Introduction
Among the most prevalent conditions affecting the digestive tract in humans is gastric ulcers. Infections, smoking, stress, extended use of NSAIDs, and excessive drinking of alcohol contribute to the pathophysiology of this disease, which is brought on by a disparity of factors that secure or destroy gastric mucosa [1]. Disrupting the gastric mucosa, which raises mucosal permeability and bleeding, causes ethanol-induced gastric ulcers. Reactive oxygen species (ROS), the primary mediators of oxidative stress, and other inflammatory factors are overproduced at the site of gastric ulcers by white blood cells, which lead to oxidative damages [2].
Proton-pump inhibitors (PPIs), antacids, H2 receptor blockers, and other modern medications are frequently used to treat gastritis. However, these drugs have their side effects that may lead to various bodily complications [3]. Conversely, herbal medicines are harmless complementary agents that often provide gastro-protective properties as well [4]. Ammi visnaga (A. visnaga), a member of the Apiaceae plants, also known as Khella in the Arab nations, is widely and effectively used in various areas and communities in Egypt. It has recently been investigated for its therapeutic benefits, of which only a few studies have focused on its antioxidant effects [5]. In this context, the A. visnaga extract is believed to contain substantial scavenging effects. Its high content of polyphenols and flavonoids are likely to have beneficial effects on human cells [6].
Aim of the study: Considering the above reviews, this study was planned to evaluate the antioxidatant effects of A. visnaga extract, and its potential gastro-protective effects on ethanol-induced gastric mucosal ulcers in rats.
Materials and Methods
Preparation of the extract: The A. visnaga seeds were extracted according to Abu-Serie, et al. [6]. The seeds were bought from a nearby market in Fayoum City, with a history and practical experience in providing various natural products. A 250 g sample of A. visnaga seeds were washed with clean water then distilled water, and left to dry out in the air. The dried seeds were ground into a fine powder and extracted twice in 500 mL of autoclaved, purified water for 72 hours at 25°C with constant agitation. Next, the concentrated liquid was filtered and lyophilized to obtain the powder. The dried powder was placed in impermeable compartments and set aside at room temperature until further experiments.
Chemical analysis: The chemical composition of A. visnaga extract and the quantities of polyphenols, flavonoids, and various antioxidants were determined by HPLC analyses. These compounds have been widely introduced and discussed in previous studies [6, 7]. From the analysis, 13 phenolic compounds were identified in the A. visnaga extract.
Calculation of the A. visnaga dosage: The A. visnaga dosage of the extract selected for the current study was based on an earlier report published recently by Kamal, et al., [8]. These authors estimated the effects of three doses of A. visnaga extract at 600, 1200, and 1800 mg/kg, and reported that the 1200 mg/kg dose resulted in the best outcomes. However, they observed signs of toxicity at the 1800 mg/kg dosage. Thus, the dose of A. visnaga extract used in the current study was 1200 mg/kg.
Experimental animals: We used 40 adult male albino rats, weighing 200-250 g, purchased from the National Research Center breeding unit in Giza, Egypt. The animals were housed under optimal laboratory and environmental conditions for two weeks for adaptation. They were fed a diet of wheat and milk-soaked bread, and water, which were freely available to them. The rats were divided into four equal groups of ten each and were treated as follows:
Group 1: (Negative controls) received distilled water at 1 mL/kg.
Group 2: (Positive controls) received omeprazole 20 mg/kg+1 mL ethanol 80%.
Group 3: (Ulcer model) received distilled water+1 mL ethanol 80%.
Group 4: (Treatment) received A. visnaga extract at 1200 mg/kg/day+1 mL ethanol 80%.
Rats in group 1 received distilled water only (1 mL/kg); however, all other rats in groups 2-4 received ethanol 80% orally (1 ml/kg) on the final day of the experiment after fasting for 18 hours. The rats in groups 2 and 4 received either omeprazole or A. visnaga extract, respectively. The omeprazole, dissolved in 1 mL distilled water, and the extract was administered via orogastric gavage once daily (at 11 AM) over two weeks. The purpose of administering 80% ethanol to the rats in groups 2-4 was to establish a model for acute gastric mucosal damage as described by an earlier study [9]. One hour after ulcer induction, the rats were sacrificed after being anaesthetized with ether and the stomach was dissected for further histopathological examinations.
Acidity of gastric secretion: The acidity of each rat’s gastric secretion was determined by dipping a pH identifier strip in it immediately after opening the stomach. The result was demonstrated as a pH value.
Ulcer area sizing: The stomach was removed, opened across the greater curvature and washed with normal saline. Each freshly opened stomach was spread on a flat dish and the mucosal lesions were identified as hemorrhages or erosions. Planimetry was used with a straightforward magnifier to calculate the gross ulcer areas (UA) [10]. The proportion of UA inhibition was calculated using Equation 1 [11] (Equation 1):
1. % UA inhibition=(UA (ethanol group)-UA (treated group))/(UA (ethanol group) )×100%
Estimation of gastric mucus content: The measurement of mucus content was performed according to a previously published method [12, 13]. Briefly, some of the stomach tissue was submerged in 10 mL of a pH 5.8 solution containing 0.02% Alcian blue, 0.16 M sucrose, and 0.05 M sodium acetate, and incubated at 25°C for 24 hours. The Alcian blue solution was then centrifuged at 3,000×g for 10 min at 4°C. Using a spectrophotometer, the supernatant›s absorbance was read at 620 nm. The amount of free mucus in the gastric content was calculated based on the amount of Alcian blue bound to the gastric mucus (ng/mg).
Histopathological examinations: The stomach tissue samples from each rat group were washed in ice-cold normal saline buffer and fixed in 10% formalin for 24 hours followed by dehydration and embedding in paraffin wax. The samples were then sectioned at 3-4 µm in thickness, and stained with hematoxylin and eosin (H&E) prior to histopathological examinations under light microscopy.
Oxidative stress markers and antioxidants: The gastric tissue samples were homogenized in super cold phosphate buffer saline (PBS) at pH 7.4 and the homogenates were centrifuged at 3000 rpm for 20 min. The supernatant was collected for the colorimetric measurement of malondialdehyde (MDA), nitric oxide (NO), reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT), using commercial kits from Biodiagnostic Co. (Cairo, Egypt), based on the manufacturer’s instructions.
Statistical analyses: The collected data were presented as the Means±SD for all parameters and used to identify the quantitative data. We used SPSS software, version 28, and one-way analysis of variance (ANOVA) followed by Tuckey’s post hoc test for multiple comparisons of the variables. The statistical differences were deemed significant at P<0.05 values.
Results
Gastric acidity: Table 1 shows that pre-treatment with A. visnaga (group 4) insignificantly improved the acidity of the gastric secretion, unlike OMZ (group 2), which increased the pH of the gastric secretion significantly compared to that of the ethanol group (group 3).
.png)
Also, ethanol induced gastric erosions with the ulcerated areas exceeding 2cm2. The ulcers had improved in rats that were pre-treated with omeprazole. Similarly, the pre-treatment with A. visnaga extract attenuated the gastric lesions by 52.4%, which was less than that observed in the OMZ treatment group (81.3%).
Gastric mucus secretion: Figure 1 demonstrates that the ethanol administration significantly reduced the gastric mucus to 1.307±0.071 ng/mg/protein (P<0.001).

