Introduction
Inflammatory bowel disease (IBD) is a chronic condition with no specific cause, which is categorized into two groups: Crohn’s disease (CD) and ulcerative colitis (UC). These conditions, prevalent at the ages of 15-40, are caused or aggravated by environmental factors, such as smoking, stress, and genotype. Symptoms of CD include persistent diarrhea, abdominal pain, weight loss, fatigue, and fever. In UC, dysentery and anemia are frequently observed in addition to the symptoms found in CD.
A rise in the global incidence of IBD has been reported over the past decade, especially in industrial Asian countries, such as China and India. In Iran, it has been estimated that 23,000 and 30,000 people lived with IBD in 2017 and 2020, respectively, and the number is projected to rise to 69,000 by the year 2035 [
1]. Due to the chronic nature of IBD, the treatments are mainly based on the severity, location, treatment history, need for hospitalization, and the associated healthcare costs. Unfortunately, IBD has imposed a high cost on the healthcare system of the countries where people with this condition reside [
2].
An imbalance between proinflammatory and anti-inflammatory cytokines in IBD prevents the termination of the inflammatory process and causes it to continue, leading to gastrointestinal (GI) damages and excessive tissue reaction. Excessive immune response to antigens in the GI lumen leads to the release of inflammatory mediators. Such cytokines, as interleukin-6 and tumor necrosis factor-alpha (TNF-α) are considered to play special roles in this disease [
3]. These cytokines activate the signaling cascades in the mucosal immune cells via Janus kinase-signal transducers and activators of transcription (JAK-STAT) pathway [
4]. Popular drugs used for the treatment of IBD are aminosalicylates (sulfasalazine), corticosteroids, thiopurines, immunosuppressants, and anti-TNF-α [
5].
Unfortunately, despite the efficiency, taking these medicines are associated with complications that limit their use. For instance, anemia and liver disorders are among the complications of sulfasalazine while renal disease and allergy limit the use of 5-aminosalicylates. Further, corticosteroids have their own side effects, such as osteoporosis, cataracts, and aseptic necrosis of the hip joint, while immunosuppressants promote anemia and infections. Among the prevalent complications of thiopurines, leukopenia and elevated liver enzymes, such as transaminases, are well known. Lastly, anti-TNF-α medications also have their side effects, such as infections, colds, otitis media, and sinusitis [
6].
Aim of the study
Considering the anti-inflammatory and anti-oxidative properties of Viola odorata and Cassia Fistula, and the therapeutic effects of the combined extracts from these plants in Persian medicine, and since colitis is a good model for exploring anti-inflammatory effect, this study was planned to experimentally investigate the anti-inflammatory effect of the extracts from both plants on induced colitis in rats.
Literature review
V. odorata is a perennial plant with dark green, elliptical leaves and long petioles usually protruding from the leaf collar or the lamina joint region. This plant contains such compounds as alkaloids, glycosides, saponin, methyl salicylates, mucilage, and vitamin C [
7]. Among its most active ingredients, this plant is known to possess valuable peptides, such as cyclotides and cycloviolacin. This plant grows in central Iran, predominantly in Kashan and the adjacent areas [
7].
C. fistula is native to tropical regions of Asia, but it also grows in South and East Africa, Mexico, and Brazil, and is the national plant of Thailand [
8]. In southwestern Iran, this plant is cultivated as an ornament [
9], and grows well in arid regions; it has tiny pinnate leaves and oval leaflets. The plant has vibrant yellow blooms, hanging down from the tree, hence its popular use as a decorative flower.
In the early centuries AD, V. odorata was used to treat diseases, such as eye inflammation and sore throat; it was also used as a laxative. In the Canon of Medicine, the Iranian physician, Ibn Sina, states that V. odorata has a cold and wet nature and heals gastritis. In the book, Makhzan-Al’ Advieh, V. odorata has been claimed to be a bile laxative, thirst-quenching, and useful medicine for cough, stomach, liver, and spleen ailments, with the flowers being antidote for toxins. In traditional medicine, this plant is used to treat cancer and migraine, and as a tranquilizer. Further, studies have shown that this plant has anti-inflammatory and anti-microbial effects [
9, 10].
V. odorata may also be used for its sedative, diuretic, anti-asthma, and laxative effects. The fruits of C. fistula are used traditionally as a safe laxative in children and pregnant women, and as a herbal tonic for liver and biliary disorders [
11, 12].
Materials and Methods
Plant collection and preparation of the extracts: The flowers and pulps of V. odorata and C. fistula, respectively,were obtained from a farmers market in Yazd, Iran. The authenticity of the plants’ flowers and pulps were confirmed by a licensed botanist, who issued the voucher numbers SSU0056 and SSU0058 to verify the products.
