Sample Collection: The garlic bulbs (A. sativum) were obtained from Kure market, in Bosso, Niger State, Nigeria, in September, 2019, and further authenticated at the Department of Plant Biology, Federal University of Technology, Minna, Niger State, Nigeria.
Experimental animals: The 38 albino mice used in this study were obtained from the National Institute for Trypanosomiasis and Onchocerciasis Research, Kaduna State, Nigeria. The mice, weighing 25.69±2.17g, were acclimatized for 6 days at the university’s animal holding unit, Department of Biochemistry, until the subsequent use for this research. All experiments on the animals were performed using standard methods and in conformation with established guidelines for the care and use of laboratory animals.
Trypanosoma Congolense: The parasites, Trypanosoma congolense (T. congolense) was obtained from the Department of Vector and Parasite, National Institute for Trypanosomiasis and Onchocerciasis Research, Kaduna State, Nigeria. They were subsequently maintained in the animal housing unit of the Department of Biochemistry by asynchronous transfer in mice.
Preparation of samples: The garlic cloves were separated and the outer layer peeled. They were sliced and dried, using a lyophilizing unit at +23oC and ground repeatedly in an electric blending machine. The ground garlic powder was stored in a refrigerator until the extraction process [
10].
Preparation of garlic extract: The powdered garlic (200 g) were measured and suspended in two liters of distilled water for 2hr. The mixture was then heated in a water bath for another two hours at 50oC for the proper extraction of the garlic’s aqueous components, and was cooled down for 10hr. The mixture was then filtered through Whatman paper filter No 1, and concentrated at 50oC on a rotary evaporator. The concentrated extract was then freeze-dried for the complete removal of its moisture [
13].
Preparation of test organism: T. congolense parasite was maintained by asynchronous passage in mice until it was needed. Twenty mL of 0.98% NaCl was used to dissolve 0.2 mL of blood from the infected mice, and the mixture was further injected into clean mice [
14].
Phytochemical screenings: A. sativum was screened for the presence and composition of chemical compounds as described by two previous studies [
15-17].
Antioxidant property of garlic extract: A 0.2 mg aliquot of the extract was weighed and poured into a vial to which 2mL methanol was added, and made up to a final volume of 100µg/mL. Additional concentrations of the extract were also prepared at 12.5, 25, and 50 µg/mL by diluting the stock solution of 100µg/mL methanol 95%. Vitamin C (ascorbic acid) was used as the standard along with the garlic extract sample and 2mL 2, 2-diphenyl-1-picrylhydrazyl (DPPH at 0.4%) was added to both the standard and the extract. The absorbance of the mixtures was read at 517 nm on a UV spectrophotometer after they were allowed to stand in dark for 20 minutes. Methanol was used as the blank while reading the absorbance [
18]. The percentage DPPH free radical scavenging activities was calculated as shown in the formula below (Equation 1):

Where, ABcontrol = Absorbance of the control (vitamin C); ABextract = Absorbance of the extract.
Acute toxicity determination: Two experimental phases were employed for the determination of the acute toxicity (LD50) of the garlic aqueous extract, using the Lorke’s method [
14,
19]. In the first phase, 9 mice were grouped into three each and were given the garlic extract orally through a cannula from 300 mg/mL stock solution prepared at a dose of 10, 100 or 1000 mg/kg, i.e., groups 1, 2 & 3, respectively. After 24 hours of the extract administration, no death occurred among the animals. Subsequently, the second experimental phase was run in three groups of three mice each that received the extract at a dose of 1,600, 2900 or 5000 mg/kg, i.e., groups 1, 2 & 3, respectively. These mice were also observed for death and behavioral changes as the signs of toxicity over the next 24 hours [
14].
Screening of trypanocidal activity: The trypanocidal activity of the garlic aqueous extract was determined in vitro based on the method described by two earlier studies [
14, 15].
Infection in mice: Blood samples were collected from the tails of highly infected mice, using EDTA coated insulin syringes. The inoculums were prepared by diluting the blood samples with normal saline. The acclimatized healthy mice, weighing 37 to 40 grams, were infected intraperitoneally with 0.2 mL of the inoculum, containing the trypanosome parasites [
14].