In contrast, OMZ at 20 mg/kg and the extract at 1200 mg/kg significantly improved the gastric mucus secretion to 5.543±0.453 and 3.795±0.070 ng/mg/protein, respectively (P<0.01).
Oxidative stress: Regarding the oxidant markers, data in Table 2 indicate that administering ethanol increased gastric MDA and reduced NO levels considerably more than that observed in group 1 rats (P<0.05).

However, compared to the group that received ethanol only, the pre-treatment with A. visnaga extract or OMZ effectively diminished the MDA and prevented the reduction of NO production induced by ethanol.
Antioxidant defense: The concentrations of SOD, GSH, and CAT in the gastric tissue samples were performed to assess the tissue antioxidant defense. Compared to group 1, the oral administration of ethanol caused a significant decline in the antioxidant enzyme activities. Pre-treatment with A. visnaga extract or OMZ significantly increased the SOD, GSH, and CAT activities (P<0.05), compared to those recorded for the group that received ethanol only (Table 2).
Histopathological findings: The histological examinations in group 1 revealed standard structures of the glandular gastric mucosa and sub-mucosa (Figures 2 and 3).


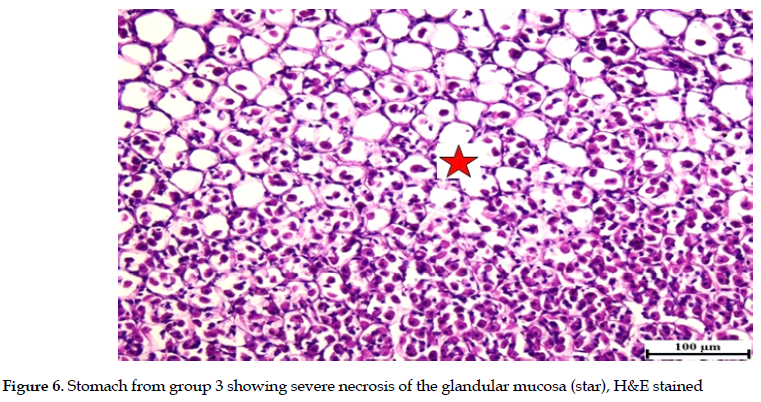
Meanwhile, severe necrosis, erosions and ulceration of the glandular mucosa were observed in rats exposed to ethanol only (group 3). These were confirmed by ample observations in histological examinations and the presence of multifocal inflammatory cell infiltrations in the glandular gastric mucosa. The sub-mucosa was severely expanded with abundant edema and variable numbers of inflammatory cell infiltrations (Figures 4, 5 & 6).


The absence of edema and inflammatory cell infiltrations demonstrated that pre-treatment with OMZ completely protected the stomach mucosa in group 2 rats (Figure 7).

Considerable improvement was also detected in rats pretreated with A. visnaga extract (group 4) associated with mild degeneration of the gastric mucosa and fewer inflammatory cell invasions, indicating that the mucosa was protected from the erosive effect of ethanol (Figures 8 & 9).

