

Oghenesuvwe Erhirhie E, Nneoma Okafor J, Chinaecherem Nwafor M, Ozioma Ajaegbo C, C. Akunne T. Toxicological Evaluations of a Popular Polyherbal Remedy-STC30 in Wistar Rats. IJT 2023; 17 (3) :1-9
URL:
http://ijt.arakmu.ac.ir/article-1-1229-en.html
1- Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Chukwuemeka Odumegwu Ojukwu University, Igbariam, Nigeria , erhirhieochuko@yahoo.com
2- Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Chukwuemeka Odumegwu Ojukwu University, Igbariam, Nigeria
3- Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, Nigeria
Full-Text [PDF 545 kb]
(10115 Downloads)
|
Abstract (HTML) (6553 Views)
Full-Text: (768 Views)
Introduction
The use of herbal remedies remains a popular approach to the treatment of several diseases from the beginning of time to date. The main incentive has been their perceived efficacy, little or no toxicity, affordability and acceptability by the consumers (1). Some commercial websites, manufacturers and distributors advertise polyherbal remedies and insist that they have no side effects without providing scientific proof, and most people are attracted by such anecdotal claims. Unfortunately, in some underdeveloped and developing countries, there are no strict and effective laws regulating the production and sales of herbal medicines (1, 2).
However, some herbal products may contain some toxic ingredients that may not be shown on their commercial labels. Some of these undisclosed additives may be done intentionally to increase the sales without considering the possible health risks (3). Superlife (STC30) is one of the leading polyherbal stem-cell natural dietary supplement, claiming that it rejuvenates, regenerates and/or repairs damaged cells, tissues, or organs by activating the stem cells in the body. Its constituents include Swiss apple, grapes, glisodin, bilberry, blackcurrant juice powder, and blueberry extract. This supplement is taken sublingually, (enabling 95% of its nutrients to be immediately absorbed). It is used in over 45 countries worldwide, including Nigeria, Ghana, Australia, Uganda, Ukraine, India, South Africa, Tiawan, China, UK and others. It is 100% natural without any side effects or risk of overdose (4).
This compound (STC-30) is the origin of stem cell in treatment and prevention of diseases. It has been claimed to activate the adult stem cells and provides robust immunity, cures a variety of diseases including diabetes, ovarian cancer, kidney, liver problems, sickle cell anemia, cataract, and other eye defects, without any side effects. Stem cells are undifferentiated special human cells that are able to develop into many different cell types, ranging from myocardial cells, to kidneys, liver, bones, skin, brain, muscle cells, among others. One of the main characteristics of stem cells is their ability to self-renew or multiply while maintaining the potential to develop into other cell types. Stem cells can fight numerous disease conditions (5).
Although some herbal remedies rarely have adverse effects, in some cases severe adverse effects of herbal medications have been reported in humans and animals, especially after prolonged use and/or overdose (3). Such situations call for screening their toxicological potential, which is a prerequisite and essential for the development of new drugs, regardless of being synthetic, semi-synthetic or natural (6). Since no toxicological studies currently exist in clinical research literature to justify the anecdotal safety claim on STC-30, the current study was planned to explore the safety profile of STC-30 in animal model.
Materials and Methods
Laboratory Materials: The equipment used in this study were weighing balance, centrifuge (SF-400, Yongkang Hengtu Technology Co., Ltd, China), and spectrophotometer (721G, Zhejiang Top Cloud- Agri Technology Co., Ltd., China). The chemicals were: formaldehyde and chloroform. The diagnostic kits used included those commercially available for assaying total protein, albumin, urea, creatinine, alinine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, chloride, sodium and potassium (Randox Laboratories Limited, Country Atrium, United Kingdom and Teco diagnostics, California, U.S.A).
Animals: A total of 34 albino Wistar rats used for this study were procured from Faculty of Pharmaceutical Sciences, Chukwuemeka Odumegwu Ojukwu University, Igbariam campus, Nigeria. In the sub-acute toxicity test, 28 rats were divided into four groups of 7 animals each.
Group 1 served as control and received 5 ml/kg of the vehicle intraperitoneally. Groups 2, 3 and 4 received 77.5, 155 and 310 mg/kg of herbal remedy respectively. Administration was given daily for a period of 30 days, which is the manufacturer’s recommended duration for use. Six animals were assigned for the acute toxicity test. The animals were fed ad libitum with standard feed and had free access to water. Also, they were maintained under standard conditions of humidity, temperature, and 12-hr of light/darkness cycles. The principles of laboratory animal care were observed in the study with the approval #: PHACOOU/AREC/2021/001issued by animal research ethics committee, Faculty of Pharmaceutical Sciences, Chukwuemeka Odumegwu Ojukwu University, Igbariam, Nigeria.