The plants’ flowers and pulps were dried, ground, powdered, and passed through a 40-mesh filter. Forty grams of the powder from each plant was dissolved in 800 mL of distilled water, and placed on a non-thermal heater continuously for 72 hours. The prepared extract was passed through Whatman paper filter and then kept in an oven at 50ºC to be concentrated and dried completely.
Measurement of total phenolic content: To measure the total flavonoid compounds, gallic acid standard solution was prepared at 10, 20, 40, 60, 80, 100, and 200 µg/mL in 60% methanol, using Folin Ciocalteu (FC) method [
13]. A 0.1 mL aliquoat of the solution was transferred to a test tube, and 0.5 mL of 10% FC solution was added. After 3-8 min, 0.4 mL sodium carbonate (75%) was added to the solution and kept at room temperature. A 300 µL aliquoat of each sample was poured into microplate wells, and the light absorbance was read three times at 760 nm with a microplate/ELISA reader, and the average absorbance values were plotted. Using the straight-line formula, the total phenolic content was determined for each sample [
13].
Investigation of the secondary metabolites
The secondry metabolites, such as alkaloides, saponins, tannins, and anthraquinons were identified, using the methods established in previous studies [
14, 15, 16, 17, 18].
Animals: In this study, 28 healthy male Wistar rats, aged 2-months, weighing 250±30 g were used. All rats were kept under standard laboratory conditions at 20-25°C with 65-75% humidity and adequate access to food and water in separate cages and alternating 12-hr light and dark cycles.
All rats were kept and handled according to the Animal Rights & Ethics Protocol of Shahid Sadoughi University of Medical Sciences. The rats were randomly divided into four groups of seven each as follows:
• Sham group: Healthy rats received distilled water through the rectum, and were given normal saline orally.
• Control group: Ill rats with colitis received no treatment and were given distilled water.
• Standard group: Ill rats with colitis were treated orally with sulfasalazine (Mehr Daroo; Tehran, Iran) at 360 mg/kg once daily.
• Experimental group: Ill rats with colitis received the combined extracts of V. odorata and C. fistula orally at 200 mg/kg three times daily.
Induction of colitis in rats: First, the animals were kept in a fasting state for 24 hours with free access to water only. Then, light anesthesia was induced in the animals with diethyl ether (Merck; Germany). A feeding tube was inserted 8 cm into the rat’s rectum. Then 1 mL acetic acid (4%; Merck, Germany) was injected into the rats’ rectum. Fifteen seconds after the presence of acetic acid in the colon, 1 mL normal saline was inoculated rectally to neutralize the acetic acid [
19, 20].
Macroscopic investigations: The animals’ weight was measured the day before the induction of colitis in various groups and at the end of the treatment period, when they were sacrificed. The presence or absence of diarrhea and rectal ulcers were also examined in all animals.
Six days after the induction of colitis, the rats were sacrificed by induction of anesthesia with ketamine and xylazine (Alfasan, Holland). The abdominal cavity was excised in the lower abdominal area to gain access to the colon. Eight centimeter from the end of the colon beyond the rectum was isolated, the colon was dissected by a longitudinal incision, rinsed in normal saline, weighed, and fixed on an Ionlite board. All samples were photographed by a digital camera from the same distance. Next, an incision was made and a 1-cm piece of tissue was cut in a specific region in all animals, fixed in 10% formalin, and used for the histopathological examinations [
21]. To determine the myeloperoxidase (MPO) activity, 1-cm of each colon tissue sample was cut out from an identical area, weighed, frozen in liquid nitrogen, and stored in a freezer at minus 70°C. The colon images were analyzed, using a Fiji software, and the extent of inflammation and macroscopic damages were scored and recorded (
Table 1).

Histopathological analyses: For histopathological analysis of the colon tissue damages and inflammation, 20×20 mm sections were made by a microtome from the samples fixed in 10% formalin. Next, we dried the sections in stages, cleaned them by xylene solution, and embedded them in paraffin wax in a tissue processor. Subsequently, we made 5-6 μ thick slices from the tissue samples, and placed them in a dish containing water and alcohol to remove the wrinkles. Next, the samples were dried and stained with hematoxylin and eosin (H&E) method. The stained sections were examined in a double-blind manner under light microscopy for histopathological features, such as crypt injury, depth of damage, and degree of inflammation, based on the Gerald’s method. Details are presented in
Table 2.

The histopathological images were taken by a digital camera attached to the microscope [
22].