 Discussion
Discussion
Gastric lesions, induced by ethanol, are a well-known experimental model to test new medications to protect gastric mucosa. Decreased blood flow and mucus secretion are linked to ethanol-induced gastric mucosal lesions. Additionally, ethanol increases the production of tissue MDA while decreasing the GSH, both of which contribute to the oxidative stress of the tissue. Further, the development of gastric ulcers is likely influenced through the production of ROS by ethanol [14]. The use of indigenous remedies in healthcare has recently attracted much attention. The growing interest may be linked to essential components in plants, such as phytochemical agents, carotenoids, terpenoids, vitamins, and other beneficial elements. Therefore, the current study aimed to investigate the ethanol-induced gastric ulcers, and the therapeutic effect of A. visnaga extract in rats.
The A. visnaga extract used in this study has been previously examined in earlier studies, showing that it contains various amounts of phenolics, flavonoids, flavonols, tannins, and vitamin C. These ingredients are well known for their anti-inflammatory and antioxidant properties. Therefore, the A. visnaga extract may have the potential to enhance human well-being, as a popular and essential medicinal agent in the coming years.
Alterations in gastric pH and mucus secretion: The current study reports that pre-treatment with A. visnaga extract reduced the ulcer areas and indices compared to the group that received ethanol only, as evident by a concomitant increase in the gastric pH. However, the gastric pH and inhibition percentage in group 2 that received OMZ were higher than that of group 4 that was treated with the extract (Table 1).
This finding was supported by an improvement in gastric wall mucus production in group 4, compared to that found for the group that received ethanol only (group 3) (Figure 1). Several studies using various types of plant extracts have reported findings similar to ours [11, 15, 16]. Another study [17] has reported that using A. visnaga extract increased the gastric pH and lowered the acid secretion in an ulcer model induced by Carbachol. The reduction in the ulcerated area is believed to be an important indicator that compounds in A. visnaga extract play beneficial roles in gastric mucosa protection, possibly due to the polyphenolic and antioxidant contents. Further, the former study [17] has concluded that the natural calcium channel blockers present in the A. visnaga extract prevent calcium ion influx and the resultant lipoxygenase pathway from getting activated during the arachidonic acid metabolism. This blockade might account for the decrease in gastric secretion and the protective effect of the extract on gastric tissue. Gastric mucus is secreted to form a gel-like, protective layer on the mucosa. This layer coats the mucosal surface and maintains a basophilic pH. Gastric mucus, produced by mucus-producing cells, effectively protects the mucosal layer from harmful stimuli such as ethanol [18].
Oxidative markers: Ethanol consumption causes gastric ulcers by stimulating oxidative stress and raising the ROS level, resulting in decreased enzymatic antioxidant activities. Excess ROS levels in gastric tissue are essential for the formation of lipid peroxides, such as MDA [19, 20]. The findings of this study are consistent with the latter fact, where the rats exposed to ethanol showed an increase in the oxidative markers, such as MDA and a reduction in the NO production.
The rats pretreated with OMZ or the extract demonstrated a considerable decrease in the MDA level, and restored the normal NO levels, consistent with similar findings reported by earlier studies [11, 15, 16]. Gastric mucosa uses NO for two distinct purposes. Initially, the endothelial nitric oxide synthase (eNOS) generates NO to stimulate gastric ulcer repair process by promoting vascularization, mucus production, and anti-inflammatory activities [21]. Abundant NO created by inducible nitric oxide synthase (iNOS), inhibits the tissue damaging events by generating ROS and fighting the potential toxicity against cells [22].
Therefore, sustaining normal NO thresholds is critical for gastric health because it restricts neutrophil infiltration and acts as a vasodilator agent in the stomach [23].