Administration of garlic extract: Twenty albino mice were divided into five groups of four each, and were infected with T. congolense. The positive and negative control groups were given diminazene aceturate (Sigma Aldrich, St. Louis, MO, USA) at 3.5 mg/kg from the stock solution of 110 mg/mL. These mice were infected but not treated with the garlic extract. Another group, consisting of three mice were assigned as the normal controls. Treatment with the garlic extract was initiated three days after infection, when the parasites were detected in the mice. Groups 1, 2 and 3 were given the aqueous extract of garlic at 100 mg/mL orally at a dose of 100, 250 or 500 mg/kg, respectively, for 16 consecutive days [
14].
Mean Parasitemia Determination: We analyzed the blood samples collected from the tails of the infected mice. These tails had been sterilized in advance with methylated spirit. The method involved the microscopic counting of the parasites at ×400 magnification per field either in pure blood or blood appropriately diluted with phosphate buffered saline (PBS) at pH 7.2. The average parasite count per mL of each blood sample was determined and recorded. This was monitored starting the second day of parasitic infection until the 16th day of treatment. This experimental step was performed to determine the rise or decline in the parasite levels and the therapeutic effect of the garlic extract [
20].
Packed Cell Volume Determination : Packed Cell Volume (PCV) is defined as the standard fraction of red cells in the total blood volume. Venous blood samples from the mice tails were collected into heparinized capillary test tubes. The blood samples were centrifuged at 11,000 rpm for 5 minutes, and the percentage of red cells was determined with a PCV reader [
21].
Body weight determination: The mice’s body weights were taken daily, using a weighing scale throughout the treatment period. This was done by spinning the mice to make them dizzy for accurate measurement of their body weights.
Preparation of samples for hematological analyses: The blood samples from the survived mice were collected on the 16th day of treatment by cardiac puncture, into EDTA coated test tubes for hematological analyses. The mice were then sacrificed by cervical incision. The hematological analyses were performed, using the standard method described by a previous study [
21].
Data analyses: The data were analyzed using the Statistical Package For Social Science (SPSS), version 23. The statistical differences found among the groups were compared, using the Analysis of Variance (ANOVA) followed by Duncan’s multiple range test at P<0.05 confidence level. Finally, the results were presented as the Mean±SD of the means.
Results
Qualitative and Quantitative Phytochemical Screening of the extract: The phytochemical components identified in the extract included alkaloid, flavonoid, tannin, saponins, glycosides, terpenoids, and ascorbic acid. However, steroids and reducing sugars were not detected (
Table 1).
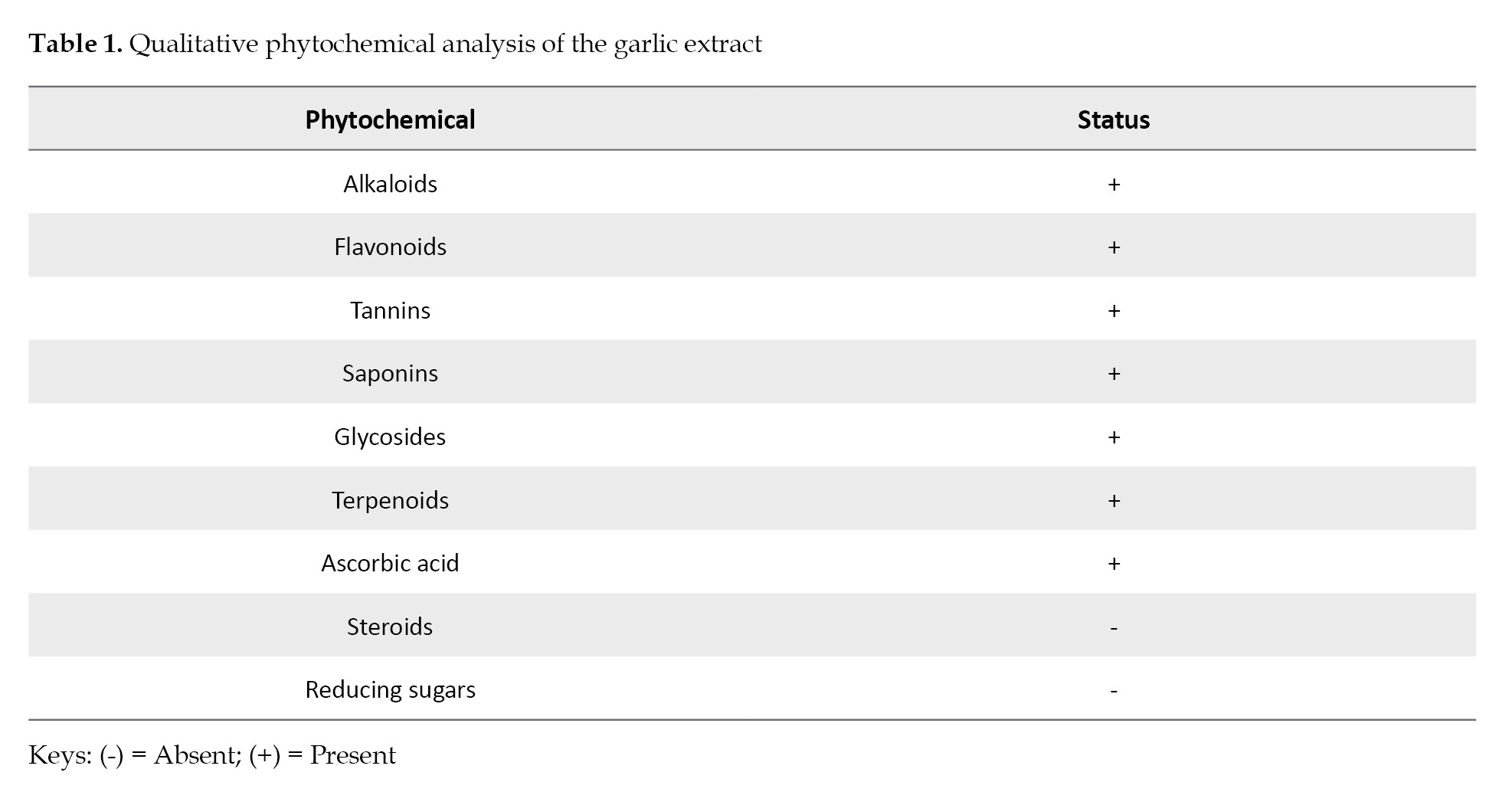 Table 2
