Introduction
Over the past 50 years, industrial development in various nations across the world has resulted in a significant increase in human exposure to toxic heavy metals. Because of the biodegradation pathways, toxic heavy metals tend to accumulate in the environment for long times, and are absorbed by the gastrointestinal, respiratory, and dermal systems. Amongst heavy metals, lead is one of the most stable and toxic elements with broad distribution in water, soil, and air [
1]. Human exposure to 400-600 μg/L of lead can result in chronic poisoning, manifested by such symptoms, as encephalopathy, lethargy, persistent vomiting, delirium, and coma [
2]. This toxic metal readily substitutes for vital cations, such as Mg+2, Ca+2, and Na+ even at picomolar concentrations [
2].
The most important mechanism of lead poisoning is the rise in its oxidative stress due to the increased generation of reactive oxygen species (ROS) in the affected organs. This event ultimately leads to carcinogenic and mutagenic alterations through the peroxidation of vital molecules and degradation of DNA strands in cells [
3]. Several defense mechanisms, including endogenous antioxidants and natural detoxification enzymes, control the ROS level in the body. Antioxidants can prevent oxidation damages by blocking the production of ROS, using beneficial oxidizers, inhibiting secondary metabolite production and inflammatory mediators within cells and repairing damaged molecules [
4].
Studies have shown that natural antioxidant compounds in plants can significantly strengthen the body’s defense mechanisms against toxins and pollutants [
5, 6]. Other studies suggest that probiotics possess high levels of antioxidant and anti-inflammatory properties. Specifically, probiotics contain lactobacilli, yeasts, and Bifidobacterium, which are parts of the natural flora in the gastrointestinal (GI) tract of living organisms [
7, 8, 9]. In our former study [
10], we reported improvement in the hematological parameters of rats exposed to lead after treatment with Lactobacillus fermentum. By monitoring the elimination of lead in feces and urine, we suggested that this probiotic might have exerted its beneficial effects by binding to and removing lead from the animals’ body [
10].
Aim of the study: Considering that kidneys play an important role in the elimination of toxins and control the ionic concentrations in the organism [
11], we planned to investigate the effect of L. fermentum on the levels of antioxidant factors and the biomarkers associated with renal function in rats exposed to lead.
Materials and Methods
Bacterial culture
The L. fermentum (ID: PTCC1638) was purchased from the Scientific Microbial Bank and the Organization of Industrial Research (Tehran, Iran). A sample of L. fermentum was inoculated in 1-ml of sterile distilled water and transferred into the MRS broth culture medium, and incubated for 24 hr at 37°C. Following McFarland standard 0.5 [
12], a bacterial aliquot equivalent to 1.5×108 CFU/mL [
13] was taken from the bacterial suspension.
Animals
Twenty-four female rats, weighing 150 ±20 grams, were purchased from the animal vivarium of the University of Tehran (Tehran, Iran). The rats were kept under identical conditions of temperature (2±23°C), humidity (45±5%) and 12 hr of light-dark cycles. After a one-week period of environmental adaptation, the rats were divided in four groups as follows:
• Control group: Rats received a daily oral treatment of 1-ml of physiological serum for 8 weeks.
• L. fermentum group: Rats received a daily treatment of 1-ml of the bacterial suspension (1.5×108 CFU/mL) for 8 weeks.
• Lead group: Rats received a daily treatment of 1-ml lead acetate, at 500 mg/kg, starting the first week to the end of the 8-week treatment period.
• Lead+L. fermentum group: Rats received an oral treatment daily of 1-mL lead acetate (500 mg/kg) and 1-ml L. fermentum (1.5×108 CFU/mL) for 8 weeks.
At the end of the 8-week treatment period, the animals were anesthetized with ketamine and xylazine, blood samples were taken directly from the heart, and subsequently, the kidneys were disceted from the rats’ body.
Measurement of renal biomarkers in the sera
In order to measure renal biomarkers in the sera, blood samples were collected in non-heparinized tubes and allowed to clot. The tubes were subsequently centrifuged at 5000 rpm, and the sera were separated. The serum creatinine (Cr) level was measured at 420 nm, using the colorimetric method of Jaffe kinetics [
14]. The blood urea nitrogen (BUN) and uric acid (UA) concentrations were monitored, using enzymatic methods as described previously [
4].
Histopathological examinations
A portion of the renal tissue from each rat was dissected, washed and fixed in 10% formalin for 48 hours. The tissue samples were then embedded in the paraffin wax. After preparing 5 μ sections from the tissue samples, they were placed on slides and stained with hematoxylin and eosin [
15]. Lastly, the histopathological alterations were examined in all slides under light microscopy.
Measuring antioxidants & lipid peroxidations
A 1-mg portion of the kidney tissue samples was isolated from each animal in the experimental groups and transferred to tubes containing
1-ml phosphate buffer saline at pH 7.4. After homogenization, the solution was centrifuged at 5000 rpm for 15 minutes at 4°C and the supernatant collected. Kits produced by Zellbio (Berlin, Germany) were used for this part of the experiments. The level of catalase (CAT) enzyme was measured at 405 nm by an ELISA reader after preparation of standards, blanks and samples (according to the kit protocol). The enzyme activity was calculated as follows (
Equation 1):
1. Catalase activity (U/mL) = (ODblank-ODsample)×
271(1/60×sample volume)
The activity level of the glutathione peroxidase (GPX) enzyme was calculated using an ELISA kit prepared from Zellbio, and was read at 412 nm, based on the following formula (
Equation 2):
2. GPX activity (U/mL)=(ODcontrol-ODsample/ODstandard-ODblank)×20×6
The glutathione (GSH) concentration was first measured at 412 nm and was then calculated using the following formula (
Equation 3):
3. Glutathione (nmol/L)=(ODsample-ODblank/ODstandard-ODblank)×1 mmol/L
The malondialdehyde (MDA) concentration was calculated after measuring the absorbance of the samples at 535nm, based on the standard curve.
