Introduction
Caseous Lymphadenitis (CLA) is one of the leading causes of economic loss in small cattle husbandry globally [
1,
2]. In Malaysia, the disease is commonly seen in goats with a prevalence of 25% and in 75% of farms in Selangor area alone [
3]. Upon introduction into a herd, CLA is difficult to eradicate because C. pseudotuberculosis responds poorly to antibiotic treatments. Typically, treatment in this disease requires long-term administration of antibiotics, which is frowned at in breeding animals for foods due to the potential impact on the human health [
1]. Other options available for their treatment involve surgical excision of the caseous lesions plus the superficial lymph nodes, and flushing them with potentiated iodine solution [
4]. The lancing further spreads C. pseudotuberculosis into the environment placing other uninfected animals at risk whereas applying potentiated iodine solution to the lesion is associated with histotoxicity and wound healing delay [
4,
5]. The spread of C. pseudotuberculosis within the animal’s body is facilitated by Phospholipase D (PLD) exotoxin and mycolic acid, which prevent the bacterial destruction by phagocytes and allow them to remain hidden in macrophages [
6].
Attempt by the body to prevent the further spread of C. pseudotuberculosis; however, results in the formation of encapsulated caseous lesions, making the treatment difficult to administer [
7]. Therefore, the need for developing alternative treatment methods is logical in the management of this disease [
7]. Previous studies have reported the efficacy of gold or silver nanoparticles as a possible treatment option against C. pseudotuberculosis [
5,
7,
8]. The application of nanoparticles to treat resistant infections is loading antibiotics unto nanoparticles for drug delivery is a new development in the field of nanomedicine [
9,
10]. Several studies have shown that calcium carbonate nanoparticles (CS-CaCO3NP) offer many advantages in the delivery of antibiotics over other inorganic nanocarriers [
11]. The advantages include synthesis of CS-CaCO3NP from inexpensive and readily available raw materials, and the ability to preserve the loaded drug’s physicochemical characteristics in nanoparticles [
11,
12].
The delivery of antibiotics, such as ciprofloxacin, vancomycin, gentamycin and tetracycline for targeted delivery against Salmonella spp, Staphylococcus aureus, Bacillus subtillis and Shigella flexineri, using CS-CaCO3NP has yielded promising results compared to the free, i.e., unloaded forms of the drugs [
12,
13,
14,
15]. Also, CS-CaCO3NP can be made functional to improve the cellular uptake, and sustained drug release [
16,
17,
18]. Lastly, CS-CaCO3NP compounds are considered biocompatible and non-toxic [
16,
17,
19]. Previous studies have investigated the OTC encapsulation in nanoparticles [
20,
21]. However, oxytetracycline loaded calcium carbonate aragonite nanoparticle (OTC-CS-CaCO3NP) against C. pseudotuberculosis remains largely unexplored in developing an effective treatment against CLA in small ruminant animals.
Aim of the study: This study aimed to investigate the potential of OTC-CS-CaCO3NP as a nano-antibiotic formulation toward developing effective treatment against CLA infection in mice.
Materials and Methods
Synthesis & characterization: The synthesis and characterization of the two compounds (CS-CaCO3NP and OTC-CS-CaCO3NP) and the in vitro release of OTC from CS-CaCO3NP were achieved as described in our previous article [
22].
Animal inoculation: The C. pseudotuberculosis used in this study was collected from the blood agar plates that had been identified and confirmed in advance. The bacteria were then sub-cultured into another blood agar plate at 37oC for 24 hours. The colonies from these plates were further cultured in Brain Heart Infusion (BHI) medium maintained at 37.0°C for 24 hours. The inoculum concentration was adjusted, using sterile saline solution (PBS 0.15M, pH 7.4) to 1×102 CFU/mL (MacFarland’s technique [
23]). Each mouse received 0.2mL of the bacterial inoculum based on the method reported by a previous study [
24].
Study design: Thirty 5-6 weeks old apparently healthy female BALB/c mice were used in this study. Adaptation of the mice was done for 1-week and they were provided with clean water and Gold coin mouse pellets diet (Gold coin feed mills, SDN BHD, Malaysia) ad libitum. The mice were randomly divided into five groups of six each. Group 1 represented the negative control and dosed with 0.2mL sterile distilled water. Group 2 were inoculated with 0.2mL of C. pseudotuberculosis suspension (positive controls). Groups 3 and 4 were inoculated with 0.2mL of C. pseudotuberculosis suspension Intraperitoneally (IP). These mice were treated with free OTC at 10mg/kg and OTC-CS-CaCO3NP at 10mg/kg IP, respectively, 30 minutes post infection. Group 5 was administered with only CS-CaCO3NP at 20mg/kg, IP.
Clinical observations: The body weights of the mice were taken weekly, and the associated clinical signs were recorded. The clinical signs observed were scored, using the method described by a previous study [
24]. The scoring for the clinical signs was from zero to three in mice, where 0=Normal; 1=Mild manifestation of clinical signs rated as 30%; 2=Moderate clinical manifestation, 60%; 3=Severe, clinical signs greater than 60%, based on the established signs for caseous lymphadenitis reported earlier in mice [
25,
26]
Blood & tissue sample collection: After the 21-day experiment, the mice were anesthetized with ketamine or xylazine, and blood samples collected through cardiac puncture. The blood samples were kept in plain and EDTA-contained test tubes for further serum biochemistry and haematological analyses, respectively. The mice were then dissected and the organs of interest harvested, such as liver, lungs and lymph nodes. These organs were fixed in 10% neutral buffered formalin for 48 hours before the subsequent histopathological examinations.
Haematological and serum biochemistry: The haematological analyses for total Red Blood Cell count (RBC), Packed Cell Volume (PCV), total White Blood Cell count (WBC) and differential cells, platelet count and erythrocyte indices (MCV & MCHC) were done, using automatic cell counter (Horiba Medical Vet. ABC Plus blood analyser, Scil Vet. ABC, USA). The determinations of serum biochemical parameters, such as Alkaline Phosphatase (ALP), Aspartate aminotransferase (AST), Alanine transaminase (ALT), urea and creatinine were performed, using an automated biochemical analyser (Dimension® EXL™ 200 integrated system, Siemens, Germany).
