Introduction
Currently, coastal ecosystems are exposed to a heavy metal pollution due to the current industrial and urban activities. This problem arises from the fact that large amounts of waste materials are dumped without proper advance treatments. Heavy metals are of special interest because they are highly persistent, toxic, and can accumulate in the exposed organisms [
1, 2]. During the transport of heavy metals to the sea, they undergo physical and chemical transformations, such as: precipitation, sedimentation and adsorption in the sediments, all of these make them available to the aquatic life [
3]. In this context, heavy metals contamination is a serious threat to aquatic systems due to their toxicity, abundance and persistence in the environment [
4].
Cadmium (Cd) is a non-essential element that is generated as a waste material from fertilizer production and other industrial activities. There are no natural sources that generate and release Cd into the environment [
5]. In species, such as Galaxias maculatus the effects of Cd on the metabolic processes have been observed, including the deterioration of oxygen consumption, stress parameters, and decreased activity of hepatic catalase enzyme [
6]. Copper (Cu) is an essential trace element that can cause harmful effects in fish and tend to accumulate heavy metals in their tissues, causing damages, such as histopathological alterations [
7]. Lead (Pb) is a potential threat to aquatic life, leading to decreases in red and white blood cells in both humans and fish. It also decreases blood hemoglobin, and is responsible for a rise in the rate of serum heterophile antigens [
8].
Catfish, Ariopsis felis (A. felis), is produced and marketed in Mexico either captured from the sea or at aquaculture farms. It is popular for its flavor and nutritional benefits, and is a source of high economic profits, its capture and production is carried out by various communities in Mexico [
9]. The American Heart Association recommends the consumption of fish for the useful fatty acids contents [
10]. There are few studies conducted in Mexico on this subject. One of the studies that was undertaken in the Gulf of Mexico, showed that the abundant toxic metals found in the catfish tissues were chrome (Cr), vanadium (V), and nickel (Ni) [
11].
Health risk analyses allow us to predict the possible adverse effects on humans arising from carcinogens and non-carcinogens due to the consumption of species that are exposed to toxic food contaminants. This study was designed to investigate the presence of specific heavy metals and the degree of contamination in edible tissue samples of catfish A. felis that grows in Mexico, and to compare the levels with the safe limits set by national and international organizations, such as Food and Agriculture Organization (FAO), World Health Organization (WHO), Ministry of Agriculture Forestry and Fisheries (MAFF), and Official Mexican Standards (NOM).
Materials and Methods
Geographic Area: Pom-Atasta system in Mexico is located in the western part of the Terminos Lagoon, between 18°, 30’ and 18°, 35’ north latitude and 91°, 50 ‘ and 92°, 20’ west longitude. It is part of the coastal plain of Campeche, supplied by the Grijalva and Usumacinta rivers. This aquatic system consists of interior lagoons of variable dimensions, with a total area of approximately 300 km2 and a depth of 2.7 meters. Atasta and Pom are the largest body of waters, and include banks where clams (Rangia cuneata), oysters (Crassostrea virginica), crab (Callinectes sapidus) and catfish are caught commercially. The water temperature in this area ranges between 25.6 and 32.6°C with the transparency of 20 to 83.3%. The oxygen content in these waters vary widely, from 4.2 to 8.2 ml/L. This aquatic system is dominated by clay silt sediments with a low content of calcium carbonate [
12].
Marine Sample Collection: In Atasta Lagoon (
Figure 1), fishing make up an important economic activity. To catch the marine organisms, two sampling campaigns were carried out in the rainy and dry seasons (
Table 1).
.jpg)
The organisms were collected through artisanal fishing techniques, using casting net. A total of 60 catfish samples were caught.
.jpg)
Sample preparation for analyses: The edible tissue was dissected and removed from each fish, using a composite sample type. These tissues were homogenized in a food processor, obtaining a sample of 20 g, which were treated according to the method suggested by the official Mexican standards [
13]. The tissue digestion was achieved by the addition of 10 ml of nitric acid (HNO3; CAS 7697-32-2; Sigma Aldrich) followed by heating the homogenate at controlled temperature and the addition of 2ml hydrogen peroxide H2O2 (CAS 7722-84-1; Sigma Aldrich) in 30% solution. The digested samples were concentrated to a volume of 1ml, and filtered through Whatman #32 paper. Finally, the filtrate was made up to 20 ml for the subsequent analyses.
Sample analyses: The edible tissue samples were analyzed in an atomic flame absorption equipment adapted with a Thermo-Scientific brand graphite furnace (iCE 3000). For the quality control of the procedures, the traceable reference materials of the ARSA spectrometric solutions were used. These were produced under the NMX-EC-17025-IMNC-2006 standard (the general requirements for the competence of testing and calibration laboratories). For the quality control and calibration of the equipment, the Limits Of Detection (LD) and Quantification (LQ) were estimated.
Statistical analyses: The variation of the mean concentrations of heavy metal contents in the edible tissue were evaluated according to the climatic season (rainy, dry) and the site, by means of a one way analysis of variance (ANOVA, α=0.05), using the statistical package, Statistica software, version 7.1.
Risk Analysis: According to the US-EPA [
14], the evaluation of health risks is based on such parameters as estimated daily intake (EDI), target hazard quotient (THQ), hazard index (HI) and target cancer risk (TCR), using the following equations (Equation 1):
.jpg)
Where:
FIR = Proportion of fish consumed (100g, children; 240g, adult men/women/week) [
9,
15].
MC = Concentration of the metal in µg/g.
Bwa = The average body weight of the population of interest (70kg, men; 60kg, women, and 16 kg, children aged 4-6 years) [
15].
Target Hazard Quotient (THQ): To set up these values, the US-EPA risk analysis standards [
14] were used. Equation 2 shows the estimate of THQ, in which the correction factors were not used [
15-17] .