Table 2 represents the various phytochemicals present in the A. sativum aqueous extract with the amounts in percentages. The data revealed the highest concentration of phenol at 291.88 mg/100g and lowest concentration of alkaloids at 13.66 mg/100g.

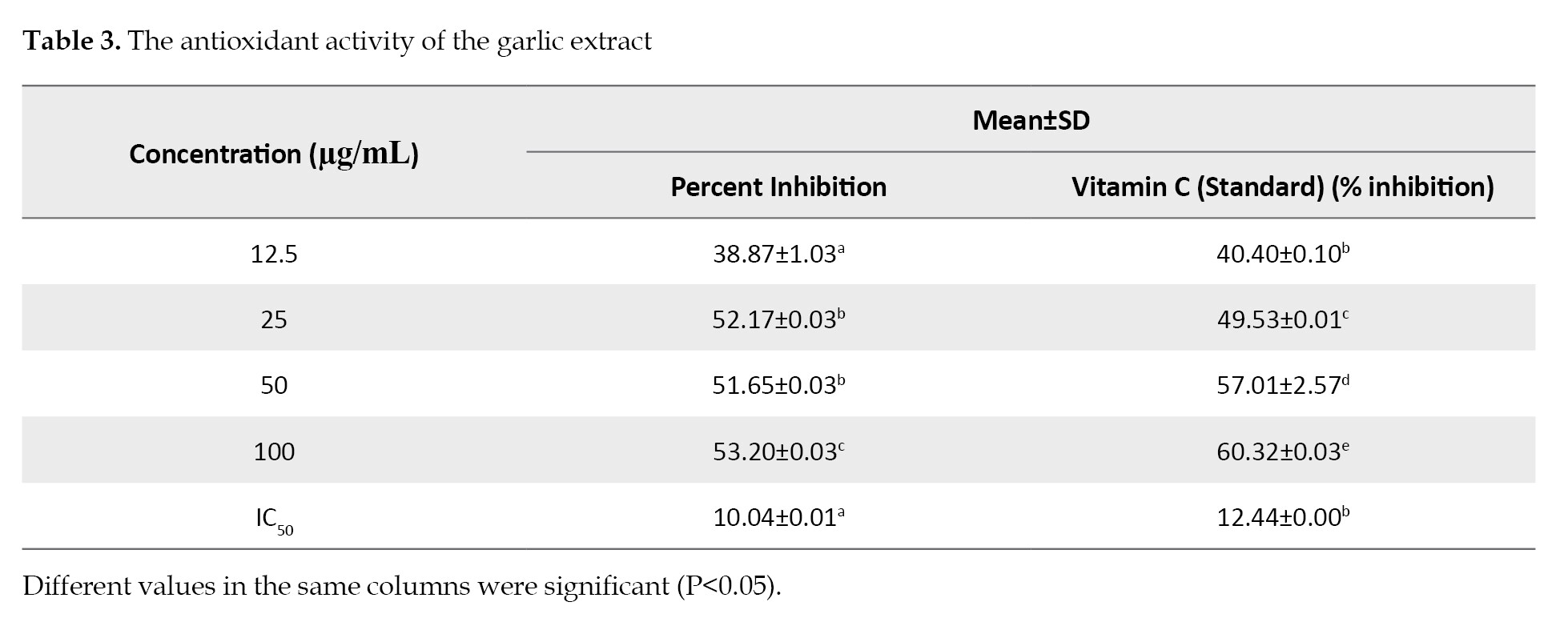
Scavenging activity of the Extract: The data demonstrated progressive rise in the extract concentration consistently increased the percent inhibition. There were significant differences (P<0.05) among the concentrations of standard (vitamin C) and the garlic extract. The IC50 values of the extract at 12.5, 25, 50 and 100 ug/mL concentrations were significantly different (P<0.05) versus the standard. The IC50 values of the extract and the standard were 12.44 and 10.04 µg/mL, respectively (
Table 3).
Acute toxicity studies of the extract:
Table 4 reflects the results of lethal dose (LD50) for the garlic extract in mice. The result signified that the estimated LD50 was more than 5000 mg/kg of the body weight since no mortality was recorded in the animals.

In vivo Anti-trypanosomal activities of the extract
Mean parasitemia counts: There was a significant increase in parasitemia in infected but untreated mice (P<0.05). However, a dose-dependent decrease in parasitemia was exhibited in the treated groups (
Figure 1). There was a significant decrease in parasitemia of the group treated with the standard drug but no complete infection clearance was observed at the end of the experiment (P<0.05).

Changes in packed cell volume:
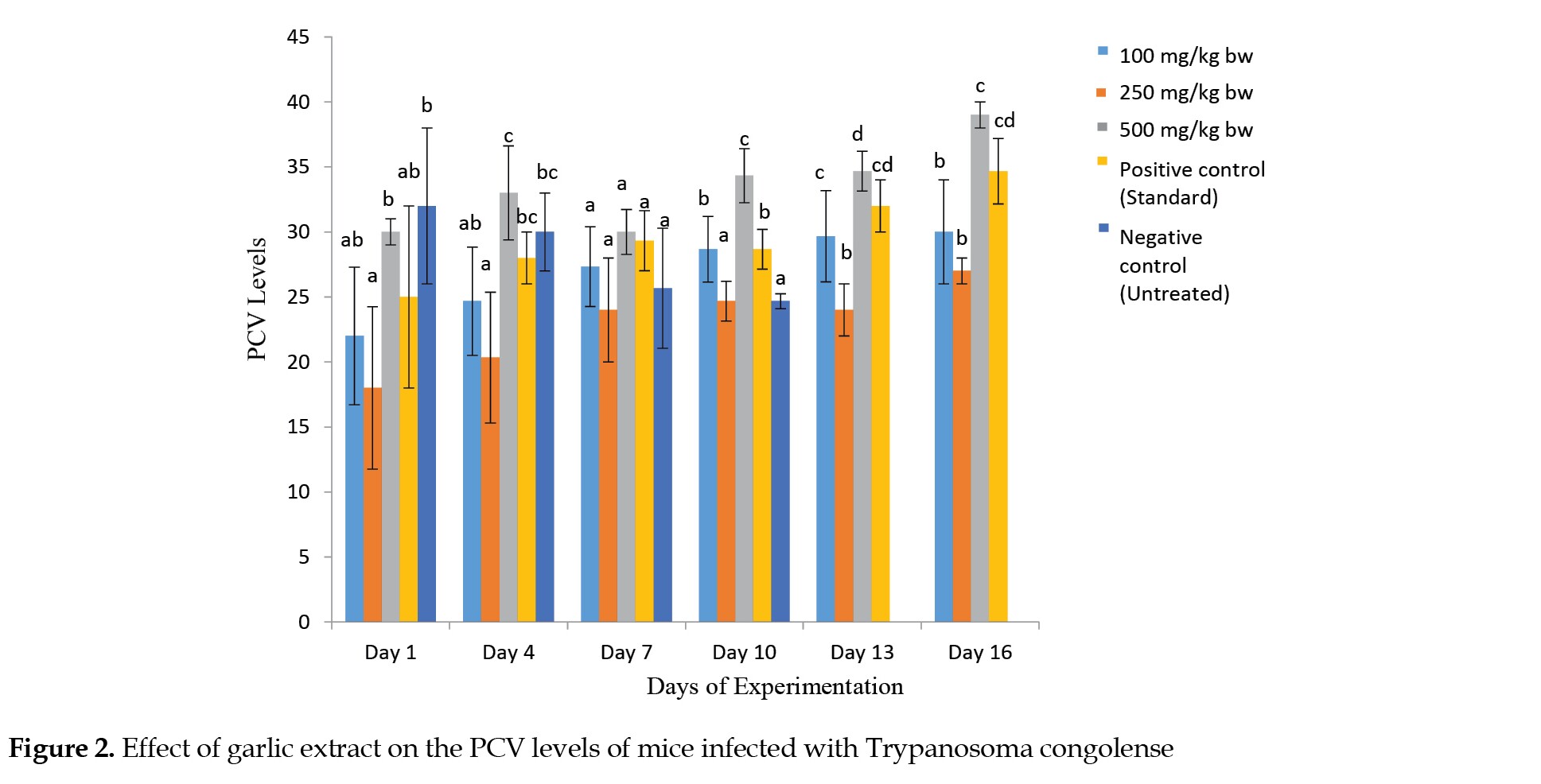