Antibacterial Efficacy of OTC-CS-CaCO3NP and OTC: The therapeutic efficacy of OTC-CS-CaCO3NP and OTC compared to untreated controls was evaluated in lymph-node, liver, and spleen of BALB/c mice infected with C. pseudotuberculosis as described earlier [
27]. The harvested organs were weighed and kept in sterile 50mL test tubes, homogenized in 0.9% sterile saline solution, and centrifuged at 10,000 rpm for 10 minutes. 200µL of the homogenate was placed in sterile 96-well plates and serially diluted in Brain Heart Infusion (BHI) broth up to a maximum of 105 dilutions. A 0.1mL aliquot of the dilution was placed on BHI agar plates and incubated for 48hr at 37°C. The Colony Forming Units (CFU) per gram of organ was determined for the lymph node, liver and spleen samples.
Histopathological alterations: The liver, kidneys, lymph nodes and spleen samples were fixed in buffered formalin for 48 hours in preparation for the histopathological examinations. The fixed tissue samples were processed, using standard protocols and embedded in paraffin wax dishes. About 5 micron-thick sections were made for each tissue sample and placed on microscope slides, dried overnight on a hotplate and stained subsequently with Haematoxylin and Eosin (H&E). The slides were examined under a light microscope (Leica DM4M, NY USA), with Moticam Pro 282A 5.0MP (Motic images Software Plus 2.0 TWAIN, Hong Kong) at a magnification of 100 x.
Statistical analyses: All of the data were expressed as the mean±SD. The weight, haematological and serum biochemical data were analyzed, using repeated measures and one-way Analysis of Variance (ANOVA), respectively. The clinical signs and histopathological scores were analyzed by Kruskal Wallis test. A P<0.05 was considered as statistically significant. All of the statistical analyses were performed, using GraphPad Prism software, version 8.
Results
Clinical signs:
Table 1 shows the mean scores of clinical signs observed during the experiments.
.jpg)
Movement in the animal groups of G2, G3 and G4 was significantly slower (P<0.0001) than those in G1 and G5 groups. However, the animals’ movement in G2 group was slower than that observed in G3 (P=0.0473) and G4 (P=0.0001) groups. There were no significant differences in the animal movement noted between G3 and G4 groups (P>0.05). Also, there were no significant differences in the ruffled fur test observed in G2 compared to the G3 (P>0.05). However, a significant increase was detected in the ruffled fur test observed in G2 group compared that of the G4 group. As expected, G1 and G5 groups had no positive ruffled fur test.
Racing over hurdles together was observed in the mice in groups G2, G3 and G4, respectively. However, the hurdling in G2 was significantly higher (P<0.0001) compared to that observed in mice in G4 group. Although, there were no significant differences recorded in hurdling between G2 and G3 groups (P>0.05). Further, the mice in G2, G3 and G4 groups were significantly depressed and duller compared to those in G1 and G5 groups (P<0.0001). Also, there were no significant differences in the observed dullness and depression between G2 and G3 groups (P>0.05). However, the dullness and depressive behavior were significantly higher in G4 group than that noted in G2 and G3 groups. However, the common clinical signs, such as diarrhoea, ocular discharge and the mortality due to C. pseudotuberculosis infection were not observed throughout the 21-day experimentation.
Mice’s body weights: There was no difference among the body weights of the mice at the first week, except for those in G4 group which was decreased (P=0.0221) compared to those in G1 group. By the second week of the study, there was a weight loss noted in the mice of G2 group (P=0.0058) compared to those in G1 group. However, the mice in all other groups were not significantly different in their body weight (P>0.05). Furthermore, at the third week, there were additional weight losses recorded in G2, G3 and G4 groups (P=0.0001, 0.0001 and 0.1013, respectively) compared to those in G1 group. However, mice in G4 group still exhibited higher body weights (P>0.05) compared to those in G2 and G3 groups. By the third week, the body weights of mice in group G5 had increased significantly (P=0.0067) compared to those in G2 and G3 groups. There was no significant difference between the body weights of mice in groups G4 and G5 at the end of the third week (
Figure 1).
Hematologic results: The erythrogram and platelet counts of the mice infected with C. pseudotuberculosis and treated with OTC-CS-CaCO3NP are shown in
Table 2.
.jpg)
The results revealed that the means for RBC, Hb, PCV, MCV and MCHC values were not significantly different in all mice groups (P>0.05). Although the means for RBC, Hb and PCV in group G4 was numerically higher than those in G2 and G3 groups, the mice in G5 group had higher means for RBC count and PCV as compared to those in G1 group.
