

Arjadi F, Murti Harini I, Muntafiah A, Setiawati S, Pangestu M. Chronic Toxicity of Purwoceng Root Extract: The Biochemical and Histopathological Effects on Rat Liver and Kidneys. IJT 2023; 17 (3) :60-69
URL:
http://ijt.arakmu.ac.ir/article-1-1230-en.html
1- Anatomy Department, Jenderal Soedirman University. Purwokerto, Indonesia , fitranto.arjadi@unsoed.ac.id
2- Department of Histology, Medical Faculty, JendralSoedirman University. Purwokerto, Indonesia
3- Department of Biochemistry, Medical Faculty, JendralSoedirman University. Purwokerto, Indonesia
4- Department of Pharmacology, Medical Faculty, Jendral Soedirman University. Purwokerto, Indonesia
5- Education Program in Reproduction and Development, Department of Obstetrics and Gynecology, Monash Clinical School, Monash University. Monash, Australia
Full-Text [PDF 680 kb]
(1087 Downloads)
|
Abstract (HTML) (1934 Views)
Full-Text: (559 Views)
Introduction
Indonesia is a country where traditional medicines, derived from plants, are commonly used. Despite the development of modern drugs, traditional medicines are still used by Indonesians due to being inexpensive and perceived efficacy (1). Purwoceng (Pimpinella pruatjan Molk.), a native Indonesian plant, is one of the sources of endemic traditional medicines still in use today. Purwoceng is primarily found in the East, West and Central Java areas, with a significant presence in Dieng highlands (2). This plant is mainly known for its aphrodisiac, diuretic, and tonic effects (3).
Pharmacological studies on Purwoceng root have revealed that it has an androgenic effect, which is useful for increasing spermatogenesis in testes, the sperm count and motility, the LH levels, and testosterone hormones. Also, it is known to reduce the testicular malondialdehyde (MDA) levels and Leydig cell apoptosis in rats subjected to sleep deprivation stress (4). These effects are related to the active compounds contained in Purwoceng roots, such as alkaloids, tannins, flavonoids, triterpenoids, steroids, glycosides, coumarins, euricomalactone, and amarolinda (2).
Purwoceng roots may also have negative effects due to the presence of xenobiotic compounds, such as phenols and lipophilic compounds, like saponins, alkaloids, tannins, and flavonoids. Similar to drugs, these compounds undergo biotransformation in the liver, and the derivatives can be toxic to the body, particularly to the liver (5). Hepatic damages can be observed through histopathological changes in the liver due to elevated levels of transaminase enzymes, such as SGOT and SGPT (6). Additionally, the sitosterol and stigmasterol found in Purwoceng can raise blood urea and creatinine levels, while the lipophilic alkaloids can irritate the cell membranes in the kidneys (7). Administration of tannins in doses exceeding 1500 mg/kg can damage the kidneys’ tubules (8).
Since the above-mentioned ingredients are present in Purwoceng, it is likely that this plant may also have potential nephrotoxic effects. The safety of a drug can be evaluated by conducting toxicity tests. These tests determine the effects of toxic substances at specific doses over a certain period of time on target organs. In an earlier study, experimental animals were used and the given dosages were adjusted based on the guidelines of the Organization for Economic Co-operation and Development (8). In another study, an acute toxicity test was conducted using doses of 5, 50, 300, and 2000 mg/kg to determine the median lethal dose (LD50). The results of the latter study showed no significant damages caused in the kidneys, liver, or reproductive system, which allowed for the conduction of a further sub-chronic toxicity test (9).
To evaluate the toxic effects of repeated administration of herbal preparations over an extended period of time and to detect any hazardous effects that were not found in the acute toxicity test, a sub-chronic toxicity test was conducted on the Purwoceng root extract (9, 10). The results demonstrated that both single and sub-chronic doses of the ethanol extract did not significantly increase SGOT and SGPT levels in white male rats. However, the smallest dose of the extract caused an increase in urea levels in white male rats at 42 mg/kg/day. However, a decline in the urea levels was observed at 168 mg/kg/day, making it impossible to determine a “no observed adverse effect-level (NOAEL) (10). The sub-chronic administration of the Purwoceng ethanol extract at 42 mg/kg/day caused liver damage, while kidney damages were observed at a dose of 84 mg/kg/day.
Aim of the Study: Given the vital role of the liver and kidneys in drug toxicity, and the results of the acute and sub-chronic toxicity tests, it is necessary to determine a dose that does not cause toxic effects in chronic usage of drugs. For this purpose, this study was planned to investigate the chronic toxicity of Purwoceng extract on kidneys and liver functions in rats after the administration at various chronic doses.
Materials and Methods
Study Design: This experimental study used a pre- and post-test with a control group design to measure serum SGOT, SGPT, urea, and creatinine levels, and a post-test only with control group designed to measure liver histopathological parameters. The study was conducted between January and October 2021 at the Anatomy Laboratory, Faculty of Medicine at the Jenderal Soedirman University, Indonesia.