Where:
Efr = Frequency of exposure (365 days/year)
EDtot = Frequency of exposure (Mexican population: 70 years).
Rfd = Reference dose of individual metal (µg/g/day); 5x10-4 for Hg; 1x10-3 for Cd; 4x10-3 for Pb; 2x10-2 for Ni and 4x10-2 for Cu [
15-17].
Atn = Average time for non-carcinogens (EfrxEDtot).
Hazard Index (HI): was determined with the individual sum of each of the analyzed factors that represent the target hazard quotient and Equation 3.

Target Cancer Risk (TCR): was based on Region III values of US-EPA criteria [
18], and Equation 4.

Where:
Carcinogenic Potency Slope (CPSo): CPSo , oral (µg/g bw/day) 8.5x10-3 for Pb, [
18] 1.7 for Ni [
19] and 2.59 x10-4 for Cd [
19].
Results
Heavy metals in edible tissue samples: In the present study, only the edible tissue (muscles), were selected for analyses. The quality control parameters are shown in
Table 2.
.jpg)
The results obtained for the edible tissues are shown in
Table 3.
.jpg)
Copper: The seasonal averages of Cu were 68.25 µg/g for the dry and 39.74 µg/g for the rainy seasons. The statistical analyses indicated that there were significant differences for the sites and the seasons (P<0.05). The Mexican legislation (NOM) does not consider Cu as a pollutant in fish; however, the international standards set by FAO and WHO allow a maximum of 20 and 30 µg/g respectively, so the detected levels were above these limits (
Table 4) [
20, 21]. The average values did not exceed any of the required limits.
.jpg)
Cadmium: The concentration of Cd was higher in the dry season with the maximum being 1.2864 µg/g (site 3) which exceeded the permissible limits of 0.5 µg/g by the Mexican legislation NOM [
21] and the international limits of FAO and MAFF whose permissible levels are 0.5 and 0.2 µg/g, respectively (
Table 4) [
20-23]. The average in the dry season were 0.223 and 0.046 µg/g in the rainy season. Statistically, there were significant differences for the climatic season (P<0.05). Total average value did not exceed any of the recommended limits.
Lead: The average value for lead, determined in the dry season, was 0.907 µg/g, which exceeded the values (0.5 µg/g) set by FAO and JECFA (
Table 4) [
20, 24]. The highest concentration found in the dry season of 3.209 µg/g (site 7) was above the permissible limits of FAO, WHO, NOM, MAFF and JECFA (
Table 4) [
20-24] whose limits are 0.5, 2.0, 1.0, 2.0 and 0.5 µg/g, respectively. In the rainy season, the average value was 0.0439 µg/g, which did not exceed any of the recommended limits.