Myeloperoxidase activity assay: Each colon sample stored at -70°C, was homogenized separately in 10 mM potassium phosphate buffer at pH 7. The buffer contained 0.5% hexadecyl trimethylammonium bromide (Merck, Germany) [
23]. The tissue samples were then centrifuged at 20000 rpm for 30 minutes at 4°C. Next, H2O2 (0.1 mM) and tetramethylbenzimide (1.6 mM; Merck, Germany) were added to the centrifuged supernatant. The supernatant’s absorbance was read at 450 nm with an ELISA reader three times, and the MPO activity was determined and recored as pg/mg of the colonic tissue samples [
24].
Data analyses: The parametric data were analyzed by one-way analysis of variance (ANOVA) and Tukey’s tests. For the analysis of non-parametric data, Mann-Whitney’s test was used. The Mean±SEM and medium ranges were used to evaluate the measured parameters. A 95% confidence interval was considered as significant to compare the means. Graphs were plotted using Excel 2013, and finally, the data were analyzed statistically on SPSS software, version 24.
Results
Total flavonoid content: The result of standardization of total flavonoid content based on the Folin Ciocalteu method was 12/63 µg/mL at 760 nm. The extract of C. fistula pulps contained alkaloid, saponin, and anthraquinone compounds.
Evaluation of colon tissue weight: The ratio of weight to length of the colon is a criterion to quantify the tissue edema. As shown in
Figure 1, the induction of colitis in the rats decreased the colon weight/length ratio in the experimental group.
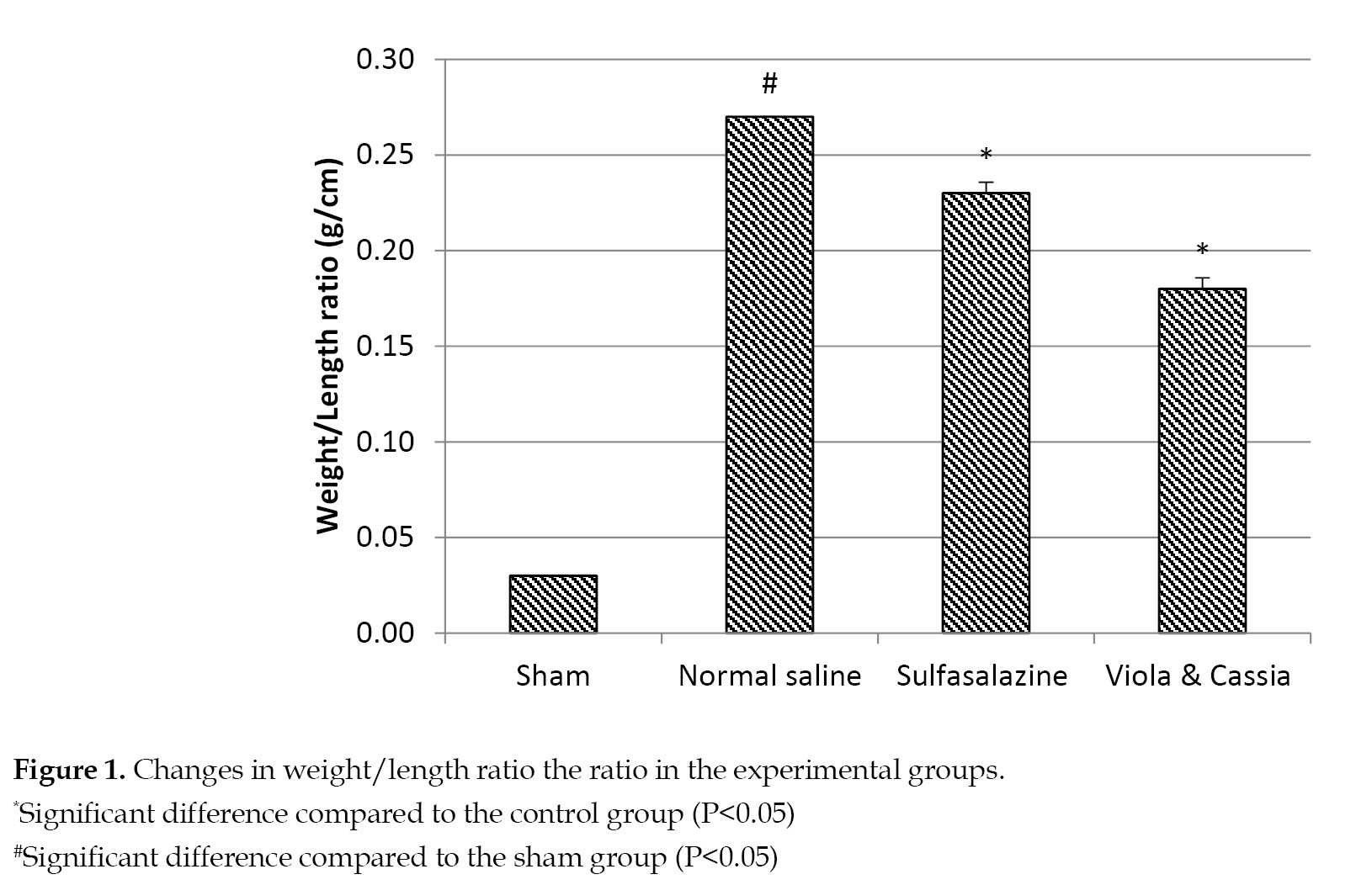
On the other hand, treating animals with the extract of V. odorata and C. fistula at 200 mg/kg (experimental group) showed a statistically significant difference between this group, and both the control and standard groups (P<0.05).
