Introduction
As identified by the World Health Organization (WHO), indoor snakebites have been well investigated as a life threatening disease in under developed nations [1]. Based on the official reports, Nigeria is ranked high among other nations with record deaths due to snakebites. The number of deaths has been as high as 25,000 annually [2], while the reported numbers could be three times as high [1]. In Nigeria, most venomous snakebites almost always occur by carpet viper snake (Echis ocellatus), cobra (Naja nigricollis) and puffadder (Bitis arietonns). It is interesting to note that these snakes belong to either viperidae or elapida species [2].
For the purpose of this study, we focussed our research on the venom from Naja nigricollis or cobra. Understandably, the venom from this snake contains up to 81 different toxins [3], with severe damaging effects on various human systems [4]. Human neurological system is the main target of cobra envomation, with the major clinical signs being eyes ptosis. The toxins gradually paralyze the skeletal and respiratory muscles, leading ultimately to death. Also, peripheral neurotoxicity can be traced to three independent toxins in the venom [5] as described below.
The first toxin resembles curare peptide, known as alpha-neurotoxin. It consists of 60-62 amino acids with the molecular weight ranging between 6-7K dalton. It has high affinity to the post-synaptic receptors in the neuromuscular junction [6]. This peptide prevents acetylcholine (Ach) from binding to the neuromuscular junction, leading to the blockade of neurotransmission [7-9].
The second toxin is phospholipase A2 (PLA2), which attacks lecithin molecules located in neurological membranes and cellular organelles [10]. This interaction usually leads to the irreversible destruction of the cells. The third toxin is known as acetylcholinesterase (AchE), which blocks neural signal transmission at the neuromuscular junction by causing destructive effects on the synaptic cleft [8].
It is a well known fact that elapid venoms, to which cobra venom belongs, have high amounts of AchE with the molecular weight being 67±2 KDa [9]. In principle, the venom toxin resembles human AchE and has catalytic activity, which breaks down acetylcholine quicker than propionylthiocholine and butyryltheocholine species [10].
However, when AchE from cobra venom is blocked, it preserves more acetylcholine at the neuromuscular junction and hence, stops the paralysis [11]. It is also known that neostigmine, used commonly in snakebite therapy, has a similar mechanism of action [12]. Further, there exists an enzyme in the cobra venom, called hyaluronidase. This enzyme promotes the venom toxins to circulate rapidly by breaking down hyaluronic acid within the extracellular matrix (ECM) [13]. This is why this enzyme is termed the circulatory factor. The rapid ECM breakdown is achieved by assisting more venom toxins to bind to sites on target cells, causing additional harmful and systemic effects [14]. It has been noted that the survival rate of mice is enhanced after cobra venom is administered to them followed by the injection of purified hyaluronidase inhibitors [14]. This suggests that any antidote with the ability to inhibit hyaluronidase can lower the local and systemic toxicity, hence providing a better clinical outcome for the human victims of cobra snakebites.
At the moment, in Nigeria, cobra snake envenomation is treated with a polyvalent anti-snake venom (ASV), which contains anti-bodies against the venom of the three species of snakes as mentioned earlier. The current ASV available in Nigeria is a purified preparation; however, it is financially out of reach for many patients and causes considerable allergic reactions or even anaphylaxis [15]. There is current information available, suggesting that high doses of ASV can result in anaphylactic response in the victims more rapidly. Not only this negative effect of ASV is established, but it also lacks the ability to break or completely inhibit the bound toxins [16]. Further, it is known that the systemic injection of ASV elicits minimal effects in terms of penetration to the site of venom’s harmful interaction [17]. Also, as much as ASV salvages lives and lowers the venom’s harmful effects locally; however, the affected tissues and organs must still be protected. Therefore, taking a different approach is warranted to compliment ASV for an appropriate anti-toxin management.
Celosia leptostachya (C. Leptostachya) is an herb, belonging to the family of amarantheceae plant. The stem has no hairs but is very slender and angled-like with decumbent base. It can project as long as 30-cm high, but reaches up to 60-cm when it is strangled at the stem, with smooth, well pronounced and ovate leaves. This plant is commonly grown in Abia State in southeast Nigeria. It is an edible plant and used commonly in vegetable soups. This plant has multiple medicinal values, of which herbal healers use it for the treatment of boils, fever, convulsion in children, eye infection, wounds, pain, and most notably, snakebites and scorpion stings. This plant is used by traditional healers to treat snakebites in Ishialangwa South of Abia State in Nigeria. Based on personal experience and interactions with traditional healers, when a person is bitten by a snake, the leaves of C. Leptostachya are manually squeezed to get the extract up to 2 mL. The victim then drinks it, the marsh is rubbed at the bitten site, and the victim goes about his or her normal business. The most interesting part of it is that one administration of this extract causes the victim recovery within 10-30 minutes of the snakebite.
Aim of the study: With all the powerful medicinal properties of this plant, no scientific research has been conducted to validate its anti-snake venom potentials. Hence, this study was designed to scientifically verify the claims regarding the antitoxic potentials of the C. leptostachya extract in mice.
Material and Methods
Collection of the venom: The lyophilized snake venom from Naja nigricollis was obtained from the Department of Veterinary Medicine, Ahmadu Bello University in Zaria, Kaduna State, Nigeria. The lyophilized venom was safely refrigerated at 40C before using it for the experiments.