Figure 2 shows the PCV level of infected mice with T. congolense. There was a decrease in PCV level in the infected but untreated mice. However, the groups of mice treated with 100, 250 and 500 mg/kg of the extract showed increases in their packed cell volume levels compared to the negative control group.
Changes in the body weight:
Figure 3 shows changes in the body weight of mice infected with T. congolense. A statistically significant decrease was noted in the body weights of the infected but untreated group. Also, significance differences in the body weights of the treated groups were observed along with a significant increase in the body weight of the mice treated with 250 and 500 mg/kg of the extract compared to those in the control groups (P<0.05).

Effect of the extract on hematological parameters: As shown in
Table 5, the extract administration in trypanosome infected mice showed significant anti-trypanosomal activity at the highest dose of 500 mg/kg, which was comparable to that observed for 3.5 mg/kg diminazene aceturate. At 500 mg/kg of the extract, the serum levels of Hb, PCV, RBC, TWBC, and neutrophils were significantly higher than those noted in the group administered with 3.5 mg/kg of diminazene aceturate.

Many of the hematological parameters did not change compared to those in normal controls administered with 500 mg/kg of the extract. However, the MCV, MCH, MCHC and lymphocytes levels were significantly higher in the group given 5 mg/kg diminazene aceturate than those documented for the group administered 500 mg/kg of the extract.