Acute Toxicity Test: The test was carried out using Up and Down Procedure as described by Erhirhie, et al., (6), where a single upper limit dose of 5000 mg/kg STC-30 was constituted at a volume of 5 ml/kg and administered to the animals (2 male and 2 females) intraperitoneally. One male and one female animal received 5 ml/kg of the vehicle. After administration, they were observed for the first for hour, thereafter for 24 hours and daily for 14 days for toxicity symptoms and/or death.
Sub-Acute Toxicity Test
Dosage Calculation, Animal Randomization and Administration: A sachet of the herbal remedy contained 1500 mg (1.5g), which is taken once daily by an adult weighing about 60 kg. The dosage per kilogram of body weight was determined to be 25 mg. Animal equivalent dose (AED) was estimated from human dosage by multiplying the human dose by a factor of 6.2, as described by Nair and Jacob (7). Thus, 25 mg x 6.2 (being 155 mg/kg) served as the medium dose, while ½ and twice the medium dosage served as the low and high doses, respectively.
Body Weight and Feed & Water Intakes: Body weights of the animals were measured in weeks 0 (before administration), 1, 2, 3 and 4 (during administration), as well as on the day animals were sacrificed (final body weight). Daily feed intake was measured by subtracting the amount of feed remaining from the initial feed weighed into the feeding trough. Daily water intake was also measured by subtracting the volume of water remaining from the initial volume of water in the trough. The average feed and water intakes per week was calculated by taking the average weight and volume of feed and water intakes, respectively over 7 days (1 week).
Samples Collection for Analyses: After the last dosing on day 30th, animals were starved overnight (with free access to water) and were euthanized under chloroform anesthesia on day 31st. Blood samples were collected from their inferior vena cava into plain tubes and allowed to clot for 45 minutes. Another smaller portion of blood sample was collected and delivered into EDTA tube and mixed gently to avoid clotting for the hematology analyses. Clotted blood samples were centrifuged at 4000 revolutions per minute (rpm) for 10 minutes and the serum was separated for the biochemical analyses.
Determination of Hematology Parameters: Well mixed blood samples in EDTA tubes were used for hematology analyses using Midray BC- 2000 Auto Hematology analyzer (Shenzhen, China). The samples were rolled for 10-15 minutes using a multifunctional mixer for proper mixing of the blood cells. A volume of 18 µL of well-mixed sample was taken into the analyzer, and the diluent was used to separate the cells in the blood as well as to create a conducive environment for cell counting. The red cells were lysed before the white blood cells were counted. All samples were run at a temperature of 24ºC.
Organ & Body Weight Gains and Histopathological Analyses: The organs were carefully dissected, cleaned of adherent tissues and weighed (absolute organ weight). Thereafter, liver, kidney and heart were placed into plain tubes containing formal saline for histopathology analyses. Relative organ weights were calculated by dividing absolute organ weights by final body weight (on the day of sacrifice) and multiplying the quotient by 100. Body weight gains were determined by subtracting the initial from the final body weights, and the differences were divided by the final body weights and multiplied by 100 (8). Relative organ weights and body weight gains were expressed in percentage. For histology analyses, liver, kidney and heart were fixed in 10% formal saline, and were processed using histokinette tissue processor (Leica, Germany). The processed tissues were then embedded in paraffin wax and 5µ thick sections were made using a rotary microtome. They were stained using hematoxylin and eosin, and stained slides were examined under optical microscope and photomicrographs of sections were taken at x400 (9).
Biochemical Parameters: The serum chloride, potassium, sodium, urea, creatinine, total protein, albumin, ALT, AST and ALP were determined, using standard procedures with the commercial kits obtained from Randox Laboratories Limited, Country Atrium, United Kingdom and Teco diagnostics, California U.S.A. Lipid peroxidation was estimated colorimetrically, using malondialdehyde (MDA) as an oxidative stress marker, by the method described by Adebayo, et al. (10).
Data Analyses: The obtained data were presented as means ± standard deviations. Statistical comparisons between the treatment groups and controls were made using one way analysis of variance (ANOVA) followed by Dunnet test. These methods compared the test groups versus the controls. The statistical significance was set at P < 0.05 while P > 0.05 was considered if the comparison of the means were not statistically significant. The software used for data analyses was the Statistical Package for Social Sciences (SPSS, version 23).
Results
Acute Toxicity Test: There were no signs of toxicity and death in all groups after 4, and 24 hours, and 7 and 14 days of treatment. Thus, the LD50 of the herbal remedy STC-30 was estimated to be greater than 5000 mg/kg, for a single or dosed treatment.
Effect on Body Weight: Table 1 depicts the body weights of the animals administered with three doses of the herbal remedy STC-30. There were progressive body weight gains in the controls and other groups. There was no statistically significant difference (P>0.05) in body weight gain at low, medium and high doses of the herbal remedy as compared to the control group.
Effect on Relative Organ Weight: There were no statistically significant differences (P>0.05) in the absolute and relative liver, kidney, heart, testes, and spleen weights in all treatment groups compared to those of the controls (Table 2).