Collection and identification of plant materials: Fresh leaves from C. leptostachya plant were collected early in the morning at Abia State, Nigeria, in June 2022. The plant was identified and authenticated by a taxonomist, Mr. Ibe K. Ndukwe, affiliated with the Herbarium Unit, Department of Forestry, Michael Okpara University of Agriculture, located in Umudike, Abia State, Nigeria (Voucher #: FHI3081).
Preparation of the extract: The leaves were collected and washed thoroughly under tap water, and dried at room temperature at 20-24ºC over two weeks. The dried leaves were powdered, using a mortar and pestle, and sieved to obtain a fine powder. Next, 300 g of the pulverized C. Leptostchya leaves was soaked in 1.5 L of ethanol for 48 hours, using maceration method. The solution was then passed through Whatman filter paper #25 and poured into a flask. The filtrate was concentrated to dryness over five days in a water bath at 400C. The greenish yield of 15.8 g extract was stored in a refrigerator at 40C.
Phytochemical analyses: The phytochemical analyses of the C. leptostachya extract was achieved using a previously described method [18]. The secondary metabolites assessed included tannins, saponins, alkaloids, flavonoids, terpenoids, steroids, phenols, glycosides, reducing sugars and resins [18].
Animals: A total of 36 male and female mice weighing 20-22 g were obtained from the animal house of Ebonyi State University, and were randomly divided into six groups of six mice each. Group 1 was the controls while groups 205 were the experimental ones. The male mice were separated from the females, but maintained under identical and standard laboratory conditions at 25-30oC, under 12 hours of light and dark cycles. The animals were kept in clean cages, each covered with a bed of saw dust, which was replaced on alternate days. The animals had free access to pellet diet and water. The National Institute of Health Guidelines for the Care and Use of Laboratory Animals were strictly followed [19].
Determination of acute toxicity: The lethal dose at 50% (LD50) was determined for the animals, using Lork’s method [20]. This test was done in two phases and the mice were deprived of food overnight prior to the administration of the extract. In phase-1, three groups of three rats per group were used. The extract was orally administered to them at increasing doses of 10, 100 or 1000 mg/kg body weight. The treated animals were monitored for signs of toxicity. Considering that there was no death among the animals during the first 24 hours, phase-2 of the study was initiated. Three mice were selected in this phase in which the extract was orally administered at 1600, 2900 or 5000 mg/kg to each mouse. The animals were then observed for the signs of toxicity and mortality over the next 24-48 hours.
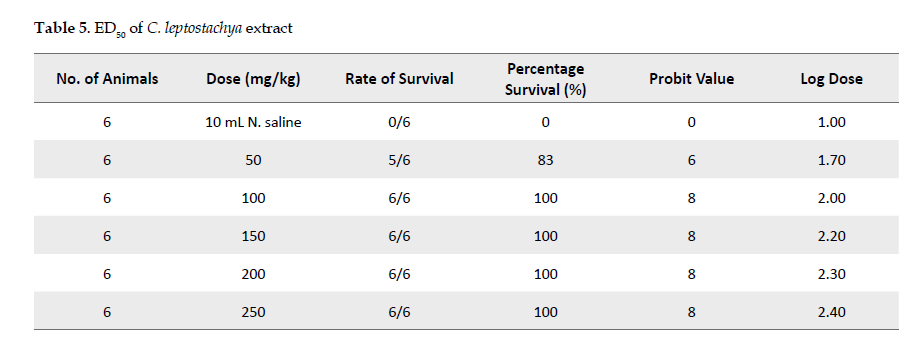
Determination of the venom’s lethal dose: The study was continued to determine the median lethal dose among 50% of the animals (LD50) and minimum lethal dose (MLD) of the venom following the method described by Theakston and Reid [21]. This method was modified slightly as described by an earlier study [22].
Assessment of the cobra venom and inhibition assays
Estimation of acetyl cholinesterase activity: An assay was conducted to determine the acetyl cholinesterase inhibitory activity, using an improved approach as described earlier by Ellman, et al. [23]. For this purpose, 200 µg of cobra venom was pre-incubated with various concentrations of the extract for 60 minutes. The supernatant was mixed with acetylcholine iodate, containing 1mL of phosphate buffer. The absorbance of the mixture was read at 412 nm on a spectrophotometer. The cobra venom without the extract was used as the control, and its percent inhibitory activity was determined based on the following formula:
% Inhibition=Control-test×100 Control
Estimation of the Proteolytic Activity: The modified experimental method of Satake, et al. [24] was used to determine the proteolytic enzyme activity of the venom. Also, 2% casein was used as the substrate in 0.02 M Tris-HCl buffer at pH 8.5. Next, 200 µg (1 mg/mL) and various concentrations of the extract were pre-incubated with 1mL of the substrate for two hours at 37ºC. The undigested casein was precipitated by the addition of 1.5 mL trichloroacetic acid (TCA; 0.44M) and centrifuged. The concentration of the digested casein in the supernatant was estimated using Folin-Ciocalteu’s reagent. Lastly, the cobra venom without the plant extract was used as the control.
Estimation of ATPase activity: The method of Vishwanath and Gowda was adopted to estimate the ATPase activity of the venom [25]. As usual, 200 µg of the cobra venom was pre-incubated with various dilutions of the extract for 30 minutes at 37ºC. Each 1-ml of the assay mixture consisted of 750 µL of 0.1M Tris buffer at pH 7.5; 100 µL of 0.1 M MgCl2, 50 µL of 0.1M ATP, and 100 µL of bovine serum albumin (BSA). The reaction was terminated at 60-minute intervals by adding 1 mL SDS solution. The resultant inorganic phosphate was estimated by the assessment method when 400 µL of the sample together with 600 µL TCA was incubated for 10 minutes at 37ºC. Next, the test tubes were centrifuged at 1500 rpm for 10 minutes. The ATPase activity was determined, using 500 µL of ferrous sulphate-ammonium molybdate reagent, and the absorbance read at 820 nm. This was done within two hours at 10 minutes intervals. The test tubes without the extract were considered as the controls. The inhibition time was estimated in percentage using mainly sodium-potassium adenosine triphosphatase (Na-K-ATPase).
Inhibitory effects of the extract against the venom: Adult mice were used to evaluate the inhibitory effects of the extract against the cobra venom. The experiment was carried out based on the method described by an earlier study [26]. For this purpose, 36 mice were used in groups of six each. The animals in each group were injected intraperitoneally with the cobra venom at 0.40 mg/kg. Twenty minutes after the injection, the animals were given normal saline or the extract. The control group received 10-mL normal saline while groups 1, 2, 3, 4 and 5 received 50, 100, 150, 200 or 250 mg/kg of the extract, respectively.
After this experimental step, the animals were returned to the cages and were allowed free access to food and water under optimal laboratory conditions. The animals in the groups that had received the plant extract were observed for toxicity signs and mortality up to 24 hours afterward. The experimental outcomes are shown in Table 1.