Lead content in the renal tissue
A 1-mg sample of each kidney tissue was isolated from each rat and washed. A 1-ml samples of nitric and perchloric acids were added at a ratio of 4:1 to each tissue sample. After digestion of the samples in the acid mixture for 48hr, the excess acid was removed under mild heat, and a 1-ml aliquot distilled water was added to each sample. The lead content was measured in the clear solutions, using an atomic absorption spectrophotometer [
16].
Results
Serum urea, creatinine and uric acid
As shown in
Table 1, the results demonstrated increases in the concentration of the three serum biomarkers in the lead-exposed rats compared to those in the controls.
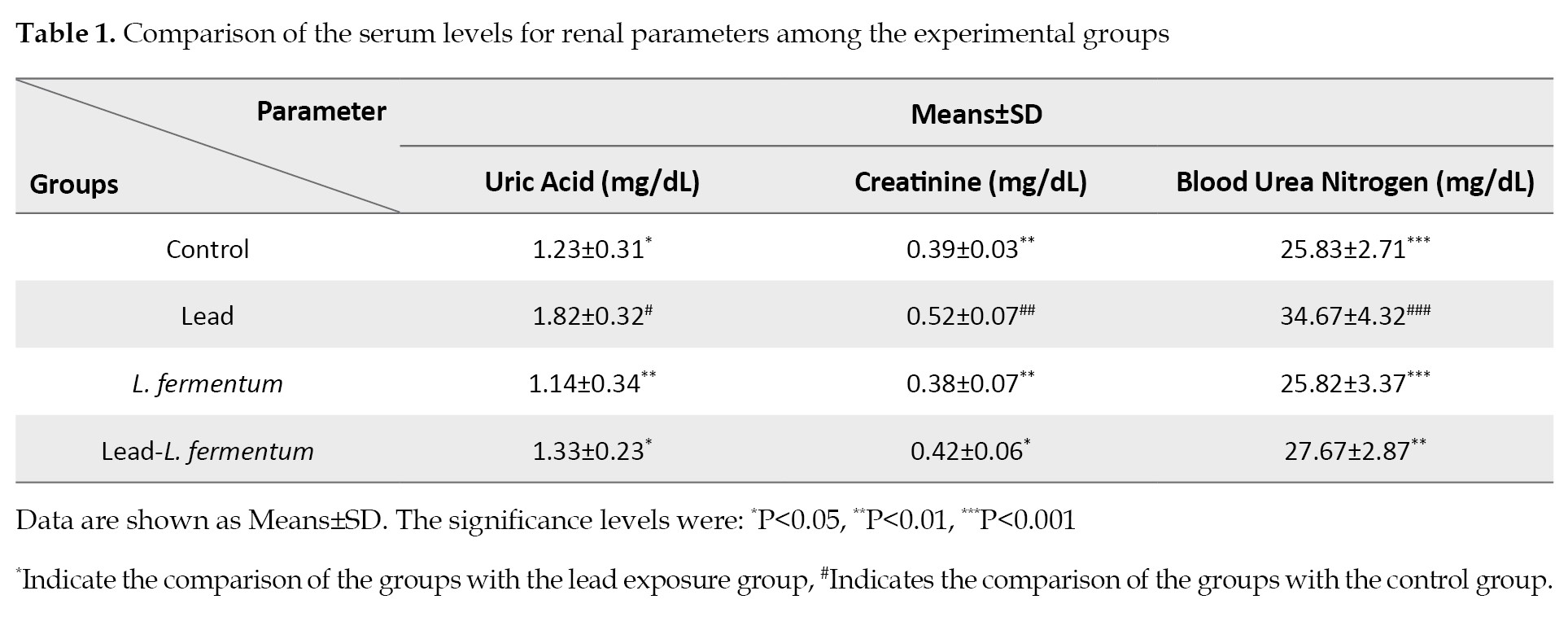
The levels of serum uric acid (P≤0.05), creatinine (P<0.01), and urea (P<0.001) were all significantly higher in the rats exposed to lead than those of the controls. The serum levels of urea, Cr, and UA in the group treated with L. fermentum and those exposed to lead plus L. fermentum were not significantly different than those of the controls. However, the concentrations of the three biomarkers in the sera of the groups that received the probiotics, compared to those exposed to lead only, were significantly lower for the uric acid and creatinine contents (P<0.05). Of note, the decline in the serum urea levels in these groups was even more significant (P≤0.01).
Pathological findings
Panels A1 and A2 in
Figure 1 illustrate parts of the renal tissue from the control rats at 100 and 400 magnifications.