Effect on feed Intake: There was no statistically significant differences (P>0.05) among the feed intakes of all treatment groups compared to the controls (Table 3).
Effect on water intake: There was increases in water intakes from week 2 to week 4, especially in rats treated with low and medium doses, the differences of which were not statistically significant (P>0.05) compared to the control group (Table 4).
Effect on Liver Biomarkers, Total Protein, Albumin and MDA: There was no statistically significant differences (P > 0.05) in ALT, ALP, Total protein, albumin and MDA levels in the treatment groups compared to the controls. However, there was a statistically significant reduction in the AST level at high STC-30 dosage compared with the control group (Table 5).
Effect on Urea, Creatinine and Electrolytes: Based on the data presented in Table 6, there was no statistically significant differences (P > 0.05) in urea, sodium, potassium and chloride levels in rats treated with low, medium and high doses of STC-30 compared to control group. On the other hand, there was a significant reduction (P < 0.05) in creatinine only in rats treated with STC-30 at high dose compared to controls.
Effect on Hematological Parameters: As shown in Table 7, there was a statistically significant (*P < 0.05) reductions in the HCT, HBG, RBC, and MCHC in rats treated with STC-30 at high dosage compared to those of the control group. There was no statistically significant difference (P > 0.05) in platelete levels of the treatment groups compared to that of the controls. Also, there is no statistically significant difference (P > 0.05) in the WBC, neutrophils, lymphocytes, and monocytes levels in animals treated with the low, medium, and high doses as compared with those found in the control group (Table 7).
Table 1. Effect of Herbal Remedy on the Body Weight in Wistar Rats.
| Body Weight (g) |
| Group |
Week 0 |
Week 1 |
Week 2 |
Week 3 |
Week 4 |
Weight Gain (%) |
| Control |
142.40 ± 10.04 |
178.00 ± 15.48 |
196.60 ± 21.64 |
209.40 ± 25.09 |
223.40 ± 28.98 |
35.78 ± 5.37 |
| Low dose |
131.80 ± 7.19 |
167.80 ± 13.10 |
185.60 ± 17.54 |
200.60 ± 13.83 |
221.20 ± 18.89 |
40.26 ± 3.04ns |
| Medium dose |
147.40 ± 6.99 |
183.40 ± 5.81 |
200.60 ± 7.57 |
223.80 ± 13.48 |
240.40 ± 12.50 |
38.64 ± 2.29ns |
| High dose |
135.60 ± 7.13 |
168.60 ± 10.90 |
185.00 ± 16.67 |
203.60 ± 20.18 |
221.60 ± 23.95 |
38.32 ± 6.52ns |
Values are presented as means ± standard deviations, n =5. P>0.05: Weight gains were not statistically & significantly different from that of the control group.
Table 2. Effect of Herbal Remedy on the Relative Organ Weights in Wistar Rats.
| Relative Organ Weight (%) |
| Group |
Liver |
Kidney |
Heart |
Lung |
Testes |
Spleen |
| Control |
3.38 ± 0.42 |
0.60 ± 0.03 |
0.38 ± 0.03 |
0.56 ± 0.09 |
1.31 ± 0.17 |
0.49 ± 0.12 |
| Low dose |
3.87 ± 0.38ns |
0.63 ± 0.07 ns |
0.40 ± 0.02 ns |
0.69 ± 0.10 ns |
1.60 ± 0.72 ns |
0.65 ± 0.25 ns |
| Medium dose |
3.65 ± 0.14 ns |
0.62 ± 0.01 ns |
0.39 ± 0.07 ns |
0.59 ± 0.12 ns |
1.04 ± 0.36 ns |
0.49 ± 0.08 ns |
| High dose |
3.83 ± 0.50 ns |
0.65 ± 0.04 ns |
0.39 ± 0.04 ns |
0.60 ± 0.14 ns |
1.27 ± 0.10 ns |
0.46 ± 0.07 ns |
Values are presented as means ± standard deviations. n =5. P>0.05: Relative organ weight were not statistically & significantly different from that of the control group.
Table 3. Effect of Herbal Remedy on the Food Intake in Wistar Rats.
| Feed Intake (g) |
| Group |
Week 1 |
Week 2 |
Week 3 |
Week 4 |
| Control |
141.86 ± 14.32 |
128.83 ± 17.87 |
162.17 ± 20.00 |
117.29 ± 29.39 |
| Low dose |
140.57 ± 23.87 ns |
130.33 ± 17.74 ns |
146.83 ± 22.81 ns |
132.86 ± 33.31ns |
| Medium dose |
152.86 ± 25.85 ns |
139.17 ± 15.45 ns |
159.50 ± 16.69 ns |
144.71 ± 28.22 ns |
| High dose |
150.57 ± 26.25 ns |
121.67 ± 32.43 ns |
161.50 ± 9.93 ns |
158.00 ± 25.06 ns |
Values are presented as means ± standard deviations. P>0.05: Relative organ weight were not statistically & significantly different from that of the control group.
