Introduction
Breast cancer is one of the most common neoplasia and the second leading cause of cancer death in women worldwide [1]. The risk factors associated with breast cancer include age, sex, family history, and genes [2]. Surgical breast resection is an effective treatment for breast cancer; however, radiotherapy and chemotherapy are also used to inhibit cancer cells and prevent tumor growth [3]. The side effects such as hair loss, drug resistance, pain, vomiting, loss of normal cells, and metastasis of the cancer cells are the main challenges in the clinical management of breast cancer [4-6]. Using natural sources, such as plants can also be used in the treatment of breast cancer. Plants have chemically active compounds known as secondary metabolites that do not play a direct role in the plant’s growth but have anti-inflammatory, anti-cancer biological properties [7, 8].
Astragalus (Fabaceae) is a large genus of herbs and small shrubs, that is well-known in traditional medicine for its anticancer properties, especially in China [9]. The active medicinal compounds that have been identified in Astragalus species include polysaccharides, saponins, and phenols [10]. One species of that has been studied extensively is Astragalus membranaceus (A. membranaceus). Astragal radix, the dried roots of A. membranaceus, has shown a number of pharmacological properties, such as anti-cancer, anti-viral and immune-modulation. The extract of this plant is believed to protect against heart disease, and antioxidant and anti-inflammatory effects [11]. Recent studies have shown that the ethanolic extract and polysaccharides fraction of different species of Astragalus reduce the growth of tumors and inhibit breast cancer cells [6, 12-14]. To date, no study has been conducted on the anti-neoplastic properties of Astragalus ovinus (A. ovinus) and its polysaccharides fraction. The only research conducted on the anti-cancer properties of A. ovinus is one by Mehraban, et al., demonstrating that the ethanolic extract of this plant has reduced the growth of breast cancer tumors in rats [12].
Aim of the study: We investigated the effects of the ethanolic extract of A. ovinus and its polysaccharides fraction on the anti-proliferation and apoptosis of MCF-7 breast cancer cell line. This article presents the promising findings of this novel research.
Materials and Methods
Plant materials: Adequate samples of A. ovinus plant roots were collected from Kakan location in Kohgiluyeh and Boyerahmad province of Iran in the spring of 2019 and were authenticated by Dr. A. Jafari, an expert from the Department of Botany at Yasuj University, Yasuj, Iran. The roots were isolated carefully, washed, dried, powdered and stored in a refrigerator before conducting the laboratory experiments.
Preparation of the ethanolic extract: A 100 gram sample of the powdered roots was dissolved in one liter of 70% ethanol and let stand at room temperature for 48 hours. Next, the solution was filtered through Whatman paper filter and the excess solvent was evaporated in a rotary apparatus at 60°C. Finally, the extract was dried, using a freeze dryer and stored in a refrigerator until further experiments [15].
Extraction of the polysaccharides: A 100 g sample of the dried roots was weighed and dissolved in 95% ethanol, and let stand at 60°C for 9 hr to remove additional impurities, such as amino acids, dyes, and small-molecule monosaccharaides. After filtering off the ethanol, the roots were dried again and powdered. The powder was dissolved in water and incubated at 80°C for 9 hr. After filtering and removing the excess water on a rotary evaporator, the protein components were removed by the Sevage method (chloroform/n-butanol 4:1, v/v). Following this step, the polysaccharides were precipitated in 95% ethanol and the solution was incubated at 4°C overnight. The polysaccharides were then precipitated, by centrifugation at 4300 rpm for 5 min [16, 17]. The precipitate was washed with acetone and dried up in a lyophilizer. The extraction rate of the polysaccharides was calculated, using Equation 1:
1. Percent yield=Dry polysaccharides weight/dry root weight×100
Determination of the polysaccharides’ sugar fraction: The total sugar content of the polysaccharides fraction was determined, using the phenol sulfuric acid method as described by a previous study [18]. The standard glucose concentrations were determined and the absorbance was read on a spectrophotometer at 490 nm (Figure 1 A).


The result was expressed as a percentage of the polysaccharides fraction.
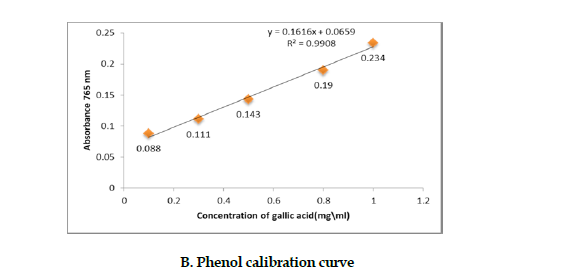
Determination of the polysaccharides’ phenol fraction: To determine the amount of phenolic compounds of the polysaccharides fraction, we used Folin-Ciocalteu method [19]. Varying concentrations of Gallic acid were prepared to plot the standard curve. The absorbance of this fraction was measured at 765 nm on a spectrophotometer, and the results were expressed as a percentage of the polysaccharides fraction (Figure 1 B).