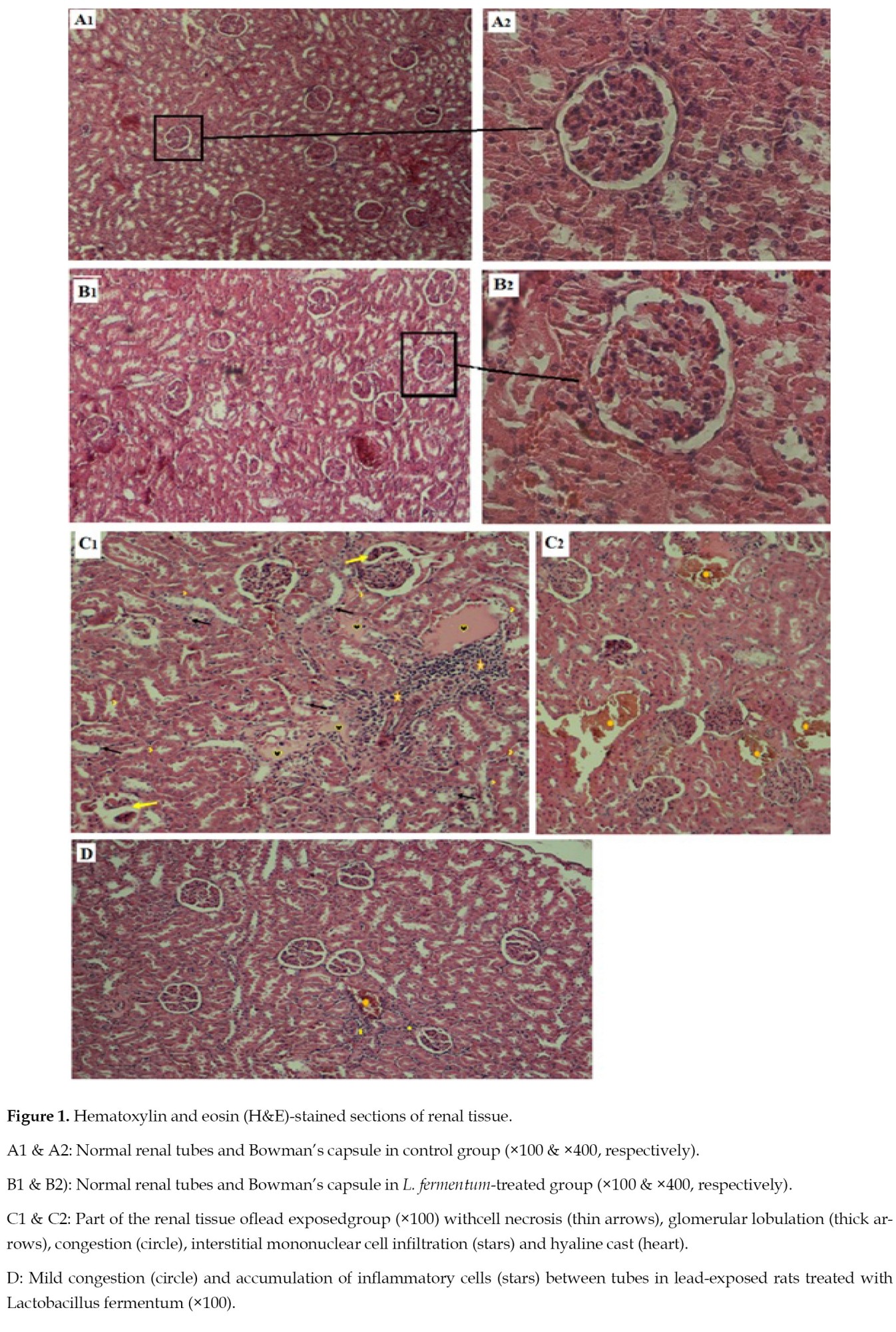
The photomicrographs had the appearance of normal tissue with intact urinary tubes and glomeruli.
Figure 1 (panels
B1 &
B2) shows parts of the renal tissue of the rats treated with L. fermentum with normal appearance without any signs of tissue damages. A number of renal lesions are observed in the renal tissue of rats exposed to lead, which include glomerular necrosis, widened Bowman’s capsules, congestion, interstitial mononuclear cell infiltrations, hyaline casts, and glomerular lobulations (
Figure 1; panels
C1 &
C2). After treating the lead-exposed rats with L. fermentum, a significant reduction in the severity of their lesions in the ureters and glomeruli were observed. These included significantly less congestion, inflammatory cells around the tubules and necrotic cells in the tissue (
Figure 1; Pane
l D).
Antioxidants and lipid peroxidation
As shown in
Figure 2 (Panels
A,
B &
C), the antioxidant biomarkers were significantly lower in the renal tissue of the lead-exposed rats than those of the controls.