Evaluation of macroscopic damage to colon: After induction of colitis, alterations were observed in the treated tissue samples, such as increased inflammation, ulcer, thickening of the colon epithelial layer, edema, and sometimes necrosis. The control group showed the highest amount of macroscopic damages in the colon compared to other groups. The experimental group was in a better shape than the control and standard groups, and the difference was statistically significant (P<0.05) compared to the sham, control, and standard groups (
Figures 2 &
3).
Extent of inflammation: As evident in
Figure 4, the control group showed the highest extent of inflammation, and the standard group was insignificantly better than the control group (P>0.05).
The administration of the extract of V. odorata and C. fistula effectively reduced the colon inflammatory areas in the group receiving the extract, and the differences were statistically significant, comparing the experimental versus other groups (P<0.05).
Histological findings: As shown in
Figure 5, the normal structures of the colon with four distinct layers and no tissue lesions were observed in the sham group.

The colon walls, mucosa, submucosa, and the muscular and serous layers in the sham group appeared normal.
As seen in
Figure 5 (at the right side of the image), significant bleeding and formation of fibrin filaments were observed in the control group. Also, extensive bleeding ulcers in all layers, together with tissue necrosis and abundant inflammation were observed in this group. The inflammation severity was consistent with the infiltration of inflammatory cells, involving various parts of the colon, i.e. the mucosa and submucosa throughout the thickness of the colon wall. These changes were indicative of necrosis across the membranes with bleeding and formation of fibrin filaments. Abundant inflammatory cells infiltration and significant edema were also observed in the submucosa. The reduced bleedings, inflammation and perforations caused by the colitis were observed in the tissue samples from the positive standard group (
Figure 5). Spot bleeding and reduced edema were also observed in the specified areas and the submucosal layer.
Variations in myeloperoxidase enzyme level: There was a statistically significant difference between the control and sham groups with respect to MPO enzyme levels. Specifically, the former was in a worse shape than the latter (P<0.05). Also, the experimental group was in a significantly better shape than the control group (P<0.05). Compared to the experimental group, the positive standard group was in a better shape, but the difference was not statistically significant (P>0.05). These results are presented in
Figure 6.

Discussion
The present study demonstrated that the extracts of V. odorata and C. fistula given to the rats at 200 mg/kg effectively reduced the tissue damages and inflammatory response caused by acetic acid-induced colitis.
The intrarectal administration of acetic acid is a valid model for inducing experimental colitis in animals. This model is used to screen the new drugs, since it develops colitis similar to that observed in humans [
25,
26]. Reactive oxygen species are the main products after the colitis induction, which generates and raises the MPO and malondialdehyde levels in the serum. Also, these enzymes can lead to increased production of nuclear factor kappa-light-chain-enhancer in activated B cells. This event also occurs due to the induction of another pathway that raises the TNF-α level [
27]. The induction of colitis in rats, using this model, causes weight loss in the animal, increased colonic tissue weight per unit area, extensive colon inflammation and ulcers, along along with thickening of the colonic epithelial layer, edema and necrosis, crypt abscesses, reduced mucin secretion in the colon, and increased MPO enzyme level [
18]. In this study, all of these manifestations were observed in the treated rats, especially those in the control group.
The experimental group showed a significant reduction in the colon weight per unit area (p<0.05) compared with the control and positive standard groups. An increase in colon weight per unit length was observed in the control group. The weight-to-length ratio measures local inflammation and tissue edema [
28]. By receiving the extracts, the experimental group had less colon tissue edema than those who received sulfasalazine (P<0.05). This is likely due to the efficacy of phytochemical compounds, such as barbalion (aloin) in the extracts that protected against edema by lowering the hydrostatic pressure of the blood while increasing the blood flow [
18,
29].