Determination of the polysaccharides’ protein fraction: We used Bradford method to evaluate the amount of protein in the polysaccharides fraction. For this purpose, varying concentrations of albumin were prepared [20]. One ml of Bradford reagent was mixed with 100 μL of the polysaccharides sample. Finally, the absorbance of the resultant solution was read at 595 nm on a spectrophotometer (Figure 1 C). Similarly, the results were expressed as a percentage of the polysaccharides fraction.
The MCF-7 cell line: The breast cancer cell line (MCF-7) was obtained from the Iranian Pasteur Institute (Tehran, Iran) and cultured in RPMI 1640 culture media (Gibco; Billings, MT, USA). It contained 10% FBS (Cegrogen,Germany), 1% penstrep (Sigma, USA) and incubated at 37°C and 5% CO2 until the cells reached the logarithmic growth phase.
MTT assay: This assay was performed based on the guidelines described in a previous study [21]. The cells were seeded at 4×104 cell per well in a 96-well plate and incubated overnight. The cells were treated with the polysaccharides fraction at concentrations of 50, 200, 400, 600, 800 or 1200 µg/mL. The media in each well contained the A. ovinus extract at 100, 200, 300, 400, 500, 600 or 700 µg/mL, and cisplatin at 2¸ 5¸ 10¸ 15¸ 20¸ 25 or 30 µg/mL. After 24 hr of incubation, 20 μL aliquot of MTT solution was added to each well at a concentration of 5 mg/mL. The plates were then incubated at 37°C for 4 hr. Finally, Formazan crystals were dissolved in DMSO and a 100 μL aliquot was added to each well. The absorption was then read at 570 nm using an ELISA reader. The cell viability was determined by Equation 2:
2. Cell viability percentage=OD of Sample/OD of the control
Cell cycle analyses: The cells were seeded at 1×105/well in 6-well plates, and were treated with cisplatin at IC50 concentration, ethanolic extract, and the polysaccharides fraction. After a 24 hr incubation, cells were trypsinized, detached and collected. The cells were washed with cold PBS, centrifuged at 1000 rpm for 5 min, and kept in 70% cold ethanol at 4°C overnight. After this step, the cells were centrifuged at 1000 rpm for 5 min again followed by incubation for 30 minutes in the dark at 37°C in PBS containing propidium iodide (PI; 50 µg/mL) and RNase (50 µg/mL). Finally, the cells were analyzed by flow cytometry based on a previously described method [22, 23].
Apoptosis assay: The cell apoptosis rate was determined by an Annexin V-FITC/PI kit. The cells were seeded (1×106/well) in 6-well plates and were treated with cisplatin at IC50 concentration. Next, the cells were treated with an aliquot of either ethanolic extract or the polysaccharides fraction and incubated for 24 hr. After the incubation, the cells in each plate were precipitated washed with PBS, and centrifuged at 1000 rpm for 5min. Subsequently, the cells were suspended in a binding buffer solution. Finally, 5 μL PI and 5 μL Annexin V-FITC aliquots were added to the cells, and were incubated for 10 minutes in the dark at room temperature. The cell apoptosis was evaluated by flow cytometry.
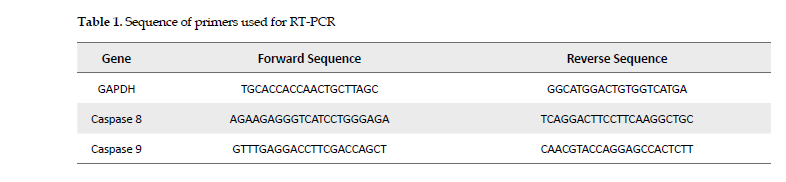
Quantitative RT-PCR: The cells were treated at the IC50 concentrations of cisplatin, followed by the polysaccharides fraction or the extract. The cells’ RNA was extracted using Trizol reagent followed by analyzing the RNA both qualitatively and quantitatively. Then, cDNA synthesis kit (Bio-Rad) was used to convert RNA to cDNA. The GAPDH gene was used as the internal control and the primers were designed, using primer-3 software (Table 1).