Table 4. Effect of Herbal Remedy on the Water Intake in Wistar Rats.
| Water Intake (mL) |
| Group |
Week 1 |
Week 2 |
Week 3 |
Week 4 |
| Control |
187.86 ± 18.68 |
200.71 ± 29.92 |
208.57 ± 45.25 |
206.43 ± 13.45 |
| Low dose |
230.71 ± 25.07 ns |
226.43 ± 31.72 ns |
260.86 ± 46.91 ns |
286.43 ± 36.71 ns |
| Medium dose |
231.86 ± 32.60 ns |
227.43 ± 30.76 ns |
273.57 ± 51.29 ns |
302.86 ± 46.27 ns |
| High dose |
194.57 ± 27.26 ns |
188.00 ± 12.03 ns |
225.71 ± 38.56 ns |
224.29 ± 19.67 ns |
Values are presented as means ± standard deviations. P>0.05: Water intake were not statistically & significantly different from that of the control group.
Table 5. Effect of Herbal Remedy on the Liver Enzymes, Total Protein, Albumin and MDA in Wistar Rats.
| Group |
ALT (U/L) |
AST (U/L) |
ALP (IU/L) |
Total Protein (mg/dl) |
Albumin (mg/dl) |
MDA (nmol/ml) |
| Control |
10.37 ± 2.61 |
32.20 ± 3.27 |
36.04 ± 11.97 |
7.16 ± 0.84 |
1.47 ± 0.25 |
0.43 ± 0.07 |
| Low dose |
7.94 ± 2.62 ns |
28.91 ± 3.11 ns |
32.39 ± 6.47 ns |
6.84 ± 0.59 ns |
1.53 ± 0.28 ns |
0.47 ± 0.07 ns |
| Medium dose |
8.74 ± 2.69 ns |
30.80 ± 3.56 ns |
32.08 ± 2.98 ns |
6.92 ± 0.63 ns |
1.47 ± 0.13 ns |
0.49 ± 0.12 ns |
| High dose |
7.17 ± 1.14 ns |
25.76 ± 2.91* |
32.89 ± 5.42 ns |
7.22 ± 0.21 ns |
1.56 ± 0.13 ns |
0.47 ± 0.11 ns |
Values are presented as means ± standard deviations. LD (Low dose), HD (High dose). P>0.05: Statistically no significant difference from that of the controls. *P<0.05: Statistically, significant difference from the controls.
Table 6. Effect of Herbal Remedy on the Urea, Creatinine and Electrolytes in Wistar Rats.
| Group |
Urea (mg/dl) |
Creatinine (mg/dl) |
Sodium (mEq/L) |
Potassium (mEq/L) |
Chloride (mEq/L) |
| Control |
10.32 ± 2.10 |
1.58 ± 0.17 |
154.32 ± 5.24 |
5.09 ± 1.15 |
88.05 ± 10.50 |
| Low dose |
7.00 ± 3.03 ns |
1.49 ± 0.24 ns |
158.97 ± 3.98 ns |
3.97 ± 1.15 ns |
93.15 ± 7.99ns |
| Medium dose |
9.22 ± 2.26 ns |
1.31 ± 0.37 ns |
155.41 ± 6.03 ns |
5.12 ± 1.56 ns |
81.61 ± 4.85 ns |
| High dose |
10.32 ± 3.35 ns |
0.98 ± 0.07* |
157.74 ± 6.17 ns |
4.56 ± 1.74 ns |
83.09 ± 14.23 ns |
Values are presented as means ± standard deviations. LD (Low dose), HD (High dose). nsP>0.05: Statistically no significant differences from controls. *P<0.05: Statistically, significant differences from that of the controls.
Table 7. Effect of Herbal Remedy on the Hematological Parameters of Wistar Rats.
| Group |
HCT (%) |
HBG (g/dl) |
RBC (1012/L) |
MCHC (%) |
Platelate (109/L) |
| Control |
39.20 ± 2.49 |
13.02 ± 0.94 |
6.66 ± 0.52 |
43.40 ± 3.10 |
292.60 ± 7.99 |
| Low dose |
37.00 ± 2.00 ns |
12.32 ± 0.64 ns |
6.20 ± 0.26 |
41.34 ± 2.29 ns |
307.60 ± 11.50ns |
| Medium dose |
38.60 ± 1.14ns |
12.76 ± 0.48 ns |
6.04 ± 0.47 |
42.54 ± 1.60 ns |
320.80 ± 28.85 ns |
| High dose |
33.40 ± 6.02* |
11.10 ± 1.96* |
5.38 ± 0.47* |
35.70 ± 4.90* |
306.60 ± 35.02 ns |
Values are presented as means ± standard deviations, n=5. nsP>0.05: Statistically, no significant differences from that of the controls. *P<0.05: Statistically significant differences from that of the controls.