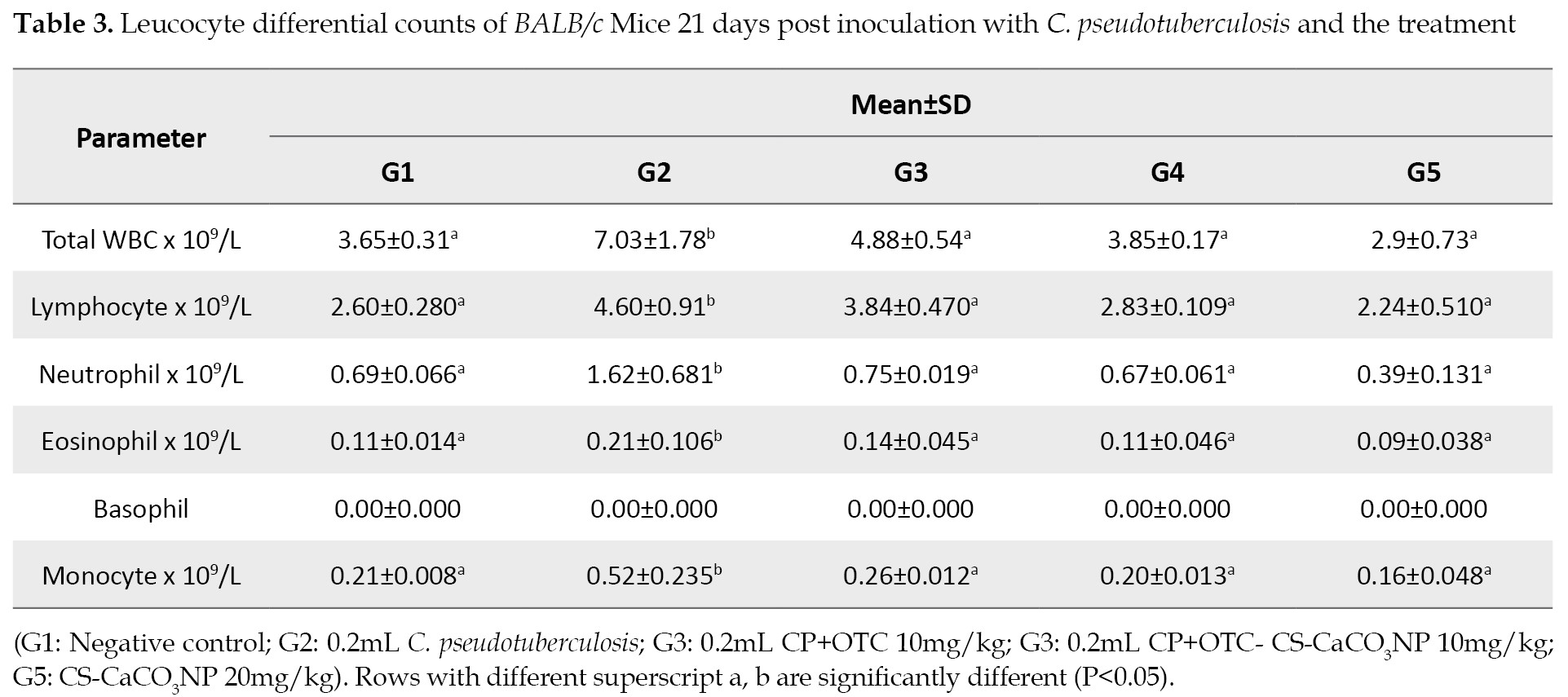
Leucocyte differential counts following inoculation & treatment: There was a significant increase (P<0.05) in the means for total WBC, lymphocyte, neutrophil, eosinophil, and monocyte counts in G2 group compared to those in other groups. However, the results of the leucocyte counts showed no statistical differences among the mice in G1, G2, G3 and G4 groups (
Table 3).
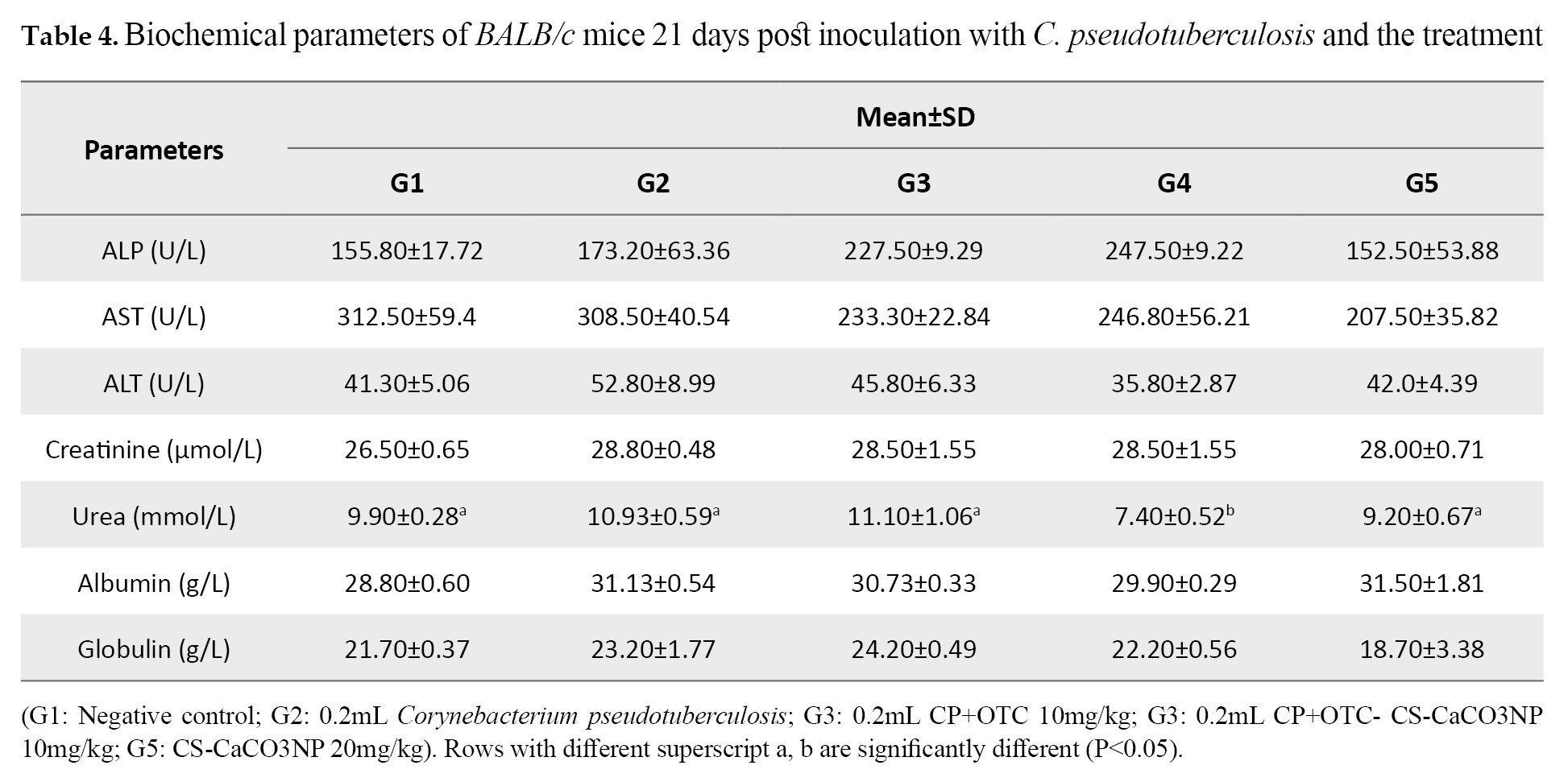
Biochemical profiles following inoculation & treatment: The results revealed that the biochemical profiles of the mice were not significantly different based on the inoculation of C. pseudotuberculosis and treatments with OTC, OTC-CS-CaCO3NP and CS-CaCO3NP (P>0.05), except the urea, for which G4 group was significantly lower than those in other groups (P=0.0116;
Table 4).