Finally, the expression of caspase-8 and -9 genes was investigated, using real-time PCR. The expression of the target genes relative to the reference gene (GAPDH) was calculated using Formula 2- ΔΔCt.
Results
Sugar, protein & phenolic contents: The sugar, protein and phenol contents of the A. ovinus’ polysaccharides were extracted, by the methods of phenol sulfuric acid and Bradford and Folin-Ciocalteu, respectively, and the amounts of each were determined as listed in Table 2.
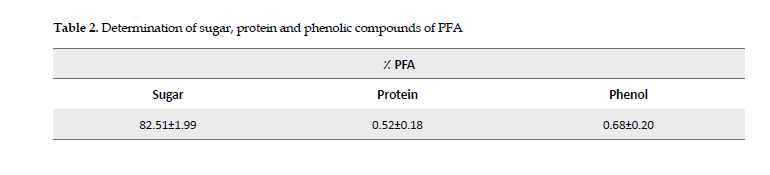
The reported results are specified as the Mean±SD in each category.
Effects on cell viability: Samples of Mcf-7 were treated with varying doses of the polysaccharide fraction, the extract, and cisplatin for 24 hr at 37ºC. The cytotoxicity results indicated that the percent inhibition of the viable MCF-7 cells increased dose-dependently after exposure to the polysaccharides fraction or the extract. Also, the results of MTT assay showed that the percent inhibition of the cells increased significantly at concentrations of polysaccharide fraction greater than 400 μgmL (Figure 2 A). The results of MTT assay with the extract demonstrated that it inhibited 50% of the MCF-7 cell line at concentrations lower than that of the polysaccharides fraction (Figure 2 B). The IC50 values for cisplatin treatment (Figure 2 C), the extract and polysaccharides fraction were 22.42, 560.9, and 961.2 in µg/mL, respectively.
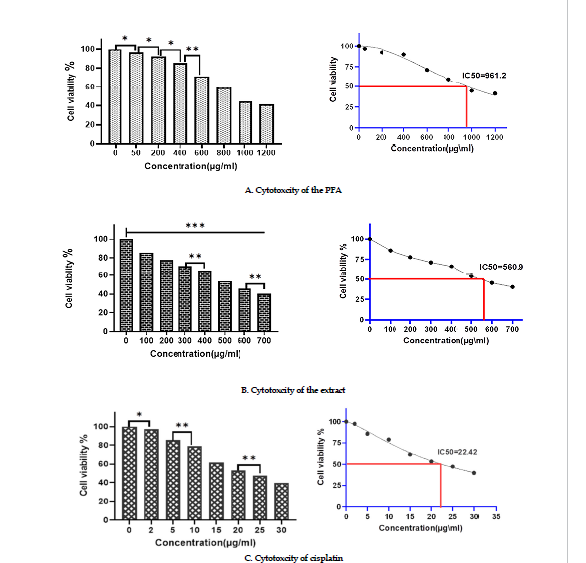
Figure 2: Effects of cytotoxicity
significant differencescompared to thecontrol groups and between groups (***P-˂0.0001¸**P˂0.001, *P˂o.05).
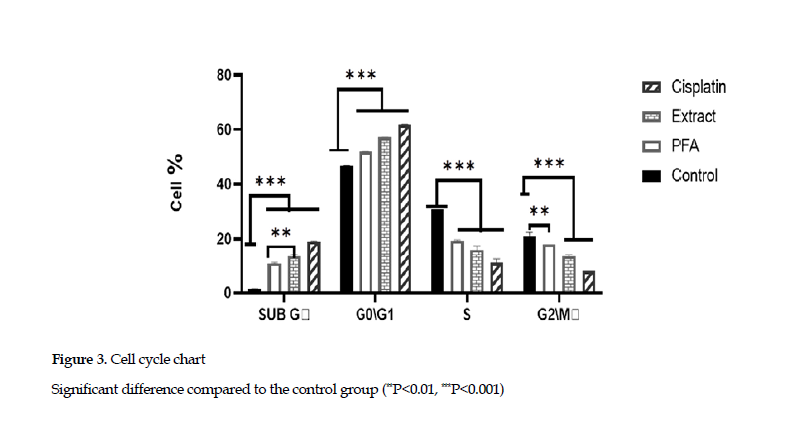
Effects on cell cycle arrest: The examination of cell cycle arrest in SubG phase showed that the extract and polysaccharide fraction increased the percentage of cells in this phase.This demonstrated that the extract and polysaccharide fraction could induce the apoptotic phase (SubG) in the cell cycle. Also, the cell cycle arrest in G0/G1 phase indicated that the percentages of cells in G0/G1 phase, based on treatment with cisplatin, the polysaccharides and extract, were 61.8%, 51.2% and 57.1%, respectively, compared to that found for the control group (46.7 %) (Figure 3).