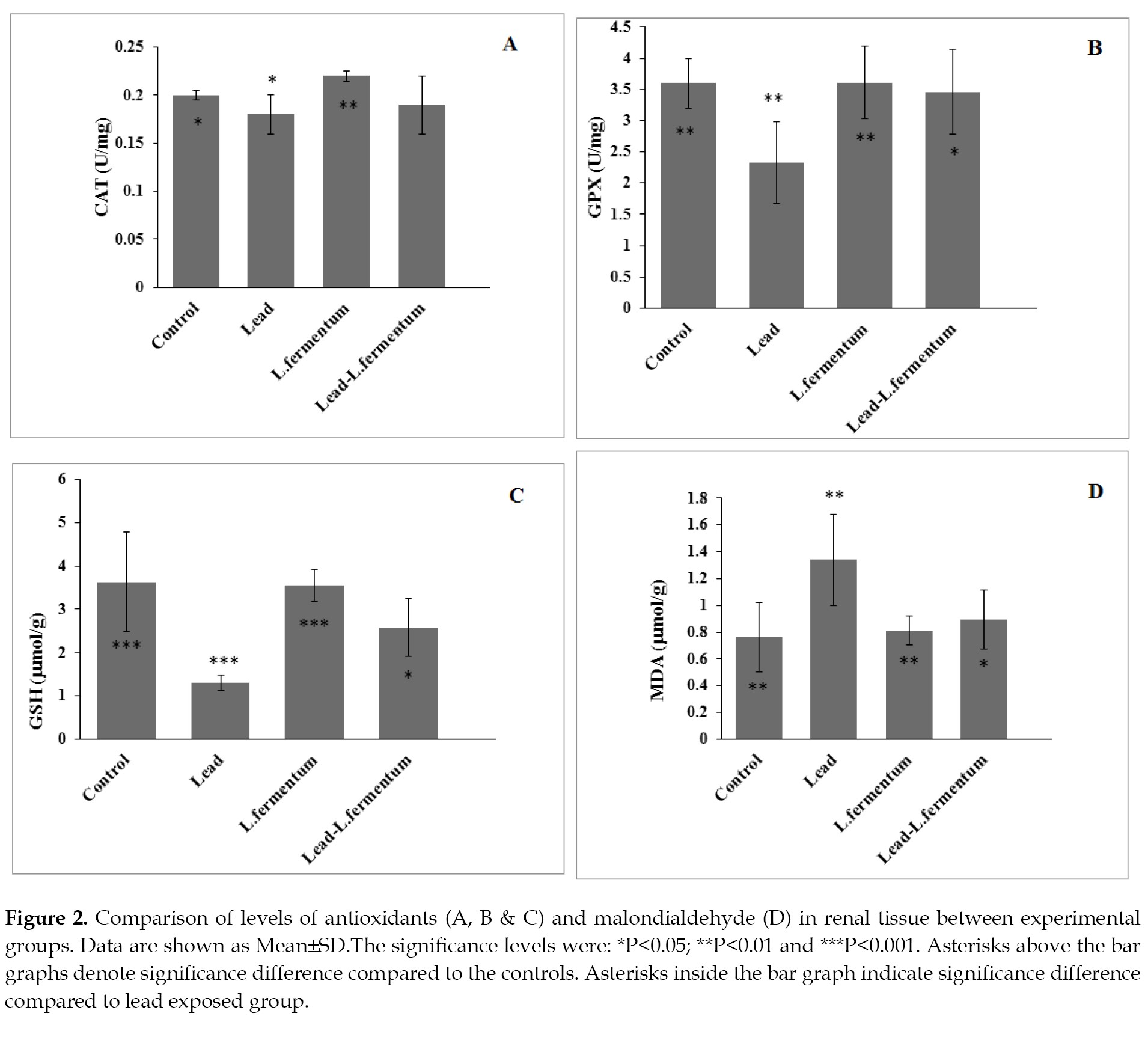
The serum levels of GSH, GPX and CAT in the experimental groups did not show a significant change compared to those of the control group. The levels of GPX and gluthatione were significantly higher in the rats exposed to lead and treated with L. fermentum compared to those treated with lead only. Despite the rise of catalase activity in this group, it was not statistically significant. The serum MDA level in the group treated with lead only showed a significant increase compared to those of the controls. The MDA level in the lead exposed group that was treated with L. fermentum showed a significant decrease compared to the rats that received lead only (
Figure 2; Panel
D).
Lead contents in renal tissue
The renal tissue lead contents in both groups that received lead only or combined with L. fermentum increased significantly (P≤0.001) compared to those of the control rats (
Figure 3).
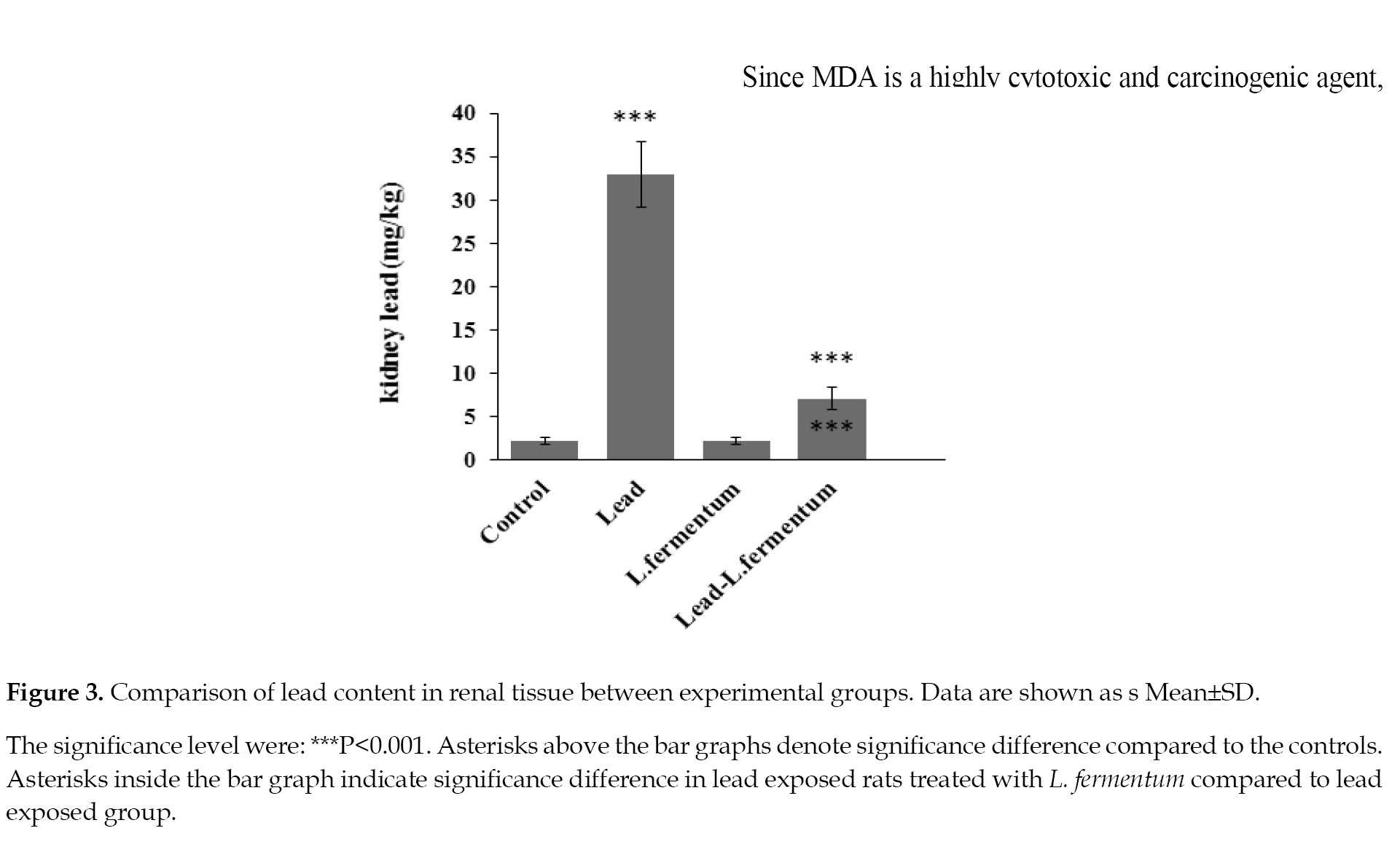
The lead content in the group treated with L. fermentum alone did not show a significant difference from the control group. Although the concentration of lead in the group exposed to lead plus L. fermentum was also high, but compared to the group exposed to lead only, the concentration of this element was significantly lower (P≤0.001).