In the current study, significant differences existed among the sites and seasons, based on the statistical analysis (P<0.05). However, the average value 0.224 µg/g did not exceed any of the recommended limits.
Nickel: The maximum concentration found for Ni (41.77 µg/g) occurred in the rainy season at site 5. The average values for the dry and the rainy seasons were 5.73 µg/g and 10.22 µg/g, respectivily. Statistically, there were significant differences for Ni per climatic season (P<0.05). The US-FDA [
25] has established a maximum limit of 70-80 µg/g of Ni in fish (
Table 4), the results obtained by our study were within the international limits.
Mercury: The maximum value of Hg was 0.03 µg/g during the rainy season (site 4) and all of the values determined were lower than the maximum permissible of 0.5 µg/g set by WHO and Mexican legislation (NOM) [
21, 22]. The international limits (
Table 4) has established a maximum level of 0.05 µg/g for Hg in fish.
Accumulation of Cu, Cd and Pb in specific sites were higher than the recommended limits set by FAO, WHO, NOM, MAFF and JECFA [
20-24]. Hence, the human health risk assessments were carried out.
Risk analysis: The evaluation of risks to human health is “The process to estimate the nature and probability of adverse effects on human health when exposed to chemicals in contaminated environments” US-EPA [
14]. The Estimated Daily Intake (EDI), Target Hazard Quotient (THQ), Hazard Index (HI) and Target Cancer Risk (TCR) are shown in
Tables 5-
7.
.jpg)
THQ in children: This value should not exceed 1; if the detected value is lower than one, it is interpreted that there is no evidence of adverse effects or potential carcinogenic risk in the exposed population. In the present study, the THQ value was higher than one for Cu at sites 4 and 7 (dry season) and 1, 3 and 5 (rainy season), for Cd at site 3 (dry season), and for Ni at sites 3 and 6 (rainy season). THQ values weare less than one for Pb and Hg in all sites and seasons.
HI in Children: In this study, 50% of the sites exceeded the values of the comparison standard, i.e., HI>1.
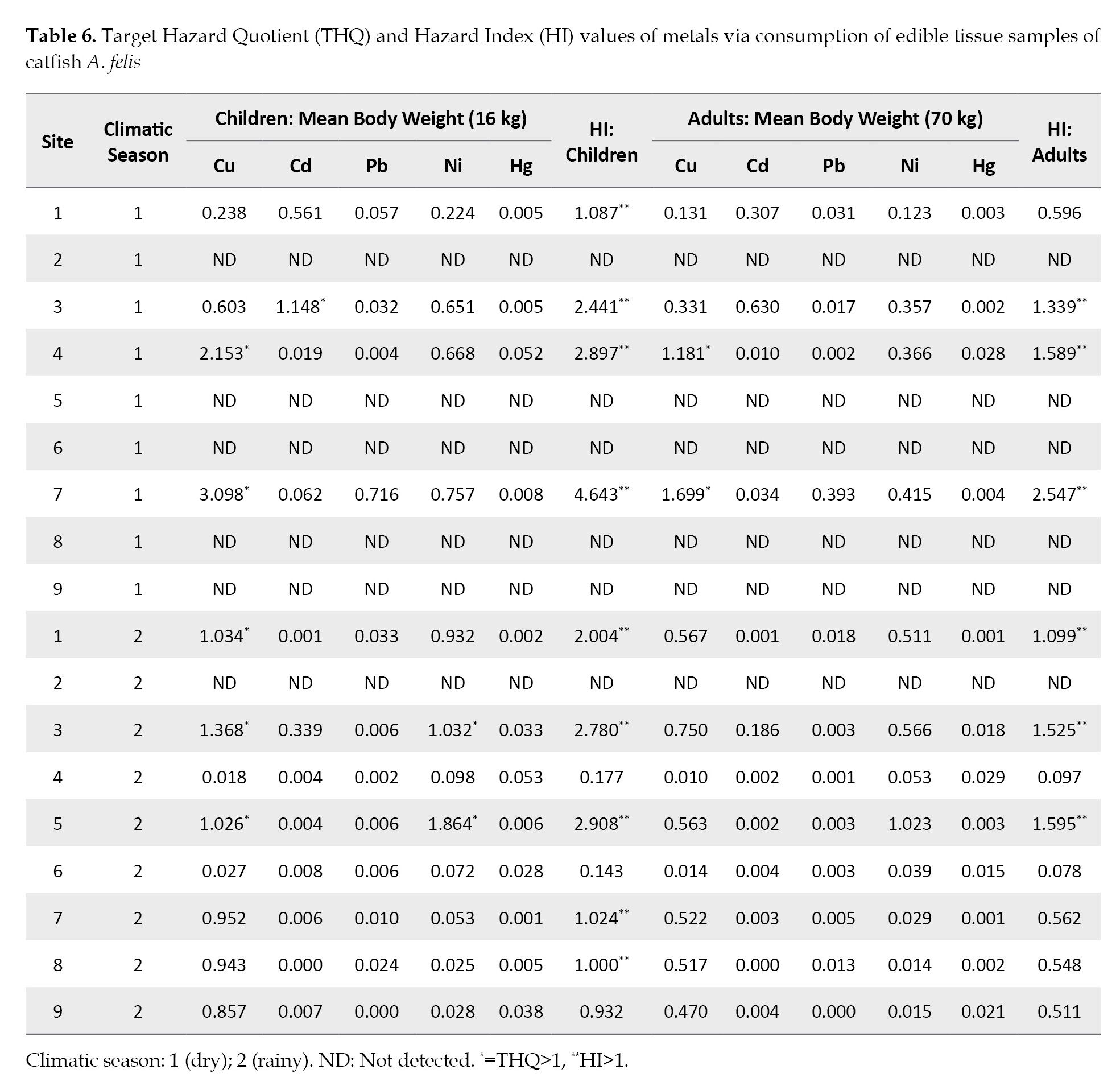
THQ in Adults: The THQ value in adults were only greater than one for Cu in sites 4 and 7 (dry season).
HI in Adults: The values obtained for 33% of the sites exceeded those of the comparison standards, i.e., HI>1.
For Cu and Hg, the TCR values wwere not determined, and there were no carcinogenic potency slope, oral (CPSo) values [
26].
 Discussion
Discussion
The internal organs of fish tend to accumulate heavy metals [
27-30]. Analogous results were shown in the fish, Pterois volitans [
28]. In an study conducted on Kyphosus vaigiensis, Stegastes rectifraenum and Balistes polylepi [