Subjects: White male Wistar rats were used with the following criteria: male, body weight of approximately 100-150 g, aged between 6-8 weeks, and healthy with no anatomical or behavioral abnormalities. Rats were excluded if they were sick or died during the research or if their body weight increased or decreased more than 20% during acclimatization. A total of 32 rats were used based on Federer’s formula and were completely randomized into four groups: control group (A), and groups receiving Purwoceng root ethanol extract at doses of 21 (B), 42 (C), or 84 mg/kg/day (D). Rats were provided with food and water ad libitum (10).
Preparation of Purwoceng Ethanol Extract: The dried Purwoceng roots from the Dieng Highlands, Wonosobo in Central Java, were authenticated at the Taxonomy Laboratory, Faculty of Biology, Jenderal Soedirman University (reference #: 094/HP.I.I/V/2021). The extracts were prepared at the Biology Laboratory, Department of Pharmacy, Faculty of Health Sciences, Jenderal Soedirman University. The Purwoceng powder was prepared by grinding it at 500 grams and sifting through a 60-mesh sieve. The powder was then macerated in 2500 mL of 96% ethanol for 24 hours, with this process repeated three times (10).
Extract Dosage Calculations: The dosage of the Purwoceng extract for group B was 21 mg/body weight/day. To calculate the dose for a 100g rat, the ethanol extract formula was 100/1,000 = x/21, with the x value being 2.1 mg. Therefore, rats weighing 100g were given 2.1 mg of the extract dissolved in 1 mL distilled water (2.1 mg/mL). For rats with varying body weights, the dosage was adjusted as follows: body weight of rats divided by 100 and multiplied by 1 mL. Thus, rats weighing 150g were given 1.5 mL of the solution. The dosage of the extract for group C rats was 42 mg/kg/day. Each 100g rat was given 4.2 mg of the purwoceng extract dissolved in 1 mL of distilled water (4.2 mg/mL). For group D rats, the dosage was 84 mg/kg/day, so a 100g rat was given 8.4 mg of the extract dissolved in 1 mL distilled water (8.4 mg/mL). The extract was administered to rats orally once a day over 90 consecutive days (8).
Liver Tissue Preparations: The histological preparations in this study were conducted at the Pathology and Anatomy Departments of the Faculty of Medicine, Jenderal Soedirman University, Indonesia. The process began by obtaining a liver tissue biopsy samples and fixed by immersing them in 10% buffered formalin solutions (1:20). The tissue samples were then cut into 1 cm thick sections, placed into an embedding cassette, and processed, using the Shandon Citadel 2000® automated tissue processor for fixation, multistage dehydration, cleaning, and paraffin infiltration. Next, the tissue samples on the insertion cassettes were transferred to the base mold and filled with liquid paraffin before being cooled and serially cut into 4 μm thick sections, using a microtome. The sections were then immersed in a floating bath at 40-45°C for 1 minute, fixed onto an objective glass, and dried in an oven at 60-70°C for 12 hours. The samples were deparaffinized with xylene solution for 10 minutes, three times, and then rehydrated with 100%, 96%, 90%, 80%, 70%, or 50% alcohol for 1 minute each. Finally, the samples were stained with Hematoxylin and Eosin (H&E) stain (11).
Experimental Protocol: The animals were acclimatized to the laboratory environment for seven days before the study began. The treatments involved daily administration of the Purwoceng roots extract at a single dose for 90 consecutive days orally at a predetermined dosage. During the treatment period, the health and mortality of the animals were monitored daily. Additionally, weekly monitoring of the weight, and food/water intakes were carried out. Blood samples were collected from the animals at the beginning and end of the study to evaluate the liver function parameters, such as SGOT, and SGPT enzymes, and urea-creatinine levels. Also, the histological features of the liver tissue samples were carefully examined under microscope.
Data Collections: The study data included measurements of the blood SGOT and SGPT enzymes, urea-creatinine levels, and histological features of the liver, kidneys, and stomach. The UV-Vis kinetic spectrophotometer method was used to collect data on SGOT/SGPT levels, and the absorbance values were determined, using factor values to derive the SGOT and SGPT concentrations. The urea levels were examined using the urease-GLDH method at λ340 nm, and serum creatinine was examined using the Jaffe method at λ492 nm, which was read on a spectrophotometer (10). The extent of liver histological damages was assessed using the Scheuer score (11). This involved observing the histological appearances of 20 hepatocytes in each visual field. The scoring was based on the degree of damages, where a score of 1 was given to normal cells, 2 for cells with parenchymal degeneration, 3 for cells with hydropic degeneration, and 4 for cellular necrosis. The scores were then added up to a total score out of five (12).
Data Analyses: The data normality was assessed using the Shapiro-Wilk’s test (13), and the variation was checked using the Levene test (14). Since the data were found to be normally distributed and homogeneous, the parametric repeated ANOVA method was also applied. Additionally, a post-hoc test with the least significant difference (LSD) was conducted to determine the significance of the differences among the treatment groups.