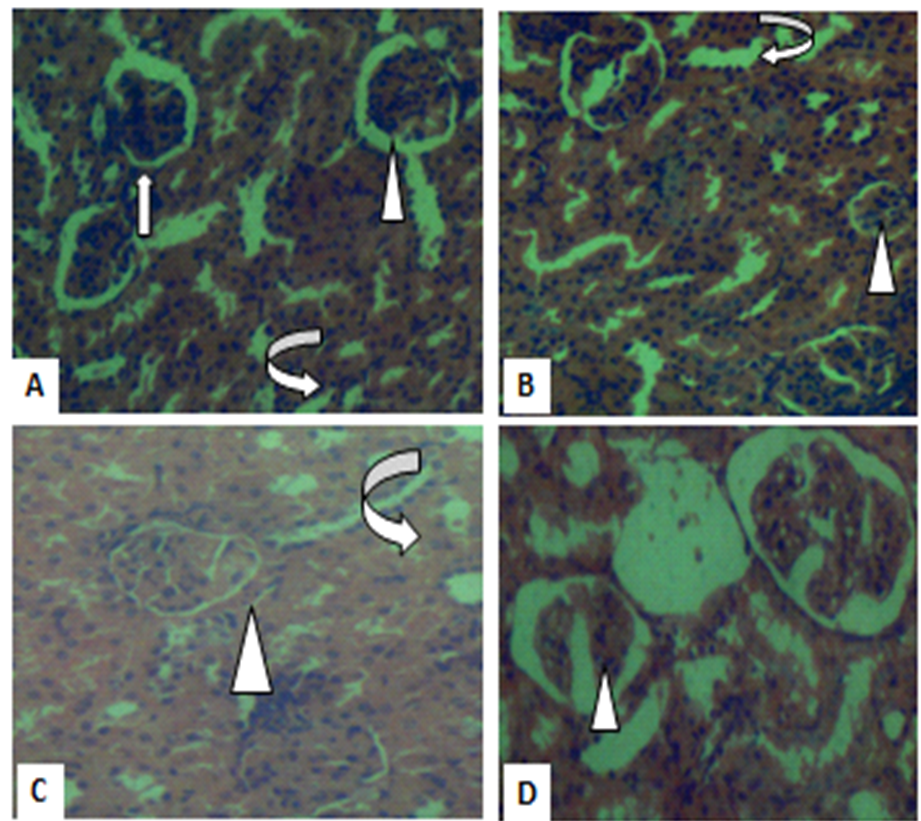
Figure 1. Photomicrographs of kidney sections in controls (A), low dose (B) and medium dose (C) show histology consistent with normal kidney morphology. But high dose (D) show mild distortions of the glomeruli within the renal capsules. Renal capsules (arrowhead) and the tubules (curved arrows). (H&E X400).
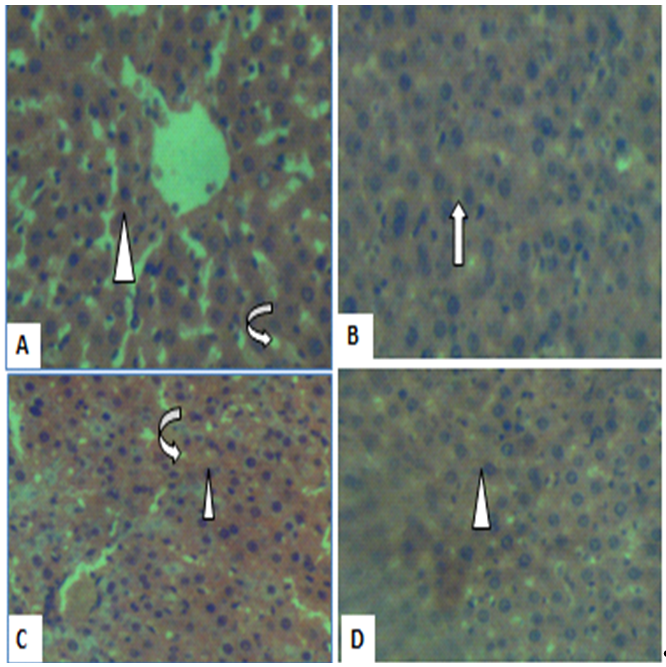
Figure 2. Photomicrograph of liver sections in controls (A), low dose (B), medium dose (C) and high dose (D) show histology consistent with liver histology. The central vein (arrowhead) and sinusoids (curved arrow) are normal with no obvious sign of injury (H&E, X400).

Figure 3. Photomicrographs of heart section of A, B, C and D show architecture consistent with normal heart histology, cardiac muscle, fibers and cells appear normal with no signs of injury (arrowhead) (H&E, X400).