Discussion
The findings of this study demonstrated that a 27.6% yield of the garlic extract was generated from 220 grams of the garlic bulb powder. Also, the qualitative phytochemical screening of the garlic extract identified a variety of phytochemicals, such as terpenoids, tannins, flavonoids, glycosides and alkaloids while steroids and reducing sugars were not detected.
The quantitative phytochemical compositions of the extract showed that alkaloids at 2.4% were the lowest while phenols at 50.9% were the highest in concentration among the screened parameters. These findings were inconsistent with those of a previous study [
22], in which tannins were reported at the highest concentration in the aqueous garlic extract. However, our findings were consistent with those of a previous study [
23] that reported phenols to be at a highest concentration in their garlic samples.
The anti-trypanosomal and anti-oxidant activities exhibited by the garlic extract may be due to the presence of biologically active components. Similarly, several researchers have reported that various phytochemicals, including flavonoids, alkaloids and polyphenols have also exhibited anti-trypanosomal activities [
24-26]. The antioxidant activity of the garlic extract, as tested by DPPH, showed that there was a significant difference in the percent inhibition of vitamin C, as the standard, and the garlic extract. The DPPH scavenging activity of A. sativum was observed to be dose-dependent with a significant difference in the percent inhibition concentration (IC50) between the extract and vitamin C.
With respect to the pathology of trypanosomiasis, oxidative stress has been shown to play an important role while flavonoids are known to provide health benefits via antioxidant mechanisms [
27, 28]. They are capable of effectively scavenging reactive oxygen species and neutralize free radicals, because of their phenolic hydroxyl groups, thereby making them potent antioxidants and potential candidates for the treatment of trypanosomiasis [
14,
29]. Therefore, the garlic extract is capable of scavenging free radicals, thereby reducing or preventing oxidative damages. The garlic extract activities against DPPH radicals could therefore be attributed to the presence of the beneficial phytochemicals reported in the current study.
This study further demonstrated that the extract is safe since the acute toxicity test of the extract at doses from 10 to 5000 mg/kg showed no abnormal physical changes or death in the mice. This is consistent with those reported by another study [
30] where no lethal outcome was recorded at similar doses in mice, although the animals were inactive for few hours. Also, other studies [
31-33] have shown that the hydromethanolic extract of E. kebericho roots does not cause signs of toxicity at varying doses of up to 5,000 mg/kg in mice.
The anti-trypanosomal activity of the garlic extract, revealed significant differences (P<0.05) for the mean parasitemia counts among the treated mice. A constant rise of the parasite in the blood samples was noted in the negative control mice that were infected but not treated until the 10th day when they all died. This finding was consistent with those of a former study [
34] where all of the untreated mice died on the 10th day of exposure to the parasite. There was no complete clearance of the parasite among the treated groups.
However, the parasitemia cell count declined in a dose dependent manner and the mice survived up to the 16th day compared to the negative controls. Our findings are also consistent with those achieved by two studies [
35, 36] that reported the anti-trypanosomal activity of A. indica to be dose-dependent. Specifically, receiving higher doses of the extract increased its efficacy and the mice survived longer. There was a significant difference in the preserved body weight of the screened animals compared to the significant decline in the negative control group. The results supported the argument that the extract significantly prevents weight loss in the mice during the trypanosome infection [
37].
Anemia has been reported to be an important feature of trypanosomes infection, and the severity is directly related to the parasite load [
27]. Anemia is induced by trypanosome species through generation of free radicals, which attack the RBC membrane, leading to oxidation and hemolysis [
38]. Evaluation of hematological parameters is a useful approach in determining the potential functions of secondary metabolites contained in plant extracts. The observed rise in the PCV level indicates that the extract possesses anti-trypanosomal property by suppressing or eliminating the parasites thus preventing the generation of free radicals by the trypanosomes. This also implies better transportation of oxygen and dissolved nutrients [
39].