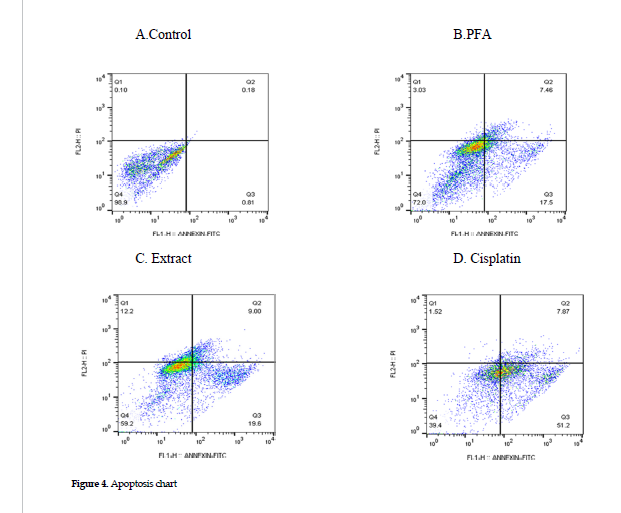
 Effects on cell apoptosis:
Effects on cell apoptosis: Cells were treated at the IC50 concentrations of cisplatin, extract or polysaccharides fraction, and the apoptosis was assessed in each category, using Annexin/PI method. The results showed that treatment with the polysaccharides fraction or the ethanol extract induced lower levels of apoptosis in the cell line than that found for the cisplatin treatment alone (positive controls). The percentages of cell apoptosis after treatment with cisplatin alone, the extract and polysaccharides fraction were 59.07%, 28.6%, and 24.96%, respectively. The data in these categories are presented in Figure 4 B, C and D.
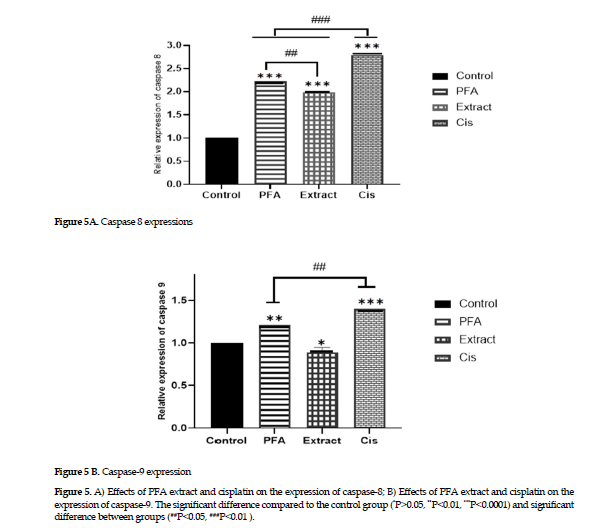
 Effects on the gene expression of caspases 8 & 9
Effects on the gene expression of caspases 8 & 9: The experimental results on gene expression showed that the extract alone affected the gene expression in caspase 8 (Figure 4 A); however, the polysaccharides fraction and cisplatin treatments affected the expression of both genes in caspases 8 and 9, with the gene expression of caspase 8 being more pronounced than that of the caspase 9 (Figure 4 B).
Discussion
Application of various Astragalus root extracts is popular in traditional Chinese medicine. Among these the extract of Astragali radix has shown significant anti-cancer effect [24]. Also, studies on other Astragalus species, including Astragalus elongatus, have discovered that the root extracts of these plant species have greater cytotoxic effects against cancer cells than those of the aerial parts, i.e. leaves and limbs [25]. In the current study, the results of MTT tests showed that the extract and polysaccharides fraction of A. ovinus inhibited the proliferation of MCF-7 cancer cell line in a dose-dependent manner. The data also showed that the extract inhibited cell proliferation at lower concentrations than the polysaccharides fraction (Figure 1).
The growth and proliferation of normal cells are regulated during the cell cycles. In this respect, P53 protein and cyclin-dependent kinases (CDK) play important roles in the regulation [26]. Disruptions in these regulatory proteins lead to uncontrolled cell growth and ultimately cancer. As a result, arresting cell cycle inhibits the growth and proliferation of cancer cells. Induction of cell cycle arrest is one of the general therapeutic strate gies in cancer treatment [27]. Other studies have shown that polysaccharide fractions from various animals, plants and fungal sources are effective in the treatment of cancer cells. This property enhances the efficacy of chemotherapy drugs, inhibits tumor metastasis, and leads to cell apoptosis while improving the immune system’s fight against cancer [28, 29].