Discussion
Upon a careful review, toxicological information on STC-30 did not exist in the research literature, hence the need for the current study in animal model. Our acute toxicity study revealed no signs of toxicity and death for STC-30 at 5000 mg/kg, suggesting that the LD50 value should be greater than 5000 mg/kg, and also considered to be practically non-toxic based on acute toxicity classification scale (6). Repeated toxicity assessment is vital in the safety profile of all pharmaceutical drugs and herbal formulations that are subject to repeated administration. Otherwise, they are likely to produce cumulative effects on blood, vital organs and vulnerable body systems (11-13).
Since STC-30 is commonly recommended for daily usage over a period of 30 days, we conducted a 30-day repeated toxicity test on this herbal preparation. Specifically, no significant alteration was recorded in the animals’ weight gain at low, medium and high doses compared to the control group. The data presented in Table 1 suggest that STC-30does not cause weight changes, due to alteration in fluid intake, and feed consumption and absorption (14). This is substantiated by the insignificant alterations in relative organ weight, and the feed and water intakes (Tables 2, 3 and 4). The findings also suggest that this polyherbal remedy does not produce anti-nutritional effects, and may not be associated with hypertrophy, hyperplasia or marked organ toxicity (15, 16).
The liver is the principal organ where biotransformation of drugs and xenobiotics occur (17). Enzymes, such as ALT, AST, and ALP are liver biomarkers used to determine the liver’s functional and metabolic capacity. The enzyme levels that are below or beyond normal may be indicative of liver damage. Based on the study results, a significant reduction in the animals’ AST levels was observed only at high dose of STC-30, suggesting that the liver cells and tissue remained intact. It has been documented that a reduction in AST implies absence of liver damage or adequate organ function (17), and thus, the polyherbal remedy may have exerted a protective effect. This argument is supported by the histology results, where the liver architecture showed no obvious injuries and inconsistencies compared with controls at low, medium and high STC-30 doses (Figure 1).
It is important to note that the plasma elevation of renal biomarkers provides evidence of renal toxicity (18). The results, shown in Table 6, revealed no significant differences in urea, electrolyte, total protein and albumin for STC-30 used at low, medium and high doses compared to those of the controls. These findings also suggest that the polyherbal remedy has no deleterious effects on renal function. However, a significant decrease in the creatinine levels only at the high dose suggests that STC-3- may pose harmful effect on the renal function when the polyherbal remedy is taken at double the recommended dose. This is justified by the kidney histological results, showing normal architecture in the kidney of control, low and medium dose, but minimal distortion in renal architecture (Figure 2). The heart remains a vital organ for blood circulation and cardiovascular function (19). Administration of the herbal remedy did not pose any deleterious effects on the heart histology, which is an indication of its safety on the cardiac function.
Hematological parameters are indicators of the blood physiological status and can directly reflect the effect of a toxicant on the body systems (20), and alterations can indicate a deviation from normal. Based on the data shown in Table 7, there were no statistically significant differences in the levels of HCT, HBG, RBC, MCHC, and platelets at the low dose and medium dose treatment groups compared to those of the controls. However, there were significant reductions in the HCT, HBG, RBC, and MCHC at the high-dose treatment group as to those of the controls. In this context, reductions in HCT, HBG, and MCHC suggest iron deficiency anemia (21). Reduced HCT may be caused by the insufficient production of healthy RBC’s due to reduced oxygen-carrying capacity of the blood. The results of this study suggest that the herbal remedy had a deleterious effect on red blood cell production only at high dosage of STC-30, leading to reduced hematocrit, hemoglobin, and MCHC. The platelets revealed no significant difference from that of the control group, suggesting that blood clotting process was unaffected by STC-30 in all of the treatment groups. Table 7 shows no significant differences in WBC, neutrophils, lymphocytes, and monocytes in any of the treatment groups compared to the controls. These findings suggest that the herbal remedy did not have an immune dysfunction effect.
Lipid peroxidation (LPO) assay is one of the reproducible and simple methods in the assessment of the toxic endpoint of polyherbal remedies (22). This is because LPO is linked to diseases as cancer, cardiovascular, diabetes among others (23). Although pro-oxidants in some polyherbal remedies have been reported to generate lipid peroxidation products which are risk factors to tissue damages (22), the insignificant alteration in the MDA level suggests that STC-30 may not produce lipid peroxidation and toxicity, as its phytocompounds may not have acted as pro-oxidants (1, 24).
Although, individual constituents of the remedies such as grape (25), bilberry (26), glisodin (27), blackcurrant (28, 29) and blueberry extract (30) have not been reported to exert deleterious effects on the biological system, it is important to note that polyherbal remedies have various bioactive constituents which may exert synergistic, antagonistic toxicological effects (22).
Conclusions
The acute toxicity test in the current study revealed no obvious toxicity and death at 5000 mg/kg. Repeated administration of recommended dose of the herbal remedy for 30 days did not produce toxic effects on hematology, liver and kidney functions. However, administration of double of recommended dose caused slight deleterious effect on the kidney and hematology parameters. Having carefully carried out this study, STC30 seems to be safe when administered at the recommended and therapeutic dosage. However, consumption of STC-30 at doubles the therapeutic dose should be avoided.