The MPO activity levels were directly related to the number of neutrophils in the inflamed colon tissue samples [
25,
26], obviously because this enzyme is released from actived neutrophils, which led to oxidative damages to the colon and induction of colitis [
25]. A significant reduction in the MPO level was observed in the experimental group compared to those in the controls (P<0.05). This suggests the efficacy of the extracts in reducing the neutrophils numbers in the inflamed tissue, which in turn reduced the oxidative reactions and the spread of the ulcers in the colon. Our macroscopic observations indicated that the experimental group had significantly lower scores than both the control and standard groups. The sham group scored zero, with similar level of oxidative reactions. The reduced MPO levels may be attributed to low neutrophils in the colon tissue, and due to the presence of phytochemical compounds i.e. barbaloin [
18,
29]. The neutrophils infiltration into the colon tissue and the low MPO level were associated with significantly lower edema and normal intercellular distances among the epithelial cells.
Oxidative enzymes are found in all body cells at normal levels. They can generate inflammation via producing ROS and nitric oxide (NO) in excess of the normal levels required by the gastrointestinal tract [
30]. Generally, the extracts of V. odorata and C. fistula are regarded as powerful antioxidants, able to inhibit diphenyl-1-picrylhydrazyl, hydroxyl and free radicals, such as NO [
31]. The extracts do these activities via their flavonoid contents, coumarin [
32, 33], vitamin C [
33], methyl esters, hexadecanoic acid, phytol [
34], and barbalion [
18]. All of these compounds have excellent antioxidant properties.
As initiators of the inflammatory process, microorganisms in IBD cause immune cells to invade the tissue due to their chemotaxis effects. This process is accelerated by releasing further inflammatory mediators, causing tissue damage and severe tissue destruction [
35]. Having antibiotic properties, with more efficacy against gram-negative bacteria than the gram-positive ones, cycloviolacin-O2 [
36] gamma-sitosterol, phytol, and hexadecanoic acid methyl ester (with antifungal effect) [
18,
34], barbalion and tannins [
37] prevent the opportunistic bacterial infections in the GI tract. Mucilage is a compound also found in V. odorata extract, which relieves the mucosa by forming a protective layer over the ulcers and inflammatory areas [
38]. Sedghi et al. investigated the histomorphological effect of V. odorata extract on skin wounds in rats. They found that the wounded areas in the experimental group were smaller, as compared to those in the control group. The authors attributed the findings to the anti-inflammatory effects of the extract [
39].
The percentage of inflamed colonic regions in the experimental group was significantly less than those found in the control and standard groups (P<0.05). The aqueous extract of C. fistula pulps showed anti-inflammatory effects [
40] due to its flavonoids, coumarin [
32,
33], vitamin C [
33], and vitamin E (tocopherol) contents [
41]. These compounds are strong antioxidants and modulators of the immune system [
42]. The presence of salicylate and barbaloin in addition to such contents as vanillic acid (inhibitor of cyclooxygenase) [
18,
43, 44, 45], and anti-inflammatory phytochemicals, such as gamma-sitosterol, phytol, methyl ester, and hexadecanoic acid in the V. odorata extract have anti-inflammatory effects.
In this study, the experimental group received a higher microscopic score than other groups, likely due to stimulation of the immune system by phytol [
34]. Long-term consumption of V. odorata extract can prevent the progress of colitis and its conversion to cancer due to the high contents of vitamin C [
33], cyclo-violacin-O2 [
31], cyclo-violacin-O8, octacosanol and gamma-sitosterol (prevents angiogenesis) [
34], barbaloin [
18], thymol, oleic acid, furanone, and rhein [
46] with anti-tumor property. In a study by Alipanah, et al., it was found that the extract of V. odorata inhibited free radicals in a dose-dependent manner. They also found that the V. odorata extract inhibited tumor growth and reduced lung and liver metastasis in animal models [
47].
Conclusions
The present preliminary study demonstrated that the hydroalcoholic extracts of V. odorata and C. fistula at 200 mg/kg significantly reduced tissue damages and inflammation caused by acetic acid-induced colitis in the colon tissue of male Wistar rats. Based on the findings of this study, the combined extracts may potentially be regarded as a complementary medicine in the clinical management of inflammatory bowel disease. Further evidence on the clinical and prognostic advantages of these extracts awaits future research in animal and human models.
Ethical Considerations
Compliance with ethical guidelines
This study was reviewed and approved by the Ethics Committee of the Shahid Sadoughi University of Medical Sciences, Yazd, Iran. (Code: IR.SSU.MEDICINE.REC.1398.340).