Discussion
Studies have shown that long-term exposure to lead, even at low levels, can lead to chronic kidney disease [
11,
17, 18, 19, 20]. In the present study, the serum creatinine, urea and uric acid levels increased in the groups that had been exposed to lead. The evaluation of the three serum biomarkers is one of the most common tests performed to assess the clinical kidney disorders [
17]. Consistent with the results of this study, Gargouri, Hasanein, and Michael have also reported similar increases in the serum levels of these factors following the exposure of experimental animals to lead at similar concentrations [
18, 19, 20].
Kidneys filter out almost all of the creatinine and uric acid. Urea is also freely filtered through the glomeruli, and some are reabsorbed passively into the tissue along with water [
21]. Increases in the serum levels of these biomarkers indicate a poor glomerular filtration rate [
21]. In the current study, the nephropathic changes observed in the renal tissue samples of the rats exposed to lead demonstrate a reduction in the glomerular filtration rate. The increased destruction of the urinary tubules, particularly the proximal parts, along with the glomerular congestion, hyaline cast production and urinary tract dilation after toxicity with lead have also been observed in previous studies [
21,
22]. Under such circumstances, the main cause of chronic kidney disorders is the overproduction of free radicals and the cellular response to ROS, as evident by a rise in lipid peroxidation [
23].
Since MDA is a highly cytotoxic and carcinogenic agent, and is a biomarker of lipid peroxidation [
22], the serum level of this agent was measured in the rats’ renal tissue samples. A significant increase in the MDA level was observed in rats exposed to lead. Lead has a high affinity for binding to unsaturated lipids in the cell membranes. By removing hydrogen ions from the plasma membrane lipids, it causes structural changes in the cells. In addition, by destroying the membranes and penetrating into the cell, it affects the function of cellular organelles and macromolecules, including antioxidant enzymes [
24]. In this study, the serum levels of GSH, CAT and GPX decreased significantly in the renal tissue samples in the lead-exposed rats. The increase in CAT was observed in the early stages of the oxidative stress. Also, this enzyme catalyzes H2O2 to oxygenated water; however, at high levels of oxidative stress, the function of this enzyme is not adequate and its serum level gradually declines. Of note, this enzyme is amply present in renal tissue, especially in the cytoplasm of the epithelial cells of the proximal tubules [
25].
Increases in catalase activity have been shown in Orechromis niloticus fish after 21 days of exposure to lead [
26]. Also, decreased levels of GSH, GPX and CAT have been reported in rodents’ organs, such as brain, kidneys and liver, as reported by two former studies [
27, 28]. Glutathione is an important non-enzymatic antioxidant agent in cells, and plays a critical role in the redox pathways. Glutathione peroxidase neutralizes peroxide and OH-ions through oxidation. The oxidized glutathione returns to the reduced glutathione form via interaction with glutathione reductase.
The reductions in the antioxidant enzymes activity and the concentration of GSH indicate the failure of endogenous antioxidant system in its fight against lead toxicity. Lead can penetrate renal tissue and weaken the body’s antioxidant system via ROS generation, thus, impairing the antioxidant enzymes due to its high affinity with their sulfhydryl groups [
26]. In our study, this argument was confirmed by a significant rise in the renal lead content found in rats exposed to lead. Kelaing and colleagues [
29] have reported a reduction in total antioxidant capacity and increase in lipid peroxidation due to lead penetration into the kidneys in animal models.
The most common treatments for lead poisoning is the use of chelating agents, such as EDTA, which binds to lead and promotes its excretion from the body, but this pathway also removes calcium ions [
30]. Studies have shown that taking probiotic supplements have modulating effects on the oxidative stress caused by various disease processes [
31, 32, 33]. In the current study, the effect of L. fermentum on lead-induced nephrotoxicity was evaluated. The results showed a significant decrease in the serum creatinine, urea, and uric acid levels following treatment of lead-exposed rats with this probiotic. The reduced levels of those biomarkers have also been reported in experimental animals poisoned with mercury following treatement with L. plantarum and B. coagalans [
34]. Similar observations have also been made in chronic adenine-induced renal failure after treatment with the probiotic, L. casei shirota [
23]. Further, reduction in serum bilirubin level has been demonstrated after treating mercury-poisoned animals with L. plantarum and B. coagalans [
34]. A similar positive effect has also been found for the chronic renal failure induced by adenine, after treatment with Lactobacillus casei shirota [
23].