Results
SGOT & SGPT Levels: Table 1 presents the SGOT levels for each study group pre- and post-treatment. While group B showed a decrease in SGOT levels post-treatment, the results of the paired-test analysis were not significantly different (P > 0.05). However, significant decreases in SGOT levels were found in groups C and D following the treatment (P < 0.05). Table 2 presents the mean SGPT levels for each group pre- and post-treatments, with a decrease in the mean SGPT levels found for all groups. A statistically significant difference was found between groups B and D (P < 0.05). Figure 1 shows the differences in the mean pre- and post-treatment for SGOT and SGPT levels among the groups, with K-independent tests and post-hoc comparisons utilized to determine the statistical significance.
Urea & Creatinine Levels: Table 3 presents the results of the paired-test analysis for pre- and post-treatment in urea levels, with a significant increase in the mean urea levels observed in all groups (A-D) (P<0.05). Table 4 represents the paired-test results for pre- and post-treatment for creatinine levels, revealing a significant difference in the creatinine levels between groups A and C (P<0.05). Although a decrease in the creatinine levels was also observed in group B, it was not statistically significant (P>0.05). The urea levels pre- and post-treatment were normally distributed (P>0.05 on Shapiro-Wilk’s test) and homogeneous (P>0.05 on Levene test) across all of the study groups. Differences in the mean pre- and post-treatment urea levels among the groups were assessed using One-Way ANOVA, followed by LSD post-hoc tests, as shown in Figure 2. The results indicated a significant difference (P<0.05) between at least two groups, with significant differences in urea levels observed between groups A and B (P=0.034), B and D (P =0.025), and C and D (P=0.037) (Figure 2). However, pre- and post-treatment for creatinine levels were not normally distributed in one of the groups (P<0.05), and the data transformation was unsuccessful. Therefore, a nonparametric Kruskal-Wallis test was conducted, revealing significant differences (P<0.05). Subsequently, the Mann-Whitney U posthoc test demonstrated significant differences (P<0.05) in creatinine levels between group pairs: A-B (P=0.002), A-D (P=0.003), B-C (P=0.002), B-D (P=0.039), and C-D (P=0.002) (Figure 3).
Liver Histological Examinations: The liver histological damages as caused by xenobiotic substances are depicted in Figure 4. In this study, histological damages were observed in all treatment groups.
Table 1. Mean SGOT levels for the experimental animals.
| Groups* |
n |
Min. |
Max. |
Mean |
Median |
Δ Mean ±SD |
Paired Test Results |
| Group A |
|
|
|
|
|
10.72 ± 53.5 |
p = 0.640 |
| Before |
7 |
123 |
288 |
169.28 |
151.64 |
|
|
| After |
7 |
120 |
287 |
180 |
170.76 |
|
|
| Group B |
|
|
|
|
|
-10.87 ± 44.6 |
p = 0.573 |
| Before |
7 |
111 |
364 |
209.71 |
167 |
|
|
| After |
7 |
129 |
254 |
198.84 |
194.89 |
|
|
| Group C |
|
|
|
|
|
-42.9 ± 47.6 |
p = 0.070* |
| Before |
7 |
104 |
341 |
186.14 |
178 |
|
|
| After |
7 |
83 |
190 |
143.24 |
149.6 |
|
|
| Group D |
|
|
|
|
|
-42.15 ± 19.9 |
p = 0.002* |
| Before |
7 |
111 |
314 |
200.42 |
198 |
|
|
| After |
7 |
85 |
287 |
158.27 |
151.35 |
|
|
*Group A (control); Group B (CMC 1% + 21 mg/kg/day extract), Group C (CMC 1% + 42 mg/kg/day extract) and Group D (CMC 1% + 84 mg/kg/day extract).
Table 2. Mean SGPT levels for the experimental animals.
| Group* |
n |
Min. |
Max. |
Mean |
Median |
Δ Mean ± SD |
Paired Test Results |
| Group A |
|
|
|
|
|
-0.73 ± 15.8 |
p = 0.913 |
| Before |
7 |
50 |
69 |
56.73 |
54.42 |
|
|
| After |
7 |
38 |
93 |
55.98 |
48.62 |
|
|
| Group B |
|
|
|
|
|
-27.24 ± 15.4 |
p = 0.005* |
| Before |
7 |
60 |
143 |
89 |
78.35 |
|
|
| After |
7 |
42 |
81 |
61.76 |
56.75 |
|
|
| Group C |
|
|
|
|
|
-7.19 ± 10.8 |
p = 0.155 |
| Before |
7 |
47 |
77 |
62.94 |
57.43 |
|
|
| After |
7 |
36 |
91 |
55.75 |
55.10 |
|
|
| Group D |
|
|
|
|
|
-9.34 ± 7.0 |
p = 0.017* |
| Before |
7 |
36 |
75 |
57.48 |
55.41 |
|
|
| After |
7 |
32 |
69 |
48.14 |
46.98 |
|
|
*Group A (control); Group B (CMC 1% + 21 mg/kg/day extract), Group C (CMC 1% + 42 mg/kg/day extract) and Group D (CMC 1% + 84 mg/kg/day extract).