Funding
This article was extracted from the results of a research project conducted by the authors at Herbal Medicines Research Center, School of Pharmacy, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran.
Authors' contributions
Conceptualization, supervision, and methodology: Rahele Zareshahi and Mohsen Zabihi; Investigation: Rahele Zareshahi, Mohsen Zabihi, Anoosheh Ahmadi & Zahra Ravaji; Writing the original draft: Anoosheh Ahmadi, Zahra Ravaji & Abolfazl Nasrollahi; Review & Editing: Rahele Zareshahi, Mohsen Zabihi and Hamed Mahmoodian; Data Collection: Anoosheh Ahmadi, Zahra Ravaji, Hamed Mahmoodian & Abolfazl Nasrollahi; Data analyses: Hamed Mahmoodian; Funding Administration: Rahele Zareshahi, Mohsen Zabihi & Abolfazl Nasrollahi.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgements
The authors wish to acknowledge the support of the management and staff of the School of Pharmacy at Shahid Sadoughi University of Medical Sciences.
References
- Olfatifar M, Zali MR, Pourhoseingholi MA, Balaii H, Ghavami SB, Ivanchuk M, et al. The emerging epidemic of inflammatory bowel disease in Asia and Iran by 2035: A modeling study. BMC Gastroenterology. 2021; 21(1):204. [PMID] [PMCID]
- Balaii H, Olfatifar M, Olianasab Narab S, Arab Hosseini A, Seyed Salehi A, Shahrokh S. Estimation the direct cost of inflammatory bowel disease in Iranian patients; the one-year follow-up. Gastroenterology and Hepatology from Bed to Bench. 2019; 12(Suppl.1):87-93. [PMID]
- Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Digestive Diseases and Sciences. 2013; 58(2):519-25. [PMID] [PMCID]
- Neurath MF. Cytokines in inflammatory bowel disease. Nature Reviews Immunology. 2014; 14(5):329-42. [DOI:10.1038/nri3661] [PMID]
- Feizi F, Mousavi M. Facilitate seed germination of the golden shower tree (Cassia fistula) in vitro using TiO2 nanoparticles and scarification treatments. Journal of Agricultural Science. 2016; 8(9):19-27. [DOI:10.5539/jas.v8n9p168]
- Tripathi K, Feuerstein JD. New developments in ulcerative colitis: Latest evidence on management, treatment, and maintenance. Drugs in Context. 2019; 8:212572. [DOI:10.7573/dic.212572] [PMID] [PMCID]
- Akhbari M, Batooli H, Kashi FJ. Composition of essential oil and biological activity of extracts of Viola odorata L. from central Iran. Natural Product Research. 2012; 26(9):802-9. [DOI:10.1080/14786419.2011.558013] [PMID]
- Seyyednejad SM, Motamedi H, Vafei M, Bakhtiari A. The antibacterial activity of Cassia fistula organic extracts. Jundishapur Journal of Microbiology. 2014; 7(1):e8921. [PMID] [PMCID]
- Besharati-Seidani A, Jabbari A, Yamini Y, Saharkhiz M. Rapid extraction and analysis of volatile organic compounds of Iranian feverfew (Tanacetum parthenium) using headspace solvent microextraction (HSME), and gas chromatography/mass spectrometry. Flavour and Fragrance Journal. 2006; 21(3):502-9. [DOI:10.1002/ffj.1650]
- Ansari M, Rafiee KH, Yasa N, Vardasbi S, Naimi SM, Nowrouzi A. Measurement of melatonin in alcoholic and hot water extracts of Tanacetum parthenium, Tripleurospermum disciforme and Viola odorata. DARU: Journal of Pharmaceutical Sciences. 2010; 18(3):173-8. [PMID]
- Saeed M, Naseer S, Hussain S, Iqbal M. Phytochemical composition and pharmacological effects of cassia fistula. Scientific Inquiry and Review. 2020; 4(1):59-69. [DOI:10.32350/sir.41.05]
- Siddiqua A, Zahra M, Begum K, Jamil M. The traditional uses, phytochemistry and pharmacological properties of Cassia fistula. Journal of Pharmacy and Pharmacology Research. 2018; 2(1):15-23. [DOI:10.26502/jppr.0006]
- Raju GS, Moghal MR, Dewan SM, Amin MN, Billah M. Characterization of phytoconstituents and evaluation of total phenolic content, anthelmintic, and antimicrobial activities of Solanum violaceum Ortega. Avicenna Journal of Phytomedicine. 2013; 3(4):313-20. [PMID]