29] the authors concluded that Cd and Cu accumulated differently in the organs. The findings agree with the bioaccumulation studies on muscle, liver and gills in Cyprinus carpio, where no significant differences were found in the metals’ levels in the muscle and liver [
30], supporting the hypothesis that fish muscle is a good indicator of heavy metals contamination [
27-30].
The bioavailability of metals in the muscles of Ictalurus punctatus, Lepomis cyanellus and Lepomis macrochirus is subject to climatic conditions [
31], and the findings are consistent with our results. In the current study, the Cu concentrations exceeded those of THQ>1 for five sites in both climatic seasons. Our findings suggest that there might have been a constant source of Cu generation. The Cd level exceeded THQ>1 at one site during the dry season. This finding may be due to the proximity to populated areas. Further, Ni exceeded the comparison values of THQ>1 at two sites during rainy season. Given our findings, the consumption of catfish A. felis should be considered a health risk in southern Mexico, mainly for the most vulnerable population, i.e., children. These results are consistent with a previous study, where THQ>1, exceeding the comparison parameters [
32] and pointing out to the vulnerability of the population to toxic and persistent bioaccumulative pollutants present in fish.
Cadmium is considered a potent carcinogen according to the International Agency for Research on Cancer (IARC) [
33]. Tolerable limits for the increased risk of cancer, according to the New York State Department of Health, (NYSDOH) [
34], the TCR categories are defined as: TCR≤10-6 low, between10-4 to 10-3 moderate, and between 10-3 to 10-1 high. Also, TCR≥10-1 is very high and with a high probability that the exposed population may develop cancer at some point in their lives. Based in our findings, the Cd, and Pb values for children were in the “low risk” category. The Ni values in children were at “moderate risk.” For adults, only the Ni concentration was classified at “moderate risk.”
The consumption of marine species caught in rivers and lakes is restricted in some countries. It is also advisable that children at ages 1 to 4 years old only should consume an average of 75 g of fish per month. In children aged 5 to 11 years, the recommended amount is 125 g per month, and for pregnant women, the permissible amount is no more than 150 g per month [
35].
Conclusions