Table 3. Mean urea levels for the experimental animals.
| Group* |
Min. |
Max. |
Median |
Mean |
Δ Mean ± SD |
Paired Test Results |
| Group A (n=7) |
|
|
|
|
35.86 ± 16.81 |
p = 0.018 |
| Before |
6 |
55 |
9 |
21.57 |
|
|
| After |
42 |
69 |
62 |
57.43 |
|
|
| Group B (n=7) |
|
|
|
|
20.72 ± 8.63 |
p = 0.018 |
| Before |
9 |
34 |
13 |
19.71 |
|
|
| After |
20 |
57 |
43 |
40.43 |
|
|
| Group C (n=7) |
|
|
|
|
22 ± 14.45 |
p = 0.007 |
| Before |
6 |
12 |
9 |
8.71 |
|
|
| After |
12 |
56 |
28 |
30.71 |
|
|
| Group D (n=7) |
|
|
|
|
36.86 ± 9.87 |
p = 0.000 |
| Before |
11 |
20 |
14 |
14.43 |
|
|
| After |
33 |
72 |
52 |
51.29 |
|
|
*Group A (control); Group B (CMC 1% + 21 mg/kg/day extract), Group C (CMC 1% + 42 mg/kg/day extract) and Group D (CMC 1% + 84 mg/kg/day extract).
Table 4. Mean creatinine levels for the experimental animals.
| Group* |
Min. |
Max. |
Median |
Mean |
Δ Mean ± SD |
Paired Test Results |
| Group A (n=7) |
|
|
|
|
0.65 ± 0.27 |
p = 0.001 |
| Before |
1 |
2.6 |
1.8 |
1.83 |
|
|
| After |
1.8 |
2.99 |
2.6 |
2.48 |
|
|
| Group B (n=7) |
|
|
|
|
-0.08 ± 0.15 |
p = 0.175 |
| Before |
1.8 |
2.41 |
2.2 |
2.15 |
|
|
| After |
1.79 |
2.41 |
2 |
2.06 |
|
|
| Group C (n=7) |
|
|
|
|
0.71 ± 0.36 |
p = 0.018 |
| Before |
1.6 |
2 |
1.8 |
1.83 |
|
|
| After |
2.2 |
3.2 |
2.2 |
2.54 |
|
|
| Group D (n=7) |
|
|
|
|
0.17 ± 0.24 |
p = 0.115 |
| Before |
1.6 |
2.41 |
1.8 |
1.97 |
|
|
| After |
1.61 |
2.59 |
2.2 |
2.14 |
|
|
*Group A (control); Group B (CMC 1% + 21 mg/kg/day extract), Group C (CMC 1% + 42 mg/kg/day extract) and Group D (CMC 1% + 84 mg/kg/day extract).
The process started with an inflammatory response, followed by necrosis, loss of liver cells, and their replacement with fibrous connective tissue, leading to hepatic cirrhosis. The severity of liver damages was evaluated using Scheuer’s score, as shown in Table 5. The mean scores for portal, lobular, and fibrosis changes were the lowest in group A and highest in group D, with a proportional increase observed with increasing the dosage. The scores for the portal, lobular, and fibrotic changes were further interpreted as the histological activity index (HAI) in Figure 5. Analyses of the total liver damage (HAI) showed statistically significant differences between group pairs of A-C and A-D. The chronic administration of Purwoceng extract at 21 mg/kg was considered safe since there was no significant difference between group A (control) and group B (21mg/kg). However, the extract at 42 and 84 mg/kg proved to be toxic, causing significant histological damages in the liver compared to those found for the control group. These results were consistent with those reported by Arjadi, et al. in 2021, who identified the extract at 42 mg/kg as being the minimally toxic dose, causing hepatic damages by the sub-chronic administration of Purwoceng root extract.
Table 5. Mean portal, lobular, and fibrotic activities for the liver based on Scheuer’s scoring system (11).
| Group* (N=6) |
Portal Activity (Mean ± SD) |
Lobular Activity (Mean ± SD) |
Fibrosis (Mean ± SD) |
| A |
0.77 ± 0.54 |
1.40 ± 0.79 |
0.63 ± 0.43 |
| B |
1.00 ± 0.55 |
1.70 ± 0.68 |
0.77 ± 0.46 |
| C |
1.97 ± 0.19 |
2.90 ± 0.17 |
1.90 ± 0.63 |
| D |
2.40 ± 0.44 |
2.93 ± 0.10 |
2.40 ± 0.25 |
*Group A (control); Group B (CMC 1% + 21 mg/kg/day extract), Group C (CMC 1% + 42 mg/kg/day extract) and Group D (CMC 1% + 84 mg/kg/day extract).