Antibacterial efficacy of OTC-CS-CaCO3NP and OTC: The efficacy of treatment with OTC-CS-CaCO3NP in lymph nodes, liver and spleen samples of the infected mice with C. pseudotuberculosis compared to OTC and untreated infected controls are shown in
Figure 2.
The results demonstrated a log reduction of 0.8 in the lymph nodes, 0.9 in the liver and 1.0 in the spleens samples of the mice treated in G4 group compared to those in G2 group. Further, in G3 mice, there was a log reduction of 0.4, 0.6 and 0.4 in the lymph nodes, liver and spleens, respectively. Mice in group G4 had significantly lower bacterial loads in their organs compared to those in G2 group (**P=0.0009, *P=0.0153 and **P=0.0049).
Histopathological evaluation of mice following inoculation & treatment: The microscopic examinations of the liver samples for G2 group revealed moderate to frequent numbers of inflammatory cells, and congestion of portal vein and sinusoids (
Figure 3B).
The liver samples from mice in group G3 exhibited moderate number of inflammatory cells with dilated and congested sinusoids (
Figure 3C). The mice in G4 group showed improved hepatic cellular architecture compared to those in G3 group with mild dilation and congestion of sinusoids (
Figure 3D). The cellular architecture of the lungs was maintained at normal level in the G1, G4 and G5 groups (
Figures 4A,
4D &
4E).
The mice in G2 group exhibited alveolar vessels congestion similar to those found in G3 mice (
Figures 4B &
4C).
The histopathological evaluations of the mesenteric lymph nodes revealed areas of necrosis and pericapsular fibrosis in group G2 mice (
Figure 5B).
Pericapsular fibrosis were also observed in the lymph node of the mice in G3 group (
Figure 5C). However, the lymph nodes architecture in G1, G4 and G5 groups was maintained at normal level (
Figures 5A,
5D &
5E).
Discussion
In the present study, an animal model was used to assess the efficacy and toxicity of OTC and OTC-CS-CaCO3NP against C. pseudotuberculosis infection. The BALB/c mice have been reported to be the most susceptible mouse strain to infection with C. pseudotuberculosis [
28]. Also, the intraperitoneal route produced the clinical signs of the disease in this animal strain [
25,
26]. The Clinical signs, weight loss and histopathology are used as the major markers to evaluate the disease severity and the progression in these animals [
29,
30].
The mice in groups G2 and G3 showed significant differences in the clinical signs of abnormal movement, ruffled fur, hurdling together, depression and dullness after the 21-day experimental treatments post inoculation compared to those in G4 group. Our results agree with those of earlier researchers [
25,
31] who also found significant clinical signs following the intra peritoneal inoculation of whole C. pseudotuberculosis bacteria in experimental animals. Although one study [
31] has reported other clinical signs, such as diarrhoea and eye discharges, which were not observed in our study. These might be due to differences in the volume and dilution of the inoculum used. The increased severity of the clinical signs in group G2 could be attributed to thriving bacterial colonization in the body and spreading through the lymphatic tracts to the rest of the animals’ body [
29]. The decline in the clinical signs and severity in both G3 and G4 groups may also be due to the antibacterial effect of OTC against the bacteria. Moreover, the decreased severity in the clinical signs noted in group G4 compared to those in G3 represents the sustained release of OTC from CS-CaCO3NP, hence the pronounced antibacterial effect evident in G4 group. Another study [
15] compared free tetracyclines and tetracycline-loaded calcium phosphate nanoparticles against Shigella flexneri 2a in similar mice groups. The authors reported a significant reduction in the clinical signs of the animals infected with Shigella.
Changes in body weight are used to express the entire loss in the body on in vivo experiments versus the experimentally produced diseases [
26]. A 10% body weight loss serves as a humane outcome for C. pseudotuberculosis studies in mice [
26]. As observed in this study, the mice in group G2 lost significant weight due to infection with C. pseudotuberculosis. This may be attributed to reduction in appetite secondary to the infection. Another study [
31] has reported that lack of appetite was also seen in animals treated with whole C. pseudotuberculosis bacteria. Furthermore, weight loss is associated with under-nourishment in mice exposed to bacterial infections that lead to sepsis [
32]. The same reason could account for the body weight loss seen in group G3 up to the third week of the study. In contrast, in G4 group, the increase in body weight might have occurred due to changes in the mice’s appetite that were affected less by the infection. The increase in the body weight of mice in G5 group might be due to the non-toxic calcium carbonate in the nanoparticle [
33], hence the recorded increases in the body weights in that study.
In this study, the infected mice with C. pseudotuberculosis did not die possibly because of the bacterial stabilization. Further, pathogenic intracellular bacteria, such as C. pseudotuberculosis, alter the host immune defence against establishing an infection [
23]. Hence the reason why the disease persists for a long time in the infected animals [
34]. Bacterial stabilization or adaptive resistance by facultative intracellular bacteria, such as Corynebacterium and Mycobacteria is an important phenomenon used to study the course of infections with pathogens. The bacteria, C. pseudotuberculosis, has been studied in CBA mice for up to two months [
23]. The disease persists long in the mice due to adaptive resistance induced by CD4 T-helper cells and cytokines to induce the bactericidal effects of macrophages [
23]. The BALB/c mice have functional CD4 T-cells which are important in maintaining C. pseudotuberculosis infection, which is why the mice did not die in the current study.