It is likely that the improvement in the renal function, as discovered in the current study, after treatment with L. fermentum, is due to the significant protective effect of this probiotic bacterium against the oxidative stress induced by lead poisoning. The histopathological improvement in the renal tissue of these rats also supports this claim. Treating lead-exposed rats with L. fermentum, significantly reduced the serum MDA level while the levels of GPX and GSH increased compared to those in the untreated, lead-poisoned rats. Although there was no significant rise in the serum CAT level compared to the group that received lead only. The level of this enzyme returned to normal similarly to that of the controls. Consistent with the results of the current study, Alla, et al. have reported increases in the activities of CAT, SOD, and GPX, but a decrease in the serum MDA level after treating experimental animals with chronic renal failure, with a variety of pro- and prebiotics. These authors have suggested that the rise in free radicals and the consequent induction of oxidative stress in rats were prevented by the antioxidant properties of L. fermentum [
23].
Considering the above facts, improvement in antioxidant factors in rats treated with L. fermentum is likely due to the inhibiting property of the probiotic against membrane lipid peroxidation. A similar argument can also be made about the inhibition of free radicals due to such agents as exopolysaccharides and their role in the production of NADH [
35, 36]. In addition, probiotics, such as Lactobacillus ruteri, L. fermentum, and Bifidobacterium, have been shown to increase the NADH levels in cells by increasing the activity of glutamate cysteine ligase, which is important in the synthesis of GSH [
37]. An additional reason in support of the effect of L. fermentum on lead-induced nephrotoxicity may be attributed to the elimination of lead from the body by this bacterium. In a previous study, we have demonstrated that the excretion of lead in faeces increased significantly in lead-poisoned rats treated with L. fermentum [
10]. In the current study, a significant reduction in the tissue lead contents was evident in all rats that received this probiotic. In support of these results, the removal of lead and cadmium from the body after treating animals with L. fermentum ME3, L. rhamnosus GG and Bifidobacterium longum 46 has previously been reported [
38, 39, 40].
The components of cellular wall structures, such as peptidoglycans, teichoic acids and polysaccharides are negatively charged because of their carboxyl, hydroxyl and phosphate groups. These elements have a strong ability to absorb positive ions, such as lead [
41]. Consequently, L. fermentum might have prevented the absorption of lead from GI and the subsequent absorption by the kidneys via lead chelation therapy, thus preventing oxidative damages to the kidneys. In light of few experimental evidence being available on the effects of probiotics and their mechanisms of efficacy [
42], we recommend that additional studies be conducted to shed light on the effects of probiotics and their potentials as adjuvant therapy to prevent or minimize renal tissue impairment in patients with a history of lead toxicity.
Conclusions
The objective of this study was to determine if L. fermentum was capable of modulating lead-induced nephrotoxicity. The results demonstrated that treating lead-poisoned rats with L. fermentum reduced the serum MDA levels while improving the antioxidant property and the biomarkers of renal impairment. Also, the histopathological images from the kidneys confirmed our laboratory results. Therefore, it is suggested that the therapeutic effects of L. fermentum is likely to be associated with its antioxidant potentials, while indirectly arising from the chelation of lead ions and its removal from the body. It also strengthens the body’s antioxidant defense system, thus reducing oxidative damages to the kidneys caused by lead poisoning.
Ethical Considerations
Compliance with ethical guidelines
The ethical guidelines on the use of laboratory animals for research were observed, as approved by the Institutional Review Board of the Islamic Azad University, Flavarjan Branch (Certificate No: IR.IAU.FALA.REC.1396.012).
Funding
This article was extracted from a student thesis project and was funded by the Islamic Azad University, Falavarjan Branch, Isfahan, Iran.
Authors' contributions
Conceptualization & supervision: Mahnoosh Fatemi & Fereshte Ghandehari; Methodology: Mahnoosh Fatemi; Histopathological examinations: Elham Ghazanfarpour & Mahnoosh Fatemi. Data collection, investigation and writing of original draft: Elham Ghazanfarpour & Mahnoosh Fatemi. Data analysis, writing, review and editing of the final draft: Mahnoosh Fatemi, Fereshte Ghandehari & Yeganeh Fatemi.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgements
The authors are grateful to the Department of Biology, Faculty of Basic Sciences for their cooperation and supplying the experimental equipments and laboratory space.