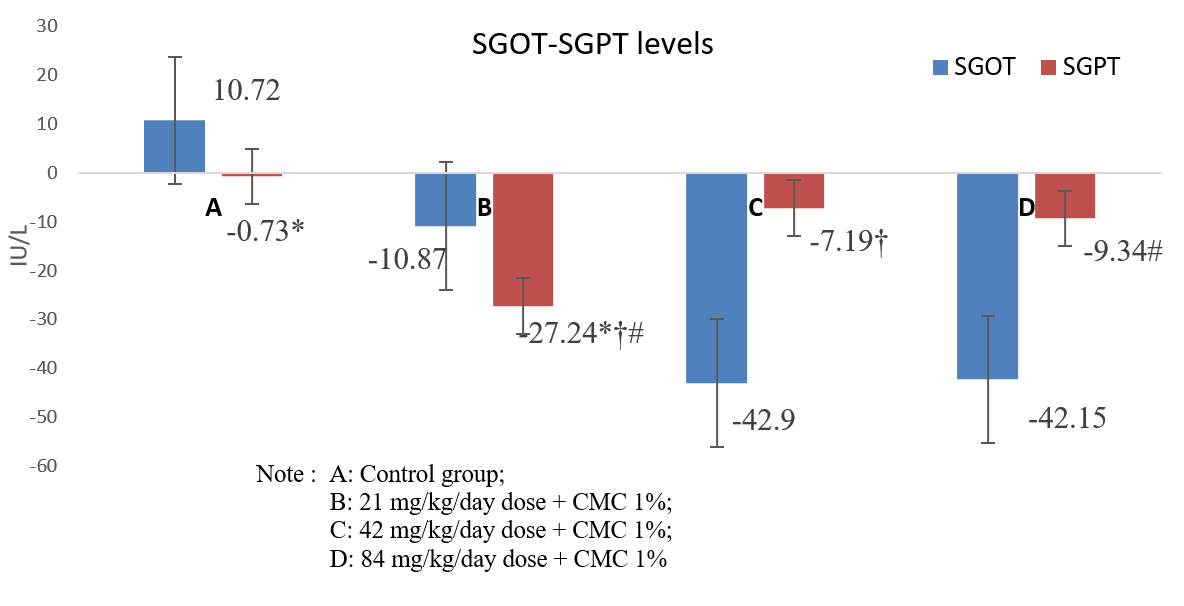
Figure 1. Δ Mean SGOT-SGPT enzyme levels for each animal group.
There was no significant difference in SGOT levels among the groups. There were significant differences in SGPT levels in group A-B (*), B-C (†) and B-D (#).
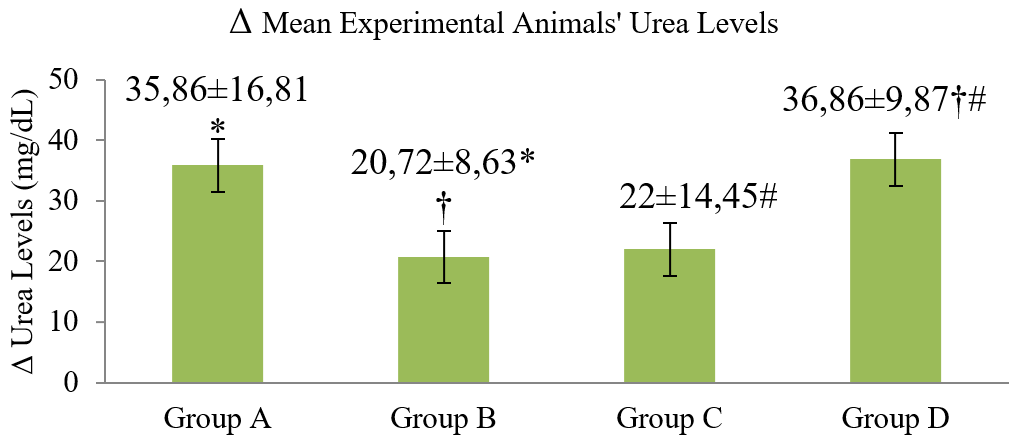
Figure 2. Δ Mean urea levels of experimental animals before and after experiments.
Group A (control); Group B (CMC 1% + 21 mg/kg/day extract), Group C (CMC 1% + 42 mg/kg/day extract) and Group D (CMC 1% + 84 mg/kg/day extract). There were significant differences between group A-B (*), B- D (†), and C- D (#).
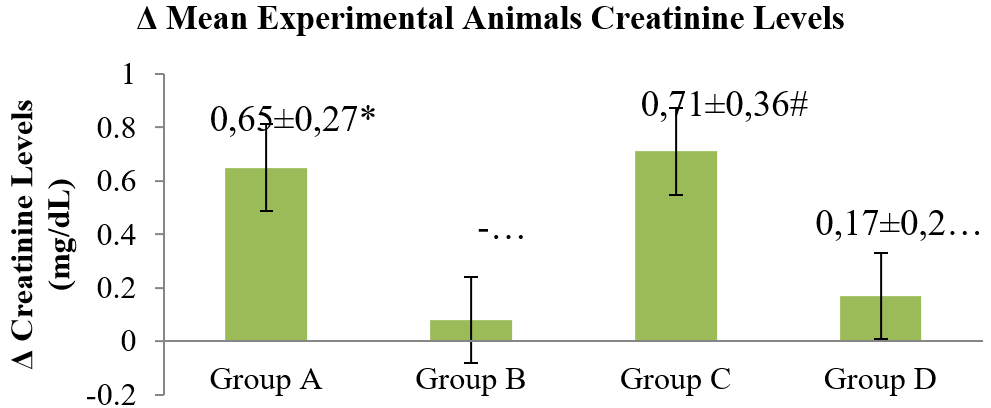
Figure 3. Mean creatinine levels for the animals before and after experiments.
