal stem cells
derived from bone marrow and enhancement of cell attachment by fibronectin.
Journal of oral science. 2004;46(4):207-13.
27.
Baksh D, Song L, Tuan R. Adult
mesenchymal stem cells: characterization, differentiation, and application in
cell and gene therapy. Journal of cellular and molecular medicine.
2004;8(3):301-16.
28.
Heino TJ, Hentunen TA, Väänänen HK.
Conditioned medium from osteocytes stimulates the proliferation of bone marrow
mesenchymal stem cells and their differentiation into osteoblasts. Experimental
cell research. 2004;294(2):458-68.
29.
Knothe Tate ML, Adamson JR, Tami AE,
Bauer TW. The osteocyte. The international journal of biochemistry & cell
biology. 2004;36(1):1-8.
30.
Kassem M, Abdallah BM, Saeed H.
Osteoblastic cells: differentiation and trans-differentiation. Archives of
biochemistry and biophysics. 2008;473(2):183-7.
31.
Elmore S. Apoptosis: a review of
programmed cell death. Toxicologic pathology. 2007;35(4):495-516.
32.
Ivanov VN, Hei TK. Sodium arsenite
accelerates TRAIL-mediated apoptosis in melanoma cells through upregulation of
TRAIL-R1/R2 surface levels and downregulation of cFLIP expression. Experimental
cell research. 2006;312(20):4120-38.
33.
Che XF, Zheng CL, Owatari S, Mutoh M,
Gotanda T, Jeung HC, et al. Overexpression of survivin in primary ATL cells and
sodium arsenite induces apoptosis by down-regulating survivin expression in ATL
cell lines. Blood. 2006;107(12):4880-7.
34.
Cai B, Xia Z. p38 MAP kinase mediates
arsenite-induced apoptosis through FOXO3a activation and induction of Bim
transcription. Apoptosis. 2008;13(6):803-10.
35.
Lau ATY, He QY, Chiu JF. A proteome
analysis of the arsenite response in cultured lung cells: evidence for in vitro
oxidative stress-induced apoptosis. Biochemical Journal. 2004;382(Pt 2):641-50.
36.
Titushkin I, Cho M. Modulation of
cellular mechanics during osteogenic differentiation of human mesenchymal stem
cells. Biophysical journal. 2007;93(10):3693-702.
37.
Li W, Chou IN. Effects of sodium
arsenite on the cytoskeleton and cellular glutathione levels in cultured cells.
Toxicology and applied pharmacology. 1992;114(1):132-9.
38.
Yancy SL, Shelden EA, Gilmont RR, Welsh
MJ. Sodium arsenite exposure alters cell migration, focal adhesion localization
and decreases tyrosine phosphorylation of focal adhesion kinase in H9C2
myoblasts. Toxicological sciences. 2005;84(2):278-86.
39.
Lorch IJ. Alkaline phosphatase and the
mechanism of ossification. Journal of Bone and Joint Surgery-British Volume.
1949;31(1):94-100.
40.
Termine JD, Kleinman HK, Whitson SW,
Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking
mineral to collagen. Cell. 1981;26(1):99-105.
41.
Almeida M, Han L, Martin-Millan M,
O'Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in
osteoblast precursors by diverting β-catenin from T cell factor-to
forkhead box O-mediated transcription. Journal of Biological Chemistry.
2007;282(37):27298-305.
42.
Hartmann C. A Wnt canon orchestrating
osteoblastogenesis. Trends in cell biology. 2006;16(3):151-8.
43.
Day TF, Guo X, Garrett-Beal L, Yang Y.
Wnt/[beta]-catenin signaling in mesenchymal progenitors controls osteoblast and
chondrocyte differentiation during vertebrate skeletogenesis. Developmental
cell. 2005;8(5):739-50.
44.
Duthie GG, Arthur JR. Free radicals and
calcium homeostasis: relevance to malignant hyperthermia? Free Radical Biology
and Medicine. 1993;14(4):435-42.
45.
Hess ML, Kukreja RC. Free radicals,
calcium homeostasis, heat shock proteins, and myocardial stunning. The Annals
of thoracic surgery. 1995;60(3):760-6.
46.
Milat F, Ng KW. Is Wnt signalling the
final common pathway leading to bone formation? Molecular and cellular
endocrinology. 2009;310(1):52-62.
47.
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH,
McGrath SP, et al. Transporters of arsenite in rice and their role in arsenic
accumulation in rice grain. Proceedings of the National Academy
of Sciences. 2008;105(29):9931-5.
48.
Ahmed K, Akhand AA, Hasan M, Islam M,
Hasan A. Toxicity of Arsenic (Sodium Arsenite) to Fresh Water Spotted Snakehead
Channa punctatus (Bloch) on Cellular Death and DNA Content. Am-Euras J Agric
Environ Sci. 2008;4(1):18-22.