Due to lack of effective seashore monitoring and environmental regulations, the lagoons in the Gulf of Mexico are contaminated with heavy metals. This study found large amounts of toxic heavy metals, including copper, lead, nickel and cadmium, at specific sites in the fishing areas along the Gulf of Mexico shoreline. Our health risk assessments of the heavy metals suggest that cadmiun and nickel may contribute to the carcinogenic conditions in humans who consume the catfish caught in these areas. The carcinogenic risk from the heavy metal contaminations to the health of children and adults was found to be from low to moderate. Based on our findings, the consumption of catfish A. felis caught in the above-mentioned areas is not recommended. This study contributes significant data to the various agencies in Mexico, other countries, and agencies, such as the US-EPA. Our data are useful to the development of toxicological standards in order to determine the risks to the human health arising from the consumption of foods contaminated with heavy metals. The data in support of the findings of this study are available from the corresponding author.
Limitations of the Study: This study showed contamination of heavy metals in catfish. However, the levels of contamination can be similar in other species in the same area.
Recommendations for Future Studies: Analyze other species such as clams (R. cuneata) and oysters (C. virginica) in heavy metal contamination studies.
Ethical Considerations
Compliance with ethical guidelines
The study did not involve human participants, therefore, the ethical guidelines did not apply. The study was approved by Autonomous University of Carmen (Code: FQ/1ERP2019/03 ).
Funding
This study was supported by Autonomous University of Carmen, Mexico (Registration: FQ/1ERP2019/03)
Authors' contributions
All authors assisted fairly equally with conducting the research, analysis, and writing the manuscript, and provided critical comments on their reviews of the drafts. All authors have read and approved the final version of the manuscript prior to submission.
Conflict of interest
The authors declare no conflicts of interests with any entities in conducting this study.
Acknowledgements
The authors gratefully acknowledge the support of the Autonomous University of Carmen, Mexico
References










































 , Claudia Aguilar Ucán *
, Claudia Aguilar Ucán * 

 2, Angel Sosa Peralta1
2, Angel Sosa Peralta1 

 , Jesus Tagle Reyes1
, Jesus Tagle Reyes1 

 , Yunuen Canedo López1
, Yunuen Canedo López1 

 , Atl Victor Córdova Quiroz1
, Atl Victor Córdova Quiroz1 

 , Alejandro Ruíz Marín1
, Alejandro Ruíz Marín1 


.jpg)
.jpg)
.jpg)



.jpg)
.jpg)
.jpg)
.jpg)