The Astragalus polysaccharides, especially those derived from Astragalus membrane species, are well known for their inhibition of cancer cells. The study of Astragalus polysaccharides on cell cycle has shown that these compounds arrest cell cycle in G2 phase in 4T1 cell line [30]. Also, they arrest the cell cycle in S phase in MGC-803 cell line [31]. In another study, Li, et al. have reported that the polysaccharides from A. membranaceus arrest the cell cycle in G1 phase and increased the percentage of SubG phase cells (apoptotic phase) in MCF-7 cell line at a concentration of 1000 μg/mL [32]. In the current study, the cell cycle evaluation showed that the extracts and polysaccharide components from A. ovinus induced cell cycle arrest in G0/G1 phases, and increased the percentage of cell apoptosis in SubG phase (Figure 3).
Apoptosis is a preprogrammed cell death and occurs via extrinsic or intrinsic pathways. The intracelular caspase 8 activates caspases 3, 6 and 7 via the extrinsic pathway while caspase 9 plays a similar role through the intrinsic pathway. Activation of these intracellular enzymes ultimately leads to cell apoptosis [33]. Induction of cancer cell apoptosis is not specific to a particular cancer type and is a major goal of drugs used against various types of cancer [34]. About 65% of the 185 anticancer drugs that were discovered between 1981 and 2019 are either natural or derived from natural sources, such as plants. Thus, plants have been important natural sources in the development of anticancer agents, many of which induce apoptosis in cancer cells [35]. Studies have shown that different compounds derived from A. membranaceus potentially induce cell apoptosis via either extrinsic or intrinsic pathway. The saponins from the Astragalus roots induce apoptosis of HT-29 cancer cell line through the intrinsic pathway [36] while Astragalus polysaccharides cause apoptosis in human hepatocellular carcinoma cells via extrinsic pathway [37].
Guo, et al. have reported that Baicalein, a flavonoid combination of extracts from the A.membranaceus roots increases the expression of caspases 8 and 9 genes in cells originated from nasopharyngeal cancer cells by inducing apoptosis via both intrinsic and extrinsic pathways [38]. The evaluation of apoptosis rate in the current study with Annexin or Pi showed that treatment with cisplatin, the extract and polysaccharide fraction of A. ovinus induced apoptosis and increased the percentage of apoptotic cells in MCF-7 cancer cell line. The rate of apoptosis after exposure to cisplatin was higher than that of other two treatments (Figure 3). Also, in our study, the results of the study on gene expressions on the extrinsic and intrinsic pathways of apoptosis provided evidence that the ethanolic extract only affected the expression of caspase 8. Thus, it can affect cell apoptosis through the activation of extrinsic pathway. Also, the polysaccharides fraction activated the gene expression in both caspases 8 and 9, hence their efficacy on inducing cell apoptosis via either intrinsic or extrinsic pathway. Finally, the polysaccharides fraction in the current study was more effective in inducing cell apoptosis through the extrinsic pathway, i.e., increasing the expression of caspase 8, more significantly than that of caspase-9 (Figure 5).
 Conclusions
Conclusions
Based on the findings of the current study, we confirmed that the ethanolic extract and polysaccharides fraction of A. ovinus provided significant anti-proliferative and cell apoptosis effects in MCF-7 cancer cell line. Therefore, we can suggest that the extract and polysaccharides fraction may be considered and tested as potential therapeutic agents toward the clinical management of breast cancer in humans. Elucidation of further mechanisms of efficacy of these potential agents awaits well-designed future studies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee, Yasuj University of Medical Sciences, Yasuj, Iran (Code: IR.YUMS.REC.1399.180).
Funding
This article was extracted from the MSc thesis project of Hashem Namazi, Department of Biochemistry, Yasuj University of Medical Sciences, and was funded by Yasuj University of Medical Sciences.
Authors' contributions
Study design and supervision: Hossein Sadeghi and Heibatollah Sadeghi; Data conduction and data analysis: Hashem Namazi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
We thank the Medicinal Plants Research Center of Yasuj University of Medical Sciences for their generous assistance with this research project.
References

















