- Khan W, Subhan S, Shams DF, Afridi SG, Ullah R, Shahat AA, et al. Antioxidant potential, phytochemicals composition, and metal contents of Datura alba. Biomed Research International. 2019; 2019:2403718. [DOI:10.1155/2019/2403718] [PMID] [PMCID]
- Varsha S, Agrawal R, Sonam P. Phytochemical screening and determination of anti-bacterial and anti-oxidant potential of Glycyrrhiza glabra root extracts. Journal of Environmental Research and Development. 2013; 7(4A):1552-8. [Link]
- Abd Elkader HAE, Abdou HM, Khamiss OA, Essawy AE. Anti-anxiety and antidepressant-like effects of astragaloside IV and saponins extracted from Astragalus spinosus against the bisphenol A-induced motor and cognitive impairments in a postnatal rat model of schizophrenia. Environmental Science and Pollution Research. 2021; 28(26):35171-87. [DOI:10.1007/s11356-021-12927-5] [PMID]
- Sakulpanich A, Gritsanapan W. Extraction method for high content of anthraquinones from Cassia fistula pods. Journal of Health Research. 2008; 22(4):167-72. [Link]
- Singh N, Goyal K, Sondhi S, Jindal S. Traditional and medicinal use of Barbaloin: Potential for the management of various diseases. Journal of Applied Pharmaceutical Research. 2020; 8(3):21-30. [DOI:10.18231/j.joapr.2020.v.8.i.3.21.30]
- Fabia R, Willen R, Ar’Rajab A, Andersson R, Ahren B, Bengmark S. Acetic acid-induced colitis in the rat: A reproducible experimental model for acute ulcerative colitis. European Surgical Research. 1992; 24(4):211-25. [DOI:10.1159/000129209] [PMID]
- Zabihi M, Hajhashemi V, Talebi A, Minaiyan M. Evaluation of central and peripheral effects of doxepin on acetic acid-induced colitis in rat and the involved mechanisms. EXCLI Journal. 2017; 16:414-22. [Link]
- Noronha-Blob L, Lowe VC, Muhlhauser RO, Burch RM. NPC 15669, an inhibitor of neutrophil recruitment, is efficacious in acetic acid-induced colitis in rats. Gastroenterology. 1993; 104(4):1021-9. [DOI:10.1016/0016-5085(93)90269-I] [PMID]
- MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978; 17(2):135-50. [PMID]
- Garrido G, González D, Lemus Y, Garcıa D, Lodeiro L, Quintero G, et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG®). Pharmacological Research. 2004; 50(2):143-9. [DOI:10.1016/j.phrs.2003.12.003] [PMID]
- Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. British Journal of Pharmacology. 2003; 139(6):1146-52. [PMID] [PMCID]
- Rashidian A, Roohi P, Mehrzadi S, Ghannadi AR, Minaiyan M. Protective effect of Ocimum basilicum essential oil against acetic acid-induced colitis in rats. Journal of Evidence-based Complementary & Alternative Medicine. 2016; 21(4):36-42. [DOI:10.1177/2156587215616550] [PMID]
- Bastaki SM, Adeghate E, Amir N, Ojha S, Oz M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. American Journal of Translational Research. 2018; 10(12):4210-22. [PMID]
- Mohamed NI, El-Kashef DH, Suddek GM. Flavocoxid halts both intestinal and extraintestinal alterations in acetic acid-induced colitis in rats. Environmental Science and Pollution Research. 2022; 29(4):5945-59. [PMID]
- Ali FEM, M Elfiky M, Fadda WA, Ali HS, Mahmoud AR, Mohammedsaleh ZM, et al. Regulation of IL-6/STAT-3/Wnt axis by nifuroxazide dampens colon ulcer in acetic acid-induced ulcerative colitis model: Novel mechanistic insight. Life Sciences. 2021; 276:119433. [DOI:10.1016/j.lfs.2021.119433] [PMID]
- Mwangi RW, Macharia JM, Wagara IN, Bence RL. The medicinal properties of Cassia fistula L: A review. Biomedicine & Pharmacotherapy. 2021; 144:112240. [DOI:10.1016/j.biopha.2021.112240] [PMID]
- Mirzaei F, Khazaei M. [Role of nitric oxide in biological systems: A systematic review (Persian)]. Journal of Mazandaran University of Medical Sciences. 2017; 27(150):192-222. [Link]