Group A (control); Group B (CMC 1% + 21 mg/kg/day extract), Group C (CMC 1% + 42 mg/kg/day extract) and Group D (CMC 1% + 84 mg/kg/day extract). There were significant differences between group A-B, A-D (*), group B-D (†), and group C-D (#).

Figure 4. Microscopic liver histological appearances in white male rats.
(A) shows portal (à) and lobular (à) inflammation in group A; (B) shows mild piecemeal necrosis (à) in Group B; (C) shows severe piecemeal necrosis (à) with multifocal lobular necrosis (à) in Group C; (D) shows septal fibrosis (à) with bridging lobular necrosis (à) in group D (at a 200-times magnification level with a scale of 10 µm).
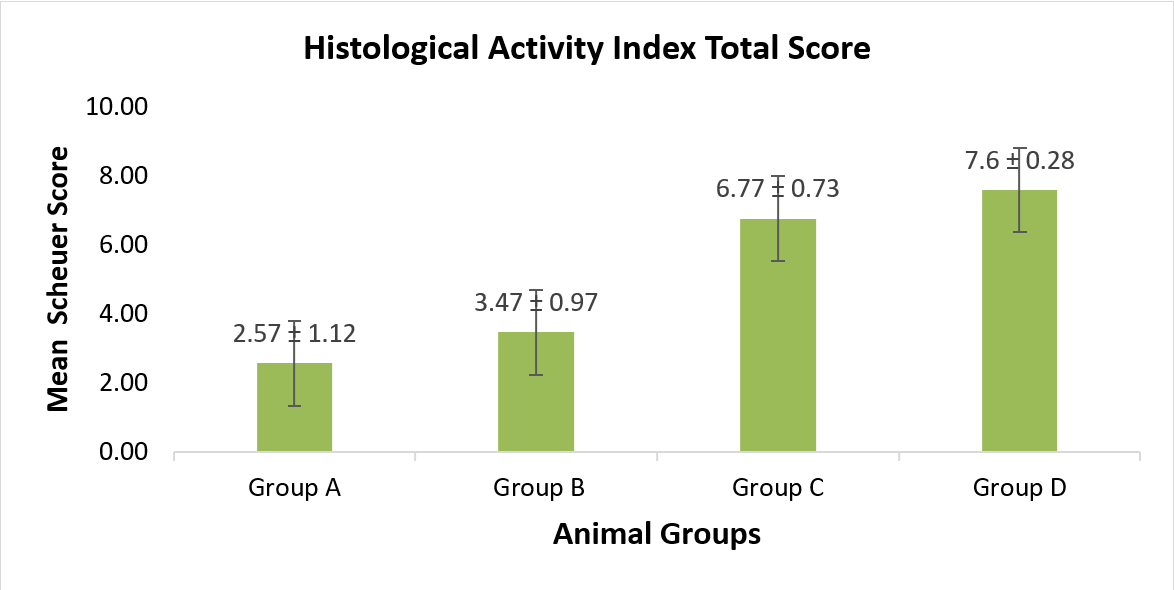
Figure 5. Mean histological activity index scores.
Group A (control); Group B (CMC 1% + 21 mg/kg/day extract), Group C (CMC 1% + 42 mg/kg/day extract) and Group D (CMC 1% + 84 mg/kg/day extract). Group C and group D were significantly different from group A and group B. (p<0.05; Post-hoc LSD).
Discussion
Based on the standard reference values, SGOT levels in this study increased only in group A. The mean SGOT level was 10.72 ± 53.5 IU/L, while the mean SGPT level was -0.73 ± 15.8 IU/L. Conversely, the treatment groups B, C, and D showed a decline in SGOT levels after being treated with the extract for 90 days. Further, the SGPT levels decreased in all treatment groups (B, C & D) compared to the reference value for group A (controls). Notably, groups B and D exhibited differences in SGPT levels. The results indicated that treatment groups B, C, and D experienced a decrease in SGOT levels after receiving the extract for 90 days.
The study results revealed a dose-dependent response for the extract, where the strength of hepatotoxic function was higher at lower doses, whereas the strength of hepatoprotective function was greater at higher doses. Pharmacodynamic changes occur in drugs with changes in dosage, as evident by non-linear dose and response relationships reported by several previous herbal drug studies (15, 16). Decreases in the means blood concentrations of SGOT and SGPT levels can also occur if hepatocyte damages accompany changes in the production capacity or progressive liver damages, such as necrosis and fibrosis (17). Extensive liver damages can reduce the levels of these enzymes, without increases in SGPT and SGOT enzymes. This may occur due to damages to hepatocytes that are increasingly widespread, reducing the production of SGPT and SGOT enzymes (18).