The RBC, Hb, and PCV values did not differ from the normal levels reported by another study conducted in BALB/c mice [
7]. The erythrocyte evaluation in the present study showed a reduction in the means of RBC, Hb and PCV in groups G2 and G3 compared to those in other groups. This could be attributed to the effect of C. pseudotuberculosis infection as described an earlier study [
31]. The reduced platelet count found in group G2 compared to those in G3 and G4 groups might be due to hypersplenism and sequestration of platelets in the spleen as observed in the gross pathology of the spleens in group G2. The higher platelet values seen in the G4 group may be attributed to recovery from CLA. This is because in diseases associated with septicaemia, one of the first sign of patient recovery is improvement in the platelets count [
35]. The findings of the present study also agree with those reported by earlier research [
36]. In that study, inoculation of mice with mildly virulent strain of C. pseudotuberculosis did not cause significant changes in the RBC, Hb, and PCV levels in C3H/HeJ mice. The erythrocytes’ counts from this study showed that there were minimal changes in the blood profile as there were no statistical significances in the erythrocyte indices (MCV & MCHC), suggesting that the mild anaemia noted in groups G2 and G3 was due to normocytic normochromic anaemia.
The values for the means of WBC counts have been reported to be within the normal range for female BALB/c mice as described by earlier studies [
7,
37]. The reported findings are consistent with our results documented for G1, G4 and G5 groups. Further in our study, the differential leucocyte counts for all groups indicated that the lymphocytes consisted approximately 80% of the total white blood cell counts whereas the neutrophils were about 20%. These results agree with those reported by a study [
38] that reported their mice generally had higher ratios of lymphocytes (70%-80%) to neutrophils (20%-30%). Interestingly, the WBC and neutrophils in the G2 group were higher, suggesting an ongoing infection due to C. pseudotuberculosis. The high WBC and neutrophil counts were also reported in BALB/c mice infected with C. pseudotuberculosis whole bacterium and PLD toxin in a previous study [
31]. The treatment administered to mice in G3 and G4 groups was possibly able to control the infection induced by C. pseudotuberculosis, hence the reason for the lower neutrophils and WBC counts.
The analysis of the blood chemistry is an important diagnostic measure which reflects the physiological health in an animal [
38]. In the present study, the liver biochemical status was evaluated by assaying the AST, ASP and ALT levels whereas the kidneys biochemical status was evaluated the creatine and urea levels. There were no significant differences across the groups for their AST, ASP and ALT. Although, there were slight variations within the groups, this was not enough to categorically diagnose liver pathology. Moreover, the AST and ALP values may have arisen from other sources like muscle stress during injection of bacterial inoculum and the treatments, hence the need to determine liver injury with ALT specific to the liver [
39]. The ALT levels were higher in groups G2 and G3 than in G4 group. This is in line with the results reported by a previous study [
31]. Increases in ALT is an indication of bacterial invasion to the liver in G2 and G3 groups. The slight reduction of ALT in G4 group is suggestive of improved antibacterial activity of OTC-CS-CaCO3NP over free OTC. The reduced ALT levels in G5 group indicates that CS-CaCO3NP did not induce hepatocellular damage in the mice, consistent with the results of previous studies [
17,
19].
The concentrations of urea in the present study in G1, G4 and G5 groups were found to be within the normal range (6.99 mm/L to 9.99 mm/L) consistent with the report of an earlier study [
40]. Though both G2 and G3 groups experienced dehydration, the slight elevation in urea concentration in G3 compared to G2 may be due to a combination of dehydration and the OTC induced oxidative stress [
41]. In group G4, the urea level was significantly reduced which may probably be due to the most prominent advantage of loading antibiotic into nanoparticle. This preparation increases the intra cellular drug concentration and reduces the drug toxicity, as opposed to that reported for the free antibiotic [
42]. The normal urea and creatine levels in the G4 group suggest the efficacy and nephroprotective effect of OTC-CS-CaCO3NP. The plasma urea levels in G5 group agree with the results of a former study [
17] where it was within the range recorded for the controls, without significant histological changes observed in the kidneys.
An insignificant effect that was found for the creatinine concentrations in all of the animal groups where the values fell to within the normal range in the mice (28.17-48.62 µmol/L) [
43]. Also, there were no significant differences in the albumin and globulin concentrations of the mice, which disagree with the findings of a former study [
31]. The differences may have arisen from the different degree and extent of liver damages in both studies.
The infection dose used in the efficacy study, based on a former work [
23], showed that C. pseudotuberculosis could be repeatedly recovered from the lymph nodes, liver and spleen of the infected mice. The significant reduction in C. pseudotuberculosis counts in the G4 group compared to that of G2 group may be due to the gradual and sustained release of OTC-CS-CaCO3NP in the mice over time, increased OTC plasma concentration, prolonged half-life (5.67hr), and increased mean residues of OTC-CS-CaCO3NP. The lower reduction in G3 group implies that there was less concentration of free OTC in their circulation. Loading antibiotics to nanoparticles have been reported by other studies to enhance their efficacy [
26,
42].
The striking histological findings in mice infected with C. pseudotuberculosis were septicaemia, congestion, infiltration of inflammatory cells, necrosis and distortion of tissue architecture. The histological changes in the liver, lungs and lymph nodes, as noted in G2 and G3 groups, were characteristic of C. pseudotuberculosis infection [
44]. Other pathological signs were the accumulation of inflammatory cells in the alveolar spaces of the lung and liver surfaces, respectively, and congestion [
25,
31]. In the lymph nodes, the pericapsular fibrosis, necrosis and enlargement of the splenic noodle present in the G2 and G3 groups could be attributed to the intracellular effects of C. pseudotuberculosis. Upon entry into the blood stream, the lymph nodes and spleen filter the bacteria, which can persist within macrophages [
44]. The macrophages cannot destroy the bacteria; thus, the body prevent further spread of infection by developing pyogranuloma [
44]. The reduced severity or absence of lesions in the G4 group compared to those in G3 group indicates that OTC-CS-CaCO3NP is effective against C. pseudotuberculosis. The in vivo antimicrobial mechanism of OTC-CS-CaCO3NP may be suggested as encapsulating OTC into CS-CaCO3NP thus enhancing the entry of OTC into C. pseudotuberculosis via destruction of cell envelope as reported in our previous publication [
45].