- Feyzabadi Z, Ghorbani F, Vazani Y, Zarshenas MM. A critical review on phytochemistry, pharmacology of Viola odorata L. and related multipotential products in traditional Persian medicine. Phytotherapy Research. 2017; 31(11):1669-75. [PMID]
- Sollai F, Zucca P, Sanjust E, Steri D, Rescigno A. Umbelliferone and esculetin: Inhibitors or substrates for polyphenol oxidases? Biological and Pharmaceutical Bulletin. 2008; 31(12):2187-93. [DOI:10.1248/bpb.31.2187] [PMID]
- Asheesh K, Suresh C, Meenakshi P. A brief knowledge of banafsha (Viola odorata linn.) & other Viola species. International Journal of Ayurveda and Pharma Research. 2017; 20: 21-32. [Link]
- Jasim SF, Baqer NN, Alraheem E. Detection of phytochemical constituent in flowers of Viola odorata by gas chromatography-mass spectrometry. Asian Journal of Pharmaceutical and Clinical Research. 2018; 11(5):262-9. [DOI:10.22159/ajpcr.2018.v11i5.24288]
- Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nature Reviews Microbiology. 2019; 17(8):497-511. [PMID] [PMCID]
- Pränting M, Lööv C, Burman R, Göransson U, Andersson DI. The cyclotide cycloviolacin O2 from Viola odorata has potent bactericidal activity against Gram-negative bacteria. Journal of Antimicrobial Chemotherapy. 2010; 65(9):1964-71. [DOI:10.1093/jac/dkq220] [PMID]
- Pizzi A. Tannins medical/pharmacological and related applications: A critical review. Sustainable Chemistry and Pharmacy. 2021; 22:100481. [DOI:10.1016/j.scp.2021.100481]
- Morton JF. Mucilaginous plants and their uses in medicine. Journal of Ethnopharmacology. 1990; 29(3):245-66. [DOI:10.1016/0378-8741(90)90036-S] [PMID]
- Sedghi E, Moghtadaei-Khorasgani E, Norbakhsh M. [The histomorphological effect of Viola odorata flower extract on skin wound healing process in Wistar rats (Persian)]. Feyz. 2020; 24(4):366-73. [Link]
- Anwikar S, Bhitre M. Study of the synergistic anti-inflammatory activity of Solanum xanthocarpum Schrad and Wendl and Cassia fistula Linn. International Journal of Ayurveda Research. 2010; 1(3):167-71. [PMID] [PMCID]
- Fazeenah AA, Quamri MA. Banafsha (Viola odorata Linn.) - A review. World Journal of Pharmaceutical Research. 2020; 9(10):514-37. [Link]
- Lewis ED, Meydani SN, Wu D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life. 2019; 71(4):487-94. [DOI:10.1002/iub.1976] [PMID] [PMCID]
- Brimson JM, Onlamoon N, Tencomnao T, Thitilertdecha P. Clerodendrum petasites S. Moore: The therapeutic potential of phytochemicals, hispidulin, vanillic acid, verbascoside, and apigenin. Biomedicine & Pharmacotherapy. 2019; 118:109319. [PMID]
- Somani SJ, Modi KP, Majumdar AS, Sadarani BN. Phytochemicals and their potential usefulness in inflammatory bowel disease. Phytotherapy Research. 2015; 29(3):339-50. [DOI:10.1002/ptr.5271] [PMID]
- Zhang D, Liu R, Sun L, Huang C, Wang C, Zhang DM, et al. Anti-inflammatory activity of methyl salicylate glycosides isolated from Gaultheria yunnanensis (French.) Rehder. Molecules. 2011; 16(5):3875-84. [PMID] [PMCID]
- Tanveer S, Latif A, Ashiq K, Qayyum M, Bajwa MA. A comprehensive review on pharmacological and phytochemical potential of Cassia Fistula Linn: A Magical Herb. International Journal of Biology, Pharmacy and Allied Sciences. 2019; 8(6):1134-57. [DOI:10.31032/IJBPAS/2019/8.6.4734]
- Alipanah H, Bigdeli MR, Esmaeili MA. Inhibitory effect of Viola odorata extract on tumor growth and metastasis in 4T1 breast cancer model. Iranian Journal of Pharmaceutical Research. 2018; 17(1):276-91. [PMID]










































 , Anoosheh Ahmadi1
, Anoosheh Ahmadi1 

 , Zahra Ravaji1
, Zahra Ravaji1 

 , Mohsen Zabihi *
, Mohsen Zabihi * 

 2, Abolfazl Nasrollahi1
2, Abolfazl Nasrollahi1 

 , Hamed Mahmoodian3
, Hamed Mahmoodian3 