The study results indicated that Purwoceng root extract contains toxic compounds, and alkaloids and flavonoids are the most abundant in it. Alkaloids, especially berberine derivatives, have nephrotoxic effects and induce cell apoptosis, leading to an inability to maintain normal cellular function (19). Conversely, flavonoids inhibit prostaglandins synthesis, which have protective effects on the kidney, by modulating vascular tone while maintaining kidney homeostasis, leading to reduced kidney functions (20). In a previous sub-chronic study on Purwoceng extract for 28 days, significant increases in the blood urea and creatinine levels were reported in Wistar rats, proving that these levels can increase after the extract administration at certain doses (10).
In the current study, the highest elevation of mean urea levels was observed in group D, in which the animals received the highest dosage of the extract (84 mg/kg/day). It is important to note that feeding experimental animals can also affect the results, as protein elements in the animal feeds can impact the distribution of blood amino acids and plasma urea levels. In this study, the protein content in the animal feeds was 15%, whereas the ideal feed for rats should be at least 12% in proteins (21).
In the control group, it is possible that the increased urea-creatinine levels were due to the chronic stresses, which raise the sympathetic impulses that cause constriction of the afferent arterioles, decrease glomerular blood flow, and reduce the glomerular filtration rate (GFR) (22). The stressors might have been caused by collecting blood samples from sinus orbitalis and the probe treatment that was done every day (10). The urea levels increased in the groups given the extract at 21 and 42 mg/kg/day but lower than that of the control group, presumably due to the damages to the liver. Hepatic damages may cause decreased urea synthesis. Also, metabolic waste substances, such as urea may not be easily excreted due to kidney damages, thus, raising the blood urea levels (8).
The administration of Purwoceng extract had mixed effects on the urea and creatinine levels. While the creatinine levels declined, this reduction was not statistically significant for the urea level, which may have been influenced by the inflammatory reaction in the liver. This organ plays a major role in producing creatinine, so when liver function falls, the creatinine production also declines (10). In the group given the extract at 42 mg/kg/day, there was a significant increase in the creatinine levels, while at a dose of 84 mg/kg/day, the increase in creatinine levels was not as significant. These results suggest that the Purwoceng extract at higher doses may have more toxic effects on organs such as the liver and kidneys.
Based on our results, the chronic administration of the extract at 21 mg/kg was found to be safe, as there was no significant difference between group A (control) and group B in terms of liver and kidney damages. However, the administration of the extract at 42 and 84 mg/kg was found to be toxic, causing significant hepatic and renal damages compared to those of the controls. These results are consistent with those reported by previous studies [10, 11] that found that the extract at 42 mg/kg to be the minimally toxic to cause liver damages after the sub-chronic administration.
Considering our findings, liver damages that started at 42 mg/kg of the extract is likely influenced by several factors, such as the type of chemical compound, dosage, and chronic exposure. In the case of Purwoceng extract, the responsible chemicals that cause organ damages are likely to the alkaloids, flavonoids, and tannins (23). Flavonoids increase reactive oxygen species (ROS), which interact with cell membrane lipids to induce peroxidation and cause oxidative stress and inflammation in the liver (24). Flavonoids directly inhibit the activity of the CYP450 enzyme, which is responsible for the metabolic barriers and elimination of various compounds, resulting in the accumulation of xenobiotic substances and damages to liver cells (25). Conversely, Alkaloids reduce the antioxidant capacity of the liver by decreasing the glutathione synthesis (GSH) (26). Tannins cause endoplasmic reticulum degranulation, inhibit protein and RNA synthesis, release of RNA from the nucleoli and polysomes disaggregation in liver cells (27).
Based on the histological damage assessment, group A (control) and group B (21 mg/kg) showed minimal organ damages, and there was no significant difference between the two groups. This may be due to the hepatoprotective and antioxidant effects of flavonoid compounds present in the extract at the given dosage (28). At certain doses, the antioxidant compounds in the extract may improve the hepatic defense mechanism, thus counteracting hydroxyl and hydrogen peroxide free radicals via metabolizing lipid peroxides and preventing the lipid peroxidases accumulation (29). For instance, a dose of 100 mg/kg of flavonoids has been shown to reduce the biomarker enzymes of liver damages, such as SGOT, SGPT, and MDA, while increasing antioxidant activity in the liver by glutathione, superoxide dismutase, and catalase (30).
There was no statistically significant difference in liver damages between groups C and D, indicating that the chronic administration of Purwoceng extract at 42 or 84 mg/kg had similar effects, resulting in extensive liver damages. This may be explained by the toxicological principle of dose-response, i.e., small doses of xenobiotics do not cause toxic effects in experimental animals, but once the dosage reaches the maximal toxic threshold, a sigmoid dose-response curve is resulted. The curve changes imply that the toxic response caused by the administration of xenobiotics exceeding the threshold will be similar to the toxic response given at that threshold dosage (8). The chronic administration of high and repeated dosages of the ethanol extract can lead to irreversible hepatic damages, such as necrosis and fibrosis, making the liver cells lose their functions in synthesizing SGOT and SGPT enzymes, leading to a decrease in the enzymes’ levels.