The extract’s effective doses (ED50) were also estimated from the plots of probit values against log doses as shown in Table 1. Probit is a unit of statistical probability, based on deviations from the mean in a normal distribution.
Results
Phytochemical screening: The result of the phytochemical screening of C. leptostchya extract revealed the presence of alkaloids, flavonoids, carbohydrates, terpenoids, steroids, saponins, balsam and resins. However, anthraquinone, cardiac glycoside and tannins were absent in this extract.
Acute toxicity study: The LD50 of the plant extract was found to be above 5000 mg/kg. Also, the values of LD50 and MLD of the cobra venom were 0.28 mg/kg and 0.40 mg/kg, respectively.
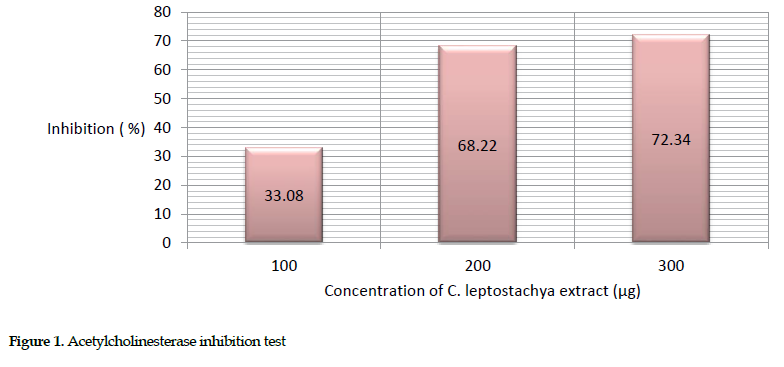
Estimation of acetyl cholinesterase activity: The crude extract of the C. leptostachya leaves was concentrated at various doses (100-300 µg) in triplicate experiments. The result showed that the extract and cobra venom elicited the greatest inhibitory effects at a concentration of 300 µg (72.35%). This activity was estimated based on the percent inhibition of the venom pre-incubated with various amounts of C. leptostachya extract versus the pure venom sample. The enzymatic response was checked every ten minutes and the absorbance was read spectrophotometrically at 412 nm. The acetylcholinesterase activity was found to be 100% (Table 2).