Compliance with Ethical Guideline
Ethical standard on the principles of laboratory animal care were observed in the study with approval number, PHACOOU/AREC/2021/001 issued by animal research ethics committee, Faculty of Pharmaceutical Sciences, Chukwuemeka Odumegwu Ojukwu University, Igbariam, Nigeria.
Conflict of Interests
Authors have no conflict of interest to disclose
Funding
No funding was received for this study.
Acknowledgment
We are grateful to the Faculty of Pharmaceutical Sciences, Chukwuemeka Odumegwu Ojukwu University, Igbariam Campus for making available the animal Facility for this study.
Authors' Contributions
EOE conceptualized the study, JNO, MCN, COA participated in animal handling, EOE wrote the manuscript and also did statistical analyses, TCA and EOE reviewed the manuscript, all authors approved the final manuscript for publication.
References
1. Neergheen-Bhujun VS. Underestimating the toxicological challenges associated with the use of herbal medicinal products in developing countries. BioMed research international. 2013; 2013:804086. [doi: 10.1155/2013/804086] [pmid: 24163821]
2. Nudrat F, Nayeem N. Toxic Effects as a Result of Herbal Medicine Intake. New Aspects to This Scientific Conundrum. 2016; 7(5):762. [doi: 10.5772/64468]
3. Mensah LK, Gustav K, Arnold DF, Cale F, Alexander KA, Rita AD. Toxicity and Safety Implications of Herbal Medicines Used in Africa. New Aspects to This Scientific Conundrum. 2019; 7(5):437. [doi: 10.5772/intechopen.72437]
4. Accessed on 5th March 2023. Available from: https://stc30-superlife-distributor.business.site/.
5. Brazier Y. What are stem cells and what do they do? Medical News Today. 2018. Retrieved on November 12 2021. Available from: www.medicalnewstoday.com.
6. Erhirhie EO, Ihekwereme CP, Ilodigwe EE. Advances in acute toxicity testing: strengths, weaknesses and regulatory acceptance. Interdisciplinary toxicology. 2018; 11(1):5-12. [doi: 10.2478/intox-2018-0001] [pmid: 30181707]
7. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. Journal of basic and clinical pharmacy. 2016; 7(2):27-31. [doi: 10.4103/0976-0105.177703] [pmid: 27057123]
8. Erhirhie EO, Ilodigwe EE. Six months chronic toxicity of Dryopterisfilix -mas (L.) Schott ethanol leaf extract on Wistar rats. Journal of Medical Herbs. 2022; 13(2):67-75.
9. Walsh HL, Sperry AJ, Blazer VS. The effects of tissue fixation on sequencing and transcript abundance of nucleic acids from microdissected liver samples of smallmouth bass (Micropterus dolomieu). PloS one. 2020; 15(8):e0236104. [doi: 10.1371/journal.pone.0236104] [pmid: 32776939]
10. Adebayo OA, Rex N, Olugbenga EB, Utibeabasi II. Effect of quercetin on liver oxidative stress parameters induced by butylparaben in male Wistar rats. Internationa Journal of Medical and Health Sciences Research. 2021; 8(1):1-7. [doi: 10.18488/journal.9.2021.81.1.7]
11. Loha M, Mulu A, Abay SM, Ergete W, Geleta B. Acute and Subacute Toxicity of Methanol Extract of Syzygium guineense Leaves on the Histology of the Liver and Kidney and Biochemical Compositions of Blood in Rats. Evidence-based complementary and alternative medicine : eCAM. 2019; 2019:5702159. [doi: 10.1155/2019/5702159] [pmid: 30956682]
12. Idagu GA, Mubarak HA. Preliminary Sub-acute toxicological assessment of methanol leaves extract of Culcasiaangolensis (Araceae) in Wistar rats. Bulletin of National Research Centers. 2021; 45(1):226. [doi: 10.1186/s42269-021-00686-9]
13. Erhirhie EO, Moke GE. Repeated Systemic Toxicity Tests: A Call for Proper Understanding of Tests Durations Nomenclature. Asian Pacific Journal of Medical Toxicology. 2022; 11(2):72-6. [doi: 10.22038/APJMT.2022.20402]
14. Otunola GA, Afolayan AJ. Assessment of oral safety profie of aqueous extract blend of three medicinal spices in Wistar rats. Tropical Journal of Pharmaceutical Research. 2017; 16(1):91-9. [doi: 10.4314/tjpr.v16i1.12]
15. Unuofin JO, Otunola GA, Afolayan AJ. Acute and subacute toxicity of aqueous extract of the tuber of Kedrostis africana (L.) Cogn in Wistar rats. Journal of complementary & integrative medicine. 2018; 15(4). [doi: 10.1515/jcim-2017-0139] [pmid: 29791313]
16. Nguenang GS, Ntyam ASM, Kuete V. Acute and Subacute Toxicity Profiles of the Methanol Extract of Lycopersicon esculentum L. Leaves (Tomato), a Botanical with Promising In Vitro Anticancer Potential. Evidence-based complementary and alternative medicine : eCAM. 2020; 2020:8935897. [doi: 10.1155/2020/8935897] [pmid: 32215048]
17. McGill MR, Jaeschke H. Biomarkers of drug-induced liver injury. Advances in pharmacology. 2019; 85:221-39. [doi: 10.1016/bs.apha.2019.02.001] [pmid: 31307588]
18. Sindete M, Gbankoto A, Osseni R, Tossavi ND, Azonbakin S, Baba-Moussa L, et al. A 90-Day Oral Toxicity Study of an Ethanolic Root Extract of Caesalpinia bonduc (L.) Roxb. in Wistar Rats. Evidence-based complementary and alternative medicine : eCAM. 2021; 2021:6620026. [doi: 10.1155/2021/6620026] [pmid: 33574881]
19. Pittman RN. Regulation of Tissue Oxygenation. San Rafael (CA): Morgan & Claypool Life Sciences; 2011. Chapter 2. The Circulatory System and Oxygen Transport. 19th March 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54112/.Accessed.