In this study, the PCV level of the group administered 500 mg/kg of the extract was significantly higher than those given 3.5 mg/kg diminazene aceturate compared to the normal control group (P<0.05). The significant increase in PCV level observed at the extract dose of 500 mg/kg indicates that it acts as an anti-trypanosomal agent, and inhibits the parasite while being able to generate red blood cells in the bone marrow more than diminazene aceturate. This action of the extract is likely due to its ability to release erythropoietin, a hormone essential for the production of red blood cells. The observed MCH and MCV levels are indicative of macrocytic anemia, as evident by reticulocytosis, which is the initial response when erythropoiesis is stimulated [
40].
Also, hemoglobin and RBCs are essential in transferring respiratory gases [
41]. These findings indicate that the oxygen-carrying capacity of the blood and the amount of oxygen delivered to tissues were not compromised by the garlic extract treatment. White blood cells are essential components to fight infections in the body, the increase in the cell numbers could be considered beneficial due to the immune roles they play. Eosinophils combat parasitic infections by releasing their specific granules rich in cationic proteins to fight against helminth parasites [
41, 42]. Thus, the increased level of white blood cells in the group administered 500 mg/kg of the extract implies that it also imparted immunostimulatory effects, in agreement with the anti-trypanosomal activity observed.
Conclusions
This study established the anti-trypanosomal and anti-oxidant activities of the garlic (A. sativum) aqueous extract. The extract was evidently able to inhibit parasitemia in the infected mice and prolonged their survival. In addition, the extract demonstrated the ability to ameliorate the anemia secondary to the trypanosomal infection. The efficacy of the garlic extract as an anti-trypanosomal agent is likely due to the presence of secondary metabolites that generate free radicals and interferes with the metabolic pathways in trypanosomes.
Hence, the garlic’s bioactive compounds are potential options for the management of trypanosomal infection, as they relieve the negative effects experienced by patients who use chemotherapeutic agents and those who face the drug resistance problems arising from the use of anti-parasitic drugs.
Ethical Considerations
Compliance with ethical guidelines
This study abided by the ethical principles governing the use of laboratory animals as set by the Federal University of Technology, Minna, Nigeria and the Ethics Committee on Medical and Scientific Research. Also, the current internationally accepted principles of care for and using laboratory animals as contained in the Canadian Council on Animal Care Guidelines and Protocol Review were duly observed.
Funding
No funding was received from any funding agency toward the conduction of this study.
Authors' contributions
Designed the study, wrote the protocol and supervised the experiments: Fatima Mohammad Madaki, Sakariu Waheed Adio and Busari Musa Bola; Carried out all of the laboratory work and performed the statistical analyses: Fatima Mohammad Madaki, Sakariu Waheed Adio and Yunusa Olatunji Ibrahim; Managed the data analyses: Authors Fatima Mohammad Madaki and Sakariu Waheed Adio; Wrote the first draft of the manuscript: Fatima Mohammad Madaki and Sakariu Waheed Adio; Managed the literature search and edited the various drafts of the manuscript: Fatima Mohammad Madaki, Busari Musa Bola, Abdullahi Mann, Adamu Yusuf Kabiru, Yunusa Olatunji Ibrahim and Emmanuel Olofo Ogbadoyi. All authors read and approved the final manuscript prior to submission.
Conflict of interest
The authors declare no conflict of interests in conducting this study with any internal or external entity.
Acknowledgements
The authors would like to appreciate the technical laboratory staff, Center for Genetic Engineering and Vaccine Production, and Department of Biochemistry, Federal University of Technology Minna for their kind assistance and support toward this study.
Ethical Approval
The Ethics Committee of the Federal University of Technology, Minna, Nigeria, approved the study design and protocol upon several expert reviews (registered code #: 000020).
References









































 1, Sakariyau Adio Waheed2
1, Sakariyau Adio Waheed2 

 , Musa Bola Busari2
, Musa Bola Busari2 

 , Yunus Olatunji Ibrahim2
, Yunus Olatunji Ibrahim2 

 , Adamu Yusuf Kabiru3
, Adamu Yusuf Kabiru3 

 , Emmanuel Olofo Ogbadoyi3
, Emmanuel Olofo Ogbadoyi3 

 , Abdullahi Mann3
, Abdullahi Mann3 