References
- Rahman Z, Singh VP. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environmental Monitoring and Assessment. 2019; 191(7):419. [PMID]
- Gupta P, Diwan B. Bacterial Exopolysaccharide mediated heavy metal removal: A Review on biosynthesis, mechanism and remediation strategies. Biotechnology Reports (Amsterdam, Netherlands). 2016; 13:58-71. [DOI:10.1016/j.btre.2016.12.006] [PMID] [PMCID]
- Fu Z, Xi S. The effects of heavy metals on human metabolism. Toxicology Mechanisms and Methods. 2020; 30(3):167-76. [DOI:10.1080/15376516.2019.1701594] [PMID]
- Fathi R, Akbari A, Nasiri K, Chardahcherik M. Ginger (Zingiber officinale roscoe) extract could upregulate the renal expression of NRF2 and TNFα and prevent ethanol-induced toxicity in rat kidney. Avicenna Journal of Phytomedicine. 2021; 11(2):134-45. [PMID]
- Omidifar N, Nili-Ahmadabadi A, Nakhostin-Ansari A, Lankarani KB, Moghadami M, Mousavi SM, et al. The modulatory potential of herbal antioxidants against oxidative stress and heavy metal pollution: Plants against environmental oxidative stress. Environmental Science and Pollution Research International. 2021; 28(44):61908-18. [DOI:10.1007/s11356-021-16530-6] [PMID]
- Bjørklund G, Shanaida M, Lysiuk R, Butnariu M, Peana M, Sarac I, et al. Natural compounds and products from an anti-aging perspective. Molecules. 2022; 27(20):7084. [DOI:10.3390/molecules27207084] [PMID] [PMCID]
- Kim KT, Kim JW, Kim SI , Kim S, Nguyen TH, Kang CH. Antioxidant and anti-inflammatory effect and probiotic properties of lactic acid bacteria isolated from canine and feline feces. Microorganisms. 2021; 9(9):1971. [DOI:10.3390/microorganisms9091971] [PMID] [PMCID]
- Tegegne BA, Kebede B. Probiotics, their prophylactic and therapeutic applications in human health development: A review of the literature. Heliyon. 2022; 8(6):e09725. [DOI:10.1016/j.heliyon.2022.e09725] [PMID] [PMCID]
- Gou HZ, Zhang YL, Ren LF, Li ZJ, Zhang L. How do intestinal probiotics restore the intestinal barrier? Frontiers in Microbiology. 2022; 13:929346. [DOI:10.3389/fmicb.2022.929346] [PMID] [PMCID]
- Ghazanfarpour E, Fatemi M, Ghandehari F. Protective effect of lactobacillus fermentum on lead-induced hematological and body weight alterations in rats. Iranian Journal of Toxicology. 2019; 13(3):15-20. [DOI:10.32598/IJT.13.3.596.1]
- Zhang Y, Gu WL, Duan L, Zhu H, Wang HY, Wang J, et al. Protective effect of dietary fiber from sweet potato (Ipomoeabatatas L.) against lead-induced renal injury by inhibitingoxidative stress via AMPK/SIRT1/PGC1a signaling pathways. Journal of Food Biochemistry. 2018; 42(3):e12513. [DOI:10.1111/jfbc.12513]
- Veeraraghavan B, Vijayakumar S, Pragasam AK, Bakthavachalam YD, Prakash JAJ. Antimicrobial Susceptibility Testing Methods for Acinetobacter spp. Methods in Molecular Biology (Clifton, N.J.). 2019; 1946:23-37. [PMID]
- Weinstein MP. Performance standards for antimicrobial susceptibility testing. Wayne: Clinical and Laboratory Standards Institute; 2018. [Link]
- Dissanayake RK, Ranaweera KPT, Dias P, Priyadarshani A. The effect of bilirubin on laboratory investigations on serum creatinine: A comparison study between jaffe reaction and creatinase enzymatic method with creatinine in phosphate buffered saline solution and serum. Clinical and Experimental Health Sciences. 2022; 12(1):11-7. [DOI:10.33808/clinexphealthsci.714844]
- Abou-Zeid SM, Ahmed AI, Awad A, Mohammed WA, Metwally MMM, Almeer R, et al. Moringa oleifera ethanolic extract attenuates tilmicosin-induced renal damage in male rats via suppression of oxidative stress, inflammatory injury, and intermediate filament proteins mRNA expression Biomedicine & Pharmacotherapy. 2021; 133:110997. [PMID]
- Fatemi M, Moshtaghian J, Ghaedi K, Jafari Dinani N, Naderi G. Effects of silver nanoparticle on the developing liver of rat pups after maternal exposure. Iranian Journal of Pharmaceutical Research. 2017; 16(2):685-93. [PMID]
- Wani N, Pasha T. Laboratory tests of renal function. Anaesthesia & Intensive Care Medicine. 2021; 22(7):393-7. [DOI:10.1016/j.mpaic.2021.05.010]
- Michael OS, Bamidele O, Ogheneovo P, Ariyo TA, Adedayo LD, Oluranti OI, et al. Watermelon rind ethanol extract exhibits hepato-renal protection against lead induced-impaired antioxidant defenses in male Wistar rats. Current Research in Physiology. 2021; 4:252-9. [DOI:10.1016/j.crphys.2021.11.002] [PMID] [PMCID]
- Hasanein P, Riahi H. Preventive use of berberine in inhibition of lead-induced renal injury in rats. Environmental Science and Pollution Research International. 2018; 25(5):4896-903. [PMID]
- Gargouri M, Soussi A, Akrouti A, Magné C, El Feki A. Ameliorative effects of spirulina platensis against lead-induced nephrotoxicity in newborn rats: Modulation of oxidative stress and histopathological changes. EXCLI Journal. 2018; 17:215-32. [PMID]
- Bai V L, Krishnan SA. Role of creatine in the body and its creatinine clearance in humans and animals. International Journal of Pharmaceutical Research and Applications. 2022; 7(5):286-96. [DOI:10.35629/7781-0705286296]