20. Etim NN. Hematological Parameters and Factors Affecting Their Values. Agriculteral Sciences. 2014; 2(1):37-47. [doi: 10.12735/as.v2i1p37]
21. Olayode OA, Daniyan MO, Olayiwola G. Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. Journal of Traditional Complement Medicine. 2020; 10(6):544-54. [doi: 10.1016/j.jtcme.2019.05.001]
22. Kale OE. Lipid Peroxidation and the Redox Effects of Polyherbal. Accenting Lipid Peroxidation. 2021. [doi: 10.5772/intechopen.97625]
23. Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. Journal of photochemistry and photobiology B, Biology. 2001; 63(1-3):103-13. [doi: 10.1016/s1011-1344(01)00207-x] [pmid: 11684457]
24. Goyal S, Gupta N, Chatterjee S, Nimesh S. Natural Plant Extracts as Potential Therapeutic Agents for the Treatment of Cancer. Current topics in medicinal chemistry. 2017; 17(2):96-106. [doi: 10.2174/1568026616666160530154407] [pmid: 27237328]
25. Nassiri-Asl M, Hosseinzadeh H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytotherapy research : PTR. 2016; 30(9):1392-403. [doi: 10.1002/ptr.5644] [pmid: 27196869]
26. Vanekova Z, Rollinger JM. Bilberries: Curative and Miraculous - A Review on Bioactive Constituents and Clinical Research. Frontiers in pharmacology. 2022; 13:909914. [doi: 10.3389/fphar.2022.909914] [pmid: 35847049]
27. Dudasova Petrovicova O, Stankovic I, Milinkovic N, Dopsaj V, Dordevic B, Dopsaj M. Effects of 6-Week Supplementation with GliSODin on Parameters of Muscle Damages, Metabolic, and Work Performance at International Level Rowers after Specific Maximal Effort. Biology. 2022; 11(10). [doi: 10.3390/biology11101437] [pmid: 36290341]
28. Cortez RE, Gonzalez de Mejia E. Blackcurrants (Ribes nigrum): A Review on Chemistry, Processing, and Health Benefits. Journal of food science. 2019; 84(9):2387-401. [doi: 10.1111/1750-3841.14781] [pmid: 31454085]
29. Pap N, Reshamwala D, Korpinen R, Kilpelainen P, Fidelis M, Furtado MM, et al. Toxicological and bioactivity evaluation of blackcurrant press cake, sea buckthorn leaves and bark from Scots pine and Norway spruce extracts under a green integrated approach. Food Chem Toxicol. 2021; 153:112284. [doi: 10.1016/j.fct.2021.112284] [pmid: 34044082]
30. Cladis DP, Li S, Reddivari L, Cox A, Ferruzzi MG, Weaver CM. A 90 day oral toxicity study of blueberry polyphenols in ovariectomized sprague-dawley rats. Food Chem Toxicol. 2020; 139:111254. [doi: 10.1016/j.fct.2020.111254] [pmid: 32165232]
Type of Study:
Research |
Subject:
General










































 , Jennifer Nneoma Okafor2
, Jennifer Nneoma Okafor2 

 , Maureen Chinaecherem Nwafor2
, Maureen Chinaecherem Nwafor2 

 , Chukwuebuka Ozioma Ajaegbo2
, Chukwuebuka Ozioma Ajaegbo2 

 , Theophine C. Akunne3
, Theophine C. Akunne3 