Conclusions
The chronic administration of Purwoceng root extract at various doses for 90 days did not significantly increase SGOT and SGPT levels in white male rats. However, the blood urea levels increased starting at 21 mg/kg/day of the extract, while the creatinine levels increased at 84 mg/kg/day. Further, liver microanatomy was damaged at 42 mg/kg/day. The minimal toxic dosage for the chronic administration of Purwoceng root extract was 21 mg/kg/day. It was difficult to determine the safe chronic dose of the extract because even the control group experienced an increase in SGOT, SGPT, urea, and creatinine levels, although a dosage of 21 mg/kg did not cause any damages to the liver, based on results from the histological examinations.
Conflict of Interests
The authors had no conflict of interests with any entities whatsoever in conducting this study.
Funding
This study was funded by a basic research grant from Research and Community Service, Jenderal Soedirman University, Indonesia (Grant #:1067/UN.23/HK.02/2021).
Acknowledgements
The authors are grateful to the Head of Research and Community Services Jenderal Soedirman University for funding from the Fundamental Research Scheme, the Dean of Faculty of Medicine, the Head of Anatomy Departemen for the permission and laboratory facility, Alvita Mega Kumala, Annisa Farah Fadhilah, Fitria Nurlaely, Halimah Chairunnisa, Rizka Khairiza, and Teofilus Kristianto for helping during experiment and collecting data.
Compliance with Ethical Guidelines
Compliance with ethical guidelines: The protocol of animal care was carried out in accordance with animal care guidelines of the insitution and under a veterinarian’s supervision. The research received ethical approval from the Medical Research Ethics Commission, Faculty of Medicine, Jenderal Soedirman University no. 1647/KEPK/IV/2021.
Authors’ Contributions
Fitranto Arjadi designed, performed and supervised the experiments; prosessed, collected, analyzed and interpreted Data; prepared, edited and revised the several drafts of the manuscript. Ika Murti Harini, Alfi Muntafiah, and Setiawati performed the experiments, prosessed the samples, collected data, analysed and interpreted the data. Mulyoto Pangestu proof-read, edited and revised the references. All authors reviewed and approved the final version of the manuscript prior to submission to this journal.
References
1. Drug and Food Control Agency. Regulation of the Head of the Drug and Food Control Agency of the Republic of Indonesia No.7/2014 about Guidelines for In Vivo Nonclinical Toxicity Testing. Indonesia2014.
2. Research Institute for Medicinal and Aromatic Plants/Balai Penelitian Tanaman Obat dan Aromatik(Balittro). Phytochemical Test Results of Purwoceng Root Laboratorium Balai Penelitian Tanam Obat dan Aromatik Bogor.2011.
3. Arjadi F, Soejono S, Pangestu M. Paradoxical sleep deprivation decreases serum testosterone and Leydig cells in male rats. Universa Medicina. 2014; 33(1):27-35.
4. Saghir S, Dorato M. Role of Absorption, Distribution, Metabolism, Excretion, and Systemic Dose in Toxicology Testing In book. Reference Module Biomedical Sciences. 2017. [doi: 10.1016/B978-0-12-801238-3.64223-X]
5. Blessing O, Nelson E. Hepatotoxic Effect of Aflatoxin-Contaminated Agro Feeds (Groundnut, Maize & Melon Seed) on Wistar Albino Rats. Agricalture Biology Science Journal. 2015; 1(5):190-6.
6. Nualkaew S, Padee P, Chusri T. Hypoglycemic activity in diabetic rats of stigmasterol and sitosterol-3-O--D-glucopyranoside isolated from Pseuderanthemum palatiferum (Nees) Radlk. leaf extract. Journal of Medical Plantet Researches. 2015; 9(20):629-35. [doi: 10.5897/JMPR2014.5722]
7. Hsu YW, Tsai CF, Chen WK, Huang CF, Yen CC. A subacute toxicity evaluation of green tea (Camellia sinensis) extract in mice. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2011; 49(10):2624-30. [doi: 10.1016/j.fct.2011.07.007] [pmid: 21771628]
8. OECD. Test No. 452: Chronic Toxicity Studies, OECD Guidelines for the Testing of Chemicals. In: OECD. Paris: Paris2018.
9. Arjadi F, Kurniawan D, Wibowo Y, Siswandari W, Rujito L. Acute Toxicity Tests of Purwoceng (Pimpinella pruatjan Molk.) Ethanolic Extract on Male Albino Rat by Determined Hepatorenal Function Test and Histopathology. Molekul. 2019; 14(2):117-25. [doi: 10.20884/1.jm.2019.14.2.542]
10. Arjadi F, Gumilas N, Harini I, Indriani V, Rujito L. The Hepatotoxic and Nephrotoxic Effects of Purwoceng (Pimpinella pruatjan Molk.) Roots Ethanol Extract Administration in Sub-chronic Dose. Molekul. 2021; 16(2):163-9. [doi: 10.20884/1.jm.2021.16.2.729]
11. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. Journal of hepatology. 1991; 13(3):372-4. [doi: 10.1016/0168-8278(91)90084-o] [pmid