The administration of 20mg/kg of CS-CaCO3NP to the mice did not induce any toxici changes hematologically, biochemically and/or histopathologically. These agree with the findings reported by two previous studies [
17,
19]. Following the intra peritoneal injection of CS-CaCO3NP, it is absorbed into the blood as ionized calcium and the remaining parts bind to other biochemical matrices that are excreted in faeces and urine [
17]. Generally, CaCO3NP has a wide safety margin and is considered biocompatible [
19].
Conclusions
This study demonstrated the potential of CS-CaCO3NP for delivering therapeutic concentrations of OTC against intracellular bacteria, such as C. pseudotuberculosis with no detectable evidence of toxicity. The OTC-CS-CaCO3NP showed better antibacterial activity than the free form of OTC in BALB/c mice, infected with C. pseudotuberculosis. This effect was demonstrated by the significant reduction of C. pseudotuberculosis counts in the lymph nodes, liver and spleen of BALB/c mice had been treated with OTC-CS-CaCO3NP. Our findings provided the experimental evidence that CS-CaCO3NP did not induce toxic haematological, biochemical and/or histopathological changes in BALB/c mice. Finally, OTC-CS-CaCO3NP has a considerable potential to be used as an antibacterial agent against C. pseudotuberculosis in BALB/c mice.
Limitations of the study: The limitation of this study was the inability to further evaluate the efficacy of OTC-CS-CaCO3NP against C. pseudotuberculosis infection in goats.
Recommendations for future research: It is recommended to follow the course of caseous lymphadenitis and OTC-CS-CaCO3NP treatment in BALB/c mice by tagging OTC-CS-CaCO3NP with a chemical agent/dye that is detected, using in vivo imaging systems which enables the monitoring of disease progression and treatment. Also, it would be informative to determine the molecular mechanisms of actions of OTC-CS-CaCO3NP in vitro and in vivo.
Ethical Considerations
Compliance with ethical guidelines
The study complied with the rules and regulations of Institutional Animal Care and Use Committee (IACUC) in University Putra Malaysia where the study was conducted (Registration #: UPM/IACUC/AUP/R050/2018). The Proposal of this work was submitted to the IACUC Committee where it was thoroughly reviewed by experts in the field, after which an approval to conduct the study was granted. This project proceeded with periodic close monitoring by IACUC Committee.
Funding
A financial grant in support of this project was provided by Geran Putra, University Putra Malaysia (Registration #: GP/2018/9616700).
Authors' contributions
Supervision: Arifah Abdul Kadir, Jesse Faez Firdaos Abdullah, Siti-Zubaidah Ramanoon, Muhammad Zuki Zackariah Abubakar; Methodology: Sherifat Banke Idris, Arifah Abdul Kadir, Muhammad Abdul Basit, Muhammad Zuki Zackariah Abubakar; Laboratory Investigation: Sherifat Banke Idris, Arifah Abdul Kadir, Muhammad Abdul Basit, Muhammad Zuki Zackariah Abubakar; Data collection and analysis: Sherifat Banke Idris, Arifah Abdul Kadir, Muhammad Abdul Basit, Muhammad Zuki Zackariah Abubakar; Original draft writing: Sherifat Banke Idris, Arifah Abdul Kadir, Muhammad Zuki Zackariah Abubakar; Review, proof reading and editing: All authors.
Conflict of interest
The authors declare no conflict of interest with any internal or external entity to disclose in conducting the current study.
Acknowledgments
The authors would like to acknowledge the financial assistance received from Geran Putra and Mr. Johari Ripin of the Pharmacology Laboratory, Faculty of Veterinary Medicine, UPM, Malaysia.
References
- Washburn KE, Fajt VR, Lawhon SD, Adams LG, Tell LA, Bissett WT. Caprine abscess model of tulathromycin concentrations in interstitial fluid from tissue chambers inoculated with Corynebacterium pseudotuberculosis following subcutaneous or intrachamber administration. Antimicrob Agents Chemother. 2013; 57(12):6295-304. [DOI:10.1128/AAC.00936-13] [PMID] [PMCID]
- Oreiby AF. Diagnosis of caseous lymphadenitis in sheep and goat. Small Rumin Res. 2015; 123(1):160-6. [DOI:10.1016/j.smallrumres.2014.11.013]
- Ismail MS, Ramanoon SZ, Lee OB. Prevalence and risk factors of caseous lymphadenitis in goats from selected farms in Selangor, Malaysia. Paper presented at: 7th Proceedings of the Seminar in Veterinary Sciences. 27 February – 02 March 2 2012; Selangor, Malaysia. https://vet.upm.edu.my/upload/dokumen/202109061031237th_Proceedings_of_the_Seminar_on_Veterinary_Sciences.pdf#page=114
- Washburn KE, Bissett WT, Fajt VR, Libal MC, Fosgate GT, Miga JA, et al. Comparison of three treatment regimens for sheep and goats with caseous lymphadenitis. J Am Vet Med Assoc. 2009; 234(9):1162-6. [DOI:10.2460/javma.234.9.1162] [PMID]