The description of this activity is presented in Figure 1.
 Estimation of protease inhibition:
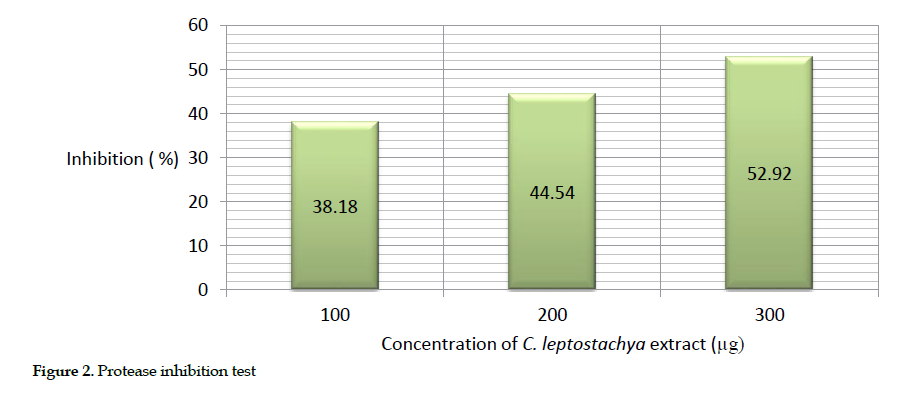
Estimation of protease inhibition: The inhibitory effect of the protease was estimated in vitro as the cobra venom broke down the substrate into peptides, and the precipitate was read at 600 nm. Similarly, the highest inhibitory effect of the protease (52.92%) was achieved at 300 µg of both the venom and the extract. The result demonstrated that as the concentration of the extract increased, a higher percentage of inhibition observed (Table 3).