Thus, adequate NO level in the tissue helps maintain the integrity of the gastric mucosal defense and the healing processes. Our current findings agree with this fact, demonstrating that the ethanol group had significantly higher ulcerated areas and lower NO levels than those observed in the controls (group 1). Conversely, the rats pretreated with OMZ or A. visnaga extract demonstrated a significant decline in their ulcer areas and recovered better NO level than those treated with ethanol only (group 3). Polyphenols interact with and disable iNOS, thereby influencing the NO output and other inflammatory mediators [24].
Antioxidant Factors: Exaggerated oxidative stress causes an increase in ROS generation, inhibiting the intrinsic antioxidant potentials. The current study found that ethanol-induced gastric injury, enhanced oxidative stress, and diminished antioxidant enzyme activities, such as SOD, CAT and GSH. However, pre-treatment with OMZ or A. visnaga extract significantly improved the antioxidant defense compared to those documented for the rat group that received ethanol only (group 3). The protective effect of A. visnaga extract was not only through decreasing the oxidative stress markers but also by increasing and improving the levels of antioxidant enzymes and non-enzymatic reactions. Studies have reported similar results after using various A. visnaga extracts [6-8]. Abu-Serie, et al. [6] found that A. visnaga extract had more potent anti-inflammatory, antioxidant, and ameliorating effects than Ketosteril and other agents.
The antioxidant properties of A. visnaga extract could be attributed to constituents, such as phenolic acids, tannins, flavonoids, flavonols, and anthocyanins. These compounds are recognized for their antioxidant properties and ability to scavenge ROS and reactive nitrogen species (RNS). Further, these agents can inhibit the activity of ROS-producing enzymes while potentiating the antioxidant defense mechanisms [24]. The presence of vitamin C in the extract enhances the antioxidant capacity of the phenolic compounds [25]. A significant correlation was reported between the antioxidant activity and the phenolic constituents. Specifically, the higher the phenolic and flavonoid levels, the better the redox ability of the scavenging effects against free radicals [26].
Histopathology: The lipid peroxides, such as MDA, are created when free radicals involved in oxidative stress interact with cell membranes. Thus, the MDA levels may be a sign of neutrophil infiltration into the gastric mucosa [20]. In the current study, an extensive inflammatory cell infiltration into the damaged gastric tissue was observed upon the histopathological analyses in the ethanol-treated rats. Also, those tissues had significantly higher MDA levels. However, the histopathological examinations showed that the gastric tissues treated with OMZ or A. visnaga extract had minimal inflammatory cell infiltrations with a significant reduction in the MDA levels compared to the rats that received ethanol only. These findings suggest that the A. visnaga extract protects against gastric injury by ethanol, and this effect is highly likely associated with its ability to reduce inflammation while having anti-lipid peroxidation effects.
Conclusions
The current study was conducted to assess the potential protective effect of A. visnaga extract on gastric erosions induced by ethanol. The A. visnaga extract significantly decreased the ulcerated areas at a 51% inhibition rate. Pre-treatment with A. visnaga extract reduced the tissue MDA and restored the NO levels, and enhanced the normal activities of the antioxidant markers. The extract’s anti-inflammatory and antioxidant properties are highly likely associated with its constituents of polyphenols and flavonoids. Therefore, it is logical to propose that the gastro-protective benefits of A. visnaga extract include the inhibition of tissue oxidative stress and enhancement of the antioxidant properties.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in this study were according to the ethical standards of the Faculty of Medicine at Fayoum University. The Ethical Committee approved the research protocol (Code: R387/101/12/2022).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The author would like to acknowledge the Forensic Medicine and Clinical Toxicology Department at the Faculty of Medicine, Fayoum University, for generously providing the laboratory and equipment that were required to successfully conducting this study.
References








































.png)