- Saka WA, Akhigbe RE, Abidoye AO, Dare OS, Adekunle AO. Suppression of uric acid generation and blockade of glutathione dysregulation by L-arginine ameliorates dichlorvos-induced oxidative hepatorenal damage in rats. Biomedicine & Pharmacotherapy. 2021; 138:111443. [DOI:10.1016/j.biopha.2021.111443] [PMID]
- Alla F, Sadeek EA. Effect of Arabic gum as prebiotics and Lactobacillus casei Shirota (LcS) as probiotic on oxidative stress and renal function in adenine-induced chronic renal failure in rats. European Journal of Nutrition & Food Safety. 2018; 8(1):29-46. [DOI:10.9734/EJNFS/2018/36022]
- Hsu PC, Guo YL. Antioxidant nutrients and lead toxicity. Toxicology. 2002; 180(1):33-44. [DOI:10.1016/S0300-483X(02)00380-3] [PMID]
- Hong YA, Park CW. Catalytic antioxidants in the kidney. Antioxidants. 2021; 10(1):130. [DOI:10.3390/antiox10010130] [PMID] [PMCID]
- Firidin G. Oxidative stress parameters, induction of lipid peroxidation, and ATPase activity in the liver and kidney of oreochromis niloticus exposed to lead and mixtures of lead and zinc. Bulletin of Environmental Contamination and toxicology. 2018; 100(4):477-84. [PMID]
- Patra RC, Swarup D, Dwivedi SK. Antioxidant effects of tocopherol, ascorbic acid andL-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology. 2001; 162(2):81-8. [DOI:10.1016/S0300-483X(01)00345-6] [PMID]
- Soussi A, Gargouri M, El Feki A. Effects of co-exposure to lead and zinc on redox status, kidney variables, and histopathology in adult albino rats. Toxicology and Industrial Health. 2018; 34(7):469-80. [DOI:10.1177/0748233718770293] [PMID]
- Kelainy EG, Ibrahim Laila IM, Ibrahim SR. The effect of ferulic acid against lead-induced oxidative stress and DNA damage in kidney and testes of rats. Environmental Science and Pollution Research International. 2019; 26(31):31675-84. [PMID]
- Wani AL, Ara A, Usmani JA. Lead toxicity: A review. Interdisciplinary Toxicology. 2015; 8(2):55-64. [PMID] [PMCID]
- Heshmati J, Farsi F, Shokri F, Rezaeinejad M, Almasi-Hashiani A, Vesali S, et al. A systematic review and meta-analysis of the probiotics and synbiotics effects on oxidative stress. Journal of Functional Foods. 2018; 46:66-84. [DOI:10.1016/j.jff.2018.04.049]
- Ağagündüz D, Kocaadam-Bozkurt B, Bozkurt O, Sharma H, Esposito R, Özoğul F, et al. Microbiota alteration and modulation in Alzheimer’s disease by gerobiotics: The gut-health axis for a good mind. Biomedicine & Pharmacotherapy. 2022; 153:113430. [DOI:10.1016/j.biopha.2022.113430] [PMID]
- Hosseini SA, Abbasi A, Sabahi S, Khani N. Application of postbiotics produced by lactic acid bacteria in the development of active food packaging. Biointerface Research in Applied Chemistry. 2022; 12:6164-83. [DOI:10.33263/BRIAC125.61646183]
- Majlesi M, Shekarforoush SS, Ghaisari HR, Nazifi S, Sajedianfard J, Eskandari MH. Effect of probiotic bacillus coagulans and lactobacillus plantarum on alleviation of mercury toxicity in rat. Probiotics and Antimicrobial Proteins. 2017; 9(3):300-9. [PMID]
- Lin MY, Chang FJ. Antioxidative effect of intestinal bacteria bifidobacterium longum ATCC 15708 and lactobacillus acidophilus ATCC 4356. Digestive Diseases and Sciences. 2000; 45(8):1617-22. [PMID]
- Yoo JW, Cho H, Lee KW, Won EJ, Lee YM. Combined effects of heavy metals (Cd, As, and Pb): Comparative study using conceptual models and the antioxidant responses in the brackish water flea. Comparative Biochemistry and Physiology. Toxicology & pharmacology: CBP. 2021; 239:108863. [PMID]
- Averina OV, Poluektova EU, Marsova MV, Danilenko VN. Biomarkers and utility of the antioxidant potential of probiotic lactobacilli and bifidobacteria as representatives of the human gut microbiota. Biomedicines. 2021; 9(10):1340. [DOI:10.3390/biomedicines9101340] [PMID] [PMCID]
- Banwo K, Alonge Z, Sanni AI. Binding capacities and antioxidant activities of lactobacillus plantarum and pichia kudriavzevii against cadmium and lead toxicities. Biological Trace Element Research. 2021; 199(2):779-91. [DOI:10.1007/s12011-020-02164-1] [PMID]
- Zhai Q, Yin R, LeileiY, Wang G, Tian F, Yu R, et al. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control. 2015; 54:23-30. [DOI:10.1016/j.foodcont.2015.01.037]
- Halttunen T, Salminen S, Tahvonen R. Rapid removal of leadand cadmium from water by specific lactic acid bacteria. International Journal of Food Microbiology. 2007; 114(1):30-5. [DOI:10.1016/j.ijfoodmicro.2006.10.040] [PMID]
- Teemu H, Salminen S, Jussi M, Raija T, Kalle L. Reversiblesurface binding of cadmium and lead by lactic acid andbifidobacteria. International Journal of Food Microbiology. 2008; 125(2):170-5. [DOI:10.1016/j.ijfoodmicro.2008.03.041] [PMID]
- Suo H, Zhao X, Qian Y, Sun P, Zhu K, Li J, et al. Lactobacillus fermentum Suo Attenuates HCl/Ethanol Induced gastric injury in mice through its antioxidant effects. Nutrients. 2016; 8(3):155. [PMID] [PMCID]









