The description of this activity is presented as bar chart in Figure 2.
 Estimation of adenosine triphosphatase activity:
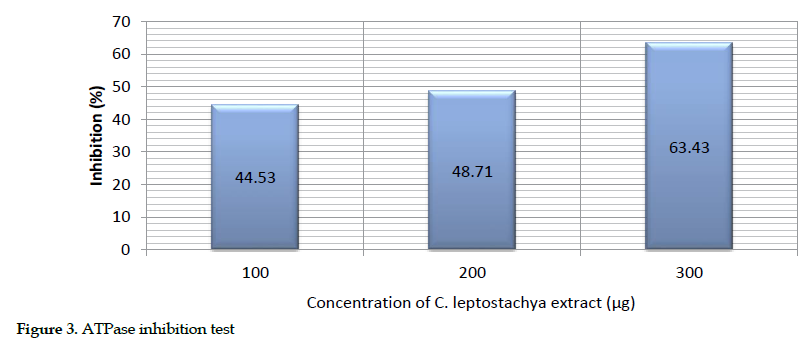
Estimation of adenosine triphosphatase activity: Various amounts of the venom and substrates were used for this purpose. The inhibition of ATPase was produced as inorganic phosphate alongside positive control of the venom (200 µg) with 10 µg ATP as the substrate. The same amounts of the venom (200 µg) and extract (100-300 µg) were pre-incubated for the response. However, a 63.48% inhibition was achieved as the maximum in the presence of the extract (300 µg; Table 4).