- Stanisic D, Fregonesi NL, Barros CH, Pontes JG, Fulaz S, Menezes UJ, et al. NMR insights on nano silver post-surgical t Treatment of superficial caseous lymphadenitis in small ruminants. RSC Adv. 2018; 8:40778-86. [DOI:10.1039/C8RA08218A]
- Stefańska I, Gieryńska M, Rzewuska M, Binek M. Survival of Corynebacterium pseudotuberculosis within macrophages and induction of phagocytes death. Pol J Vet Sci. 2010; 13(1):143-9. [PMID]
- Santos LM, Stanisic D, Menezes UJ, Mendonça MA, Barral TD, Seyffert N, et al. Biogenic silver nanoparticles as a post-surgical treatment for Corynebacterium pseudotuberculosis infection in small ruminants. Front Microbiol. 2019; 10:824. [DOI:10.3389/fmicb.2019.00824] [PMID] [PMCID]
- Mohamed MM, Fouad SA, Elshoky HA, Mohammed GM, Salaheldin TA. Antibacterial effect of gold nanoparticles against Corynebacterium pseudotuberculosis. Int J Vet Sci Med. 2017; 5(1):23-9. [PMID]
- Zazo H, Colino CI, Lanao JM. Current applications of nanoparticles in infectious diseases. J Control Release. 2016; 224:86-102. [DOI:10.1016/j.jconrel.2016.01.008] [PMID]
- Aderibigbe BA. Metal-based nanoparticles for the treatment of infectious diseases. Molecules. 2017; 22(8):1370. [DOI:10.3390/molecules22081370] [PMID] [PMCID]
- Maleki Dizaj S, Barzegar-Jalali M, Zarrintan MH, Adibkia K, Lotfipour F. calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin Drug Deliv. 2015, 12(10):1649-60. [PMID]
- Pan X, Chen S, Li D, Rao W, Zheng Y, Yang Z, et al. The synergistic antibacterial mechanism of gentamicin-loaded CaCO3 nanoparticles. Front Chem. 2018; 5:130. [DOI:10.3389/fchem.2017.00130] [PMID] [PMCID]
- Saidykhan L, Abu Bakar MZ, Rukayadi Y, Kura AU, Latifah SY. Development of nanoantibiotic delivery system using cockle shell-derived aragonite nanoparticles for treatment of osteomyelitis. Int J Nanomedicine. 2016; 11:661-73. [PMID]
- Isa T, Zakaria ZA, Rukayadi Y, Mohd Hezmee MN, Jaji AZ, Imam MU, et al. Antibacterial activity of ciprofloxacin-encapsulated cockle shells calcium carbonate (aragonite) nanoparticles and its biocompatibility in macrophage J774A.1. Int J Mol Sci. 2016; 17(5):713. [DOI:10.3390/ijms17050713] [PMID] [PMCID]
- Mukherjee R, Dutta D, Patra M, Chatterjee B, Basu T. Nanonized tetracycline cures deadly diarrheal disease shigellosis in mice, caused by multidrug-resistant Shigella flexneri 2a bacterial infection. Nanomedicine. 2019; 18:402-13. [PMID]
- Fu W, Mohd Noor MH, Yusof LM, Ibrahim TA, Keong YS, Jaji AZ, et al. In vitro evaluation of a novel pH sensitive drug delivery system-based cockle shell-derived aragonite nanoparticles against osteosarcoma. J Exp Nanosci. 2017; 12(1):161-87. [DOI:10.1080/17458080.2017.1287965]
- Hammadi NI, Abba Y, Hezmee MNM, Razak ISA, Jaji AZ, Isa T, et al. Formulation of a Sustained release docetaxel loaded cockle shell-derived calcium carbonate nanoparticles against breast cancer. Pharm Res. 2017; 34(6):1193-203.[DOI:10.1007/s11095-017-2135-1] [PMID]
- Min KH, Jang EY, Lee HJ, Hwang YS, Ryu JI, Moon JH, et al. pH-Responsive mineralized nanoparticles for bacteria-triggered topical release of antibiotics. J Ind Eng Chem. 2019; 71:210-9. [DOI:10.1016/j.jiec.2018.11.027]
- Jaji AZ, Zakaria ZAB, Mahmud R, Loqman MY, Hezmee MNM, Abba Y, et al. Safety assessments of subcutaneous doses of aragonite calcium carbonate nanocrystals in rats. J Nanopart Res. 2017; 19(5):175. [DOI:10.1007/s11051-017-3849-z] [PMID] [PMCID]
- Georgescu D, Brezoiu AM, Mitran RA, Berger D, Matei C, Negreanu-Pirjol B. Mesostructured silica titania composites for improved oxytetracycline delivery systems. C R Chim. 2017; 20(11-12):1017-25. [DOI:10.1016/j.crci.2017.09.006]
- Mishra D, Khare P, Shanker K, Singh DK, Luqman S. Controlled delivery systems of cellulose matrix for oxytetracycline: In vitro dissolution. New Horiz Transl Med. 2016; 3(2):66-72. [DOI:10.1016/j.nhtm.2016.06.001]
- Idris SB, Arifah AK, Jesse FF, Ramanoon S, Basit M, Zakaria Z, et al. Synthesis, characterization, and in vitro release of oxytetracycline loaded in pH-responsive CaCO3 nanoparticles. J Appl Pharm Sci. 2019; 9(11):19-27. [DOI:10.7324/JAPS.2019.91103]
- de Souza AP, Vale VL, Silva Mda C, Araújo IB, Trindade SC, de Moura-Costa LF, et al. MAPK involvement in cytokine production in response to Corynebacterium pseudotuberculosis infection. BMC Microbiology. 2014; 14:230. [PMID]
- Jesse FFA, Randolf PSS, Saharee AA, Wahid AH, Zamri-Saad M, Jasni S, et al. Clinico-pathological response of mice following oral route infection of C. pseudotuberculosis. IOSR-J Agric Vet Sci. 2013; 2(2):38-42. https://www.iosrjournals.org/iosr-javs/papers/vol2-issue2/I0223842.pdf?id=1818
- Jesse FFA, Sang SL, Saharee AA, Shahirudin S. Pathological changes in the organs of mice model inoculated with Corynebacterium pseudotuberculosis organism. Pertanika J Trop Agric Sci. 2011; 34(1):145-9. http://psasir.upm.edu.my/id/eprint/10392/1/Pathological%20C%20ganism.pdf