The description of this activity is presented as bar chart in Figure 3.
Inhibitory potential of C. leptostachya extract against cobra venom: As presented in Table 1, the results revealed that the extract at 100, 150, 200 or 250 mg/kg inhibited the toxic effect of the venom by 100% after 20 minutes of its administration to the animals. However, at 50mg/kg, it inhibited the venom up 83.3%, suggesting that the extract functions dose dependently.
The median effective dose of the extract: The median effective dose (ED50) of the C. leptostachya extract was estimated from the data in Table 5.
The effective dose (ED50) and the median effective dose of C. leptostachya extract Vs probit are shown in Figure 4.

The percentage curve was converted to probit and plotted against the log doses of the extract. The probit-5 was considered a 50% response (survival).
Based on the graph in Figure 4, it was evident that the effective dose was 20 mg/kg, i.e. as deduced from probit-5 to the log dose. On the other hand, the therapeutic index as the ratio of LD50 to effective dose (ED50) was found to be 63.0 (ED50=Antilog of 1.3 from the graph=20 mg/kg).
Discussion
The quest for new remedies through the assessment and confirmation of traditional medicine presents an opportunity and a reliable avenue to the development of more effective drugs. The major benefit of such development is the availability of effective drugs in remote places where snakebite is a serious health issue. Also, the fact that such agents can be derived from available plants is an added advantage. This study carefully chose procedures for the selection and screening of plants with medicinal properties and evaluated the anti-snake venom effects that are claimed by traditional healers in Nigeria.
Certain information obtained from the well-known traditional healers and plant taxonomists formed the background of this study, which was properly referenced. The facts were carefully separated from fictions, because the use of medicinal plants by traditional healers may not always be scientifically based. However, the physical characteristics of the plants were also important in the process of collecting the materials for this study. It was particularly important whether they were flowering and healthy, i.e., free from disease or contamination. The time of plant collection was also well considered. These factors influence the plant’s secondary metabolites and their active constituents [27].
Further, the choice of the extraction procedures was important since they can influence the quality and quantity of the active metabolites that would be present in the final extract. Although, traditional healers usually use simple method for extraction, either by soaking them in water or other solvents, and sometimes administer them in raw forms, as is the case with C. Leptostachya. Thus, the use of ethanol as the solvent was considered to extract all of the plant’s active ingredients [27].
The snakebites usually occur as an emergency incidence, and the treatments require urgent care by the native healers. In this context, the local healers use the plant’s leaves quickly after they squeeze them manually to get the raw extract.
Based on findings from acute toxicity studies, we believe it is reasonable to declare the LD50 of C. leptostachya extract in rats as being ≥5000 mg/kg body weight. Therefore, this plant and its extract could be considered nontoxic. In order to determine the safety of the plant extract, the therapeutic index (TI) was estimated. The TI is the ratio of the dose required to kill 50% of the animals to the dose that cures 50% of the population. This value was estimated to be 250 mg/kg, indicating that any anti-snake venom developed from the C. Leptostachya extract must be multiplied by 250 before it becomes toxic. Therefore, it is fair to say that C.
Leptostachya extract has a high margin of safety. In this context, the lethal doses of the cobra venom for LD50 and MLD were found to be 0.28 and 0.40 mg/kg, respectively.
Inhibitory effects of the extract: The inhibitory effect of the extract against cobra venom was chosen to investigate its anti-venom activity, since it is used by the local healers in the treatment of people suffering from snakebites. In the current study, the efficacy of the extract against cobra venom was evaluated by comparing the status of the rats treated with this preparation versus those with normal saline (controls). On this basis, a 40% inhibition or greater was considered significant. The C. leptostachya extract neutralized the crude venom at 0.40 mg/kg with an LD50 value of 0.28 mg/kg. This was expressed by the ability of the treated mice to successfully fight off the venom at a lethal dose.
Effect on mortality: Based on the findings, the extract at 50 mg/kg significantly reduced the mortality rate among the treated mice. Also, based on our findings, an MLD dose of the venom (0.40 mg/kg) dropped the mortality rate in rats from 100% to 83%. Further, the IP administration of the extract at 100, 150, 200 or 250 mg/kg converted the 100% mortality rate to zero percent in mice pre-treated with the cobra venom. Although various graded doses of the extract were used, a 100% mortality inhibition occurred at 100 mg/kg or greater. This provides experimental evidence that the extract’s efficacy is directly dose dependent.
Biochemical inhibitory effects: We observed that the greatest inhibition of acetyl cholinesterase (72.34%), protease (52.92%) and cholinesterase (63.48%) in mice occurred at 300 µg concentration of the extract. This further suggests that the extract was able to inhibit acetyl cholinesterase, protease and ATPase activities. Obviously, the increasing inhibition rate of the venom enzymes by raising the extract concentration indicates its dose dependency.
Pain relieving effect: It is important to note that one of the symptoms being presented by the patients of cobra envenomation is severe pain at the site of snakebite [28]. However, the conventional antivenom agents used, do not always relieve the pain satisfactorily. The use of plant extracts with analgesic effect can resolve this problem, thus being an additional benefit to the victims of snakebite. In this context, the scratching behaviour observed in mice could be an indication of pain. This behavior declined at greater percentages in the mice treated with the extract. On this basis, the extract of C. Leptostachya is likely to contain some chemical compounds that inhibit pain as suggested by the reduction in the scratching behavior in mice. Thus, our results suggest that the C. Leptostachya extract is highly likely to contain agents that inhibit pain, in addition to being an effective antitoxic treatment to fight off the venom due to cobra snakebites.
Anti-inflammatory effect: The local swelling that occurs at the site of cobra snakebite is also one of the effects of the envenomation. Thus, using the plant extract for its anti-inflammatory effect would be of additional benefit to the victims. The evidence in support of this effect came from significant relief of the swellings observed in the mice treated with the extract within minutes after its administration. Thus, our findings in this regard suggest that the plant extract may also be used for its anti-inflammatory property.
Effect on muscles: One of the major outcomes of cobra snakebite is the subsequent paralysis of voluntary muscles. This condition is directly caused by the post synaptic neurotoxicity of the venom, which blocks post-synaptic, cholinergic receptors [28]. In this context, the muscles supplied by cranial nerves are usually paralyzed first, then by those located in the chest and diaphragm, which are the most resistant ones, and lastly, followed by the muscles of the limbs. Later, as these events progress, the paralysis affects the respiratory system, leading to its arrest. Hence, the use of this extract to prevent respiratory failure during snakebite supported our interest in conducting this research. Therefore, the impacts of the extract on all of the parameters as discussed above warrant further systematic research on the therapeutic potentials of C. leptostachya extract in the management of patients suffering from cobra snakebites (Table 4).
Conclusions
The fact that snakebite is a serious medical problem especially in communities where anti-snake venom is not easily available or is financially out of reach, the quest for inexpensive and effective drugs for the treatment of urgent cases is crucial. The outcome of the current study showed that the leaf extract of C. Leptostachya has inhibitory effects against the bioactive ingredients of cobra venom, which are of vital need in areas where cobra snakebites pose serious medical problems. Consequently, the use of this plant extract is justified in the treatment of cobra snakebites since it effectively inhibits the toxins in the venom.
Ethical Considerations
Compliance with ethical guidelines
The current study was undertaken after the approval granted by the Ethical Committee of the Ebonyi State University at Abakaliki, Nigeria (Code: EBSU/DRIC/UREC/VOL.04/098).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Study design: Eugene Ohanme; Statistical analyses: Eugene Ohanme, Omotayo Erejuwa and Mansur Ramalan; The literature search and editing of the manuscript: Eugene Ohanme, Godwin Akuodor, and Omotayo Erejuwa; Experiments and final approval: All authors;
Conflict of interest
The authors declared no conflict interests in conducting this study.
Acknowledgements
Authors express their profound gratitude to iyen B. Auta and Sunday Azi of University of Jos in Nigeria for their generous technical assistance provided during this study.
References










































 , Godwin C. Akuodor2
, Godwin C. Akuodor2 

 , Casimir C. Ofor3
, Casimir C. Ofor3 

 , Kenneth E. Etu4
, Kenneth E. Etu4 

 , Mansur A. Ramalan5
, Mansur A. Ramalan5 

 , Donatus O. Anele6
, Donatus O. Anele6 

 , Omotayo O. Erejuwa4
, Omotayo O. Erejuwa4 