- de Oliveira Silva MT, de Pinho RB, Bezerra FSB, Campos VF, Azevedo V, Borsuk S. Establishment of an objective endpoint in mice model for caseous lymphadenitis vaccine trials. Vet Microbiol. 2019; 230:86-9. [DOI:10.1016/j.vetmic.2019.01.017] [PMID]
- Imbuluzqueta E, Gamazo C, Lana H, Campanero MÁ, Salas D, Gil AG, et al. Hydrophobic gentamicin-loaded nanoparticles are effective against Brucella melitensis infection in mice. Antimicrob Agents Chemother. 2013; 57(7):3326-33. [DOI:10.1128/AAC.00378-13] [PMID] [PMCID]
- Droppa-Almeida D, Vivas WL, Silva KK, Rezende AF, Simionatto S, Meyer R, et al. Recombinant CP40 from Corynebacterium pseudotuberculosis confers protection in mice after challenge with a virulent strain. Vaccine. 2016; 34(8):1091-6. [DOI:10.1016/j.vaccine.2015.12.064] [PMID]
- Oliveira Neto MG, Santos HA, Fraga RE, Pacheco AS, Sampaio GP, Moura-Costa LF, et al. Nitric oxide and immune response in infection control of Caseous Lymphadenitis. Arq Bras Med Vet Zootec. 2017; 69(6):1565-72. [DOI:10.1590/1678-4162-9023]
- Norville IH, Hatch GJ, Bewley KR, Atkinson DJ, Hamblin KA, Blanchard JD, et al. Efficacy of liposome-encapsulated ciprofloxacin in a murine model of Q fever. Antimicrob Agents Chemother. 2014; 58(9):5510-8. [DOI:10.1128/AAC.03443-14] [PMID] [PMCID]
- Abdinasir YO, Jesse FFA, Saharee AA, Jasni S, Khairani-Bejo S, Haron AW. Clinico-pathological changes in mice following experimental infection with whole cell and exotoxin (PLD) extracted from Corynebacterium pseudotuberculosis. J Anim Vet Adv. 2012; 11(21):4064-72. [DOI:10.3923/javaa.2012.4064.4072]
- Wu D, Zhou S, Hu S, Liu B. Inflammatory responses and histopathological changes in a mouse model of Staphylococcus aureus -induced bloodstream infections. J Infect Dev Ctries. 2017; 1(4):294-305. [PMID]
- Horie M, Nishio K, Kato H, Endoh S, Fujita K, Nakamura A, et al. Evaluation of cellular influences caused by calcium carbonate nanoparticles.Chem Biol Interact. 2014; 210:64-76. [PMID]
- Oreiby AF, Hegazy YM. Diagnosis of ovine caseous lymphadenitis by blood and milk gamma interferon assays. Small Rumin Res. 2016; 144:109-12. [DOI:10.1016/j.smallrumres.2016.08.005]
- Wickramashinghe SN. Haematological aspects of infection. London: Bailliere Tindall; 2000. https://www.google.com/books/edition/Haematological_Aspects_of_Infection/1L_bQgAACAAJ?hl=en
- Nieto NC, Foley JE, MacLachlan NJ, Yuan T, Spier SJ. Evaluation of hepatic disease in mice following intradermal inoculation with Corynebacterium pseudotuberculosis. Am J Vet Res. 2009; 70(2):257-62. [PMID]
- Nemzek JA, Bolgos GL, Williams BA, Remick DG. Differences in normal values for murine white blood cell counts and other haematological parameters based on sampling site. Inflamm Res. 2001; 50(10):523-7. [PMID]
- Fernandes DP, Pimentel MML, Santos FAD, Praxedes ÉA, Brito PD, Lima MA, et al. Haematological and biochemical profile of BALB/c nude and C57BL/6 SCID female mice after ovarian xenograft. An Acad Bras Cienc. 2018; 90(4):3941-8. [PMID]
- Ivica J, Hill S. The potential of reducing AST testing in hospital settings. Clin Biochem. 2019; 64:57-9. [PMID]
- Barbosa BDS, Praxedes EA, Érika A, Lima MA, Pimentel MML, Santos FA, et al. Haematological and Biochemical Profile of BALB/c Mice. Acta Scientiae Veterinariae. 2017; 45(1):1-5. https://seer.ufrgs.br/ActaScientiaeVeterinariae/article/view/80473/0
- Gnanasoundari M, Pari L. Impact of naringenin on oxytetracycline-mediated oxidative damage in kidney of rats. Ren Fail. 2006; 28(7):599-605. [PMID]
- Gounani Z, Asadollahi MA, Pedersen JN, Lyngsø J, Skov Pedersen J, Arpanaei A, et al. Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf B Biointerfaces. 2019; 175:498-508. [PMID]
- Barbosa BD, Praxedes ÉA, Lima MA, Pimentel MM, Santos FA, Brito PD, et al. Haematological and biochemical profile of BALB/c mice. Acta Scientiae Veterinariae. 2017; 45(1):5. [DOI:10.22456/1679-9216.80473]
- Bastos BL, Dias Portela RW, Dorella FA, Ribeiro D, Seyffert N, Castro TLP, et al. Corynebacterium pseudotuberculosis: Immunological responses in animal models and zoonotic potential. J Clin Cell Immunol. 2012; S4:005. https://www.longdom.org/open-access/corynebacterium-pseudotuberculosis-imal-models-2155-9899.S4-.pdf
- Banki IS, Kadi AA, Jesse FF, Ramanoon SZ, Basi MA, Zakaria MZ. Antibiofilm Activity of Oxytetracycline Loaded Calcium Carbonate Aragonite Nanoparticle Against Corynebacterium pseudotuberculosis. Int J Pharm. 2021; 17(2):73-83. [DOI:10.3923/ijp.2021.73.83]










































 , Arifah Abdul Kadir *2
, Arifah Abdul Kadir *2 

 , Jesse Faez Firdaos Abdullah3
, Jesse Faez Firdaos Abdullah3 

 , Siti-Zubaidah Ramanoon4
, Siti-Zubaidah Ramanoon4 

 , Muhammad Abdul Basit1
, Muhammad Abdul Basit1 

 , Muhammad Zuki Zackariah Abubakar1
, Muhammad Zuki Zackariah Abubakar1 


.jpg)
.jpg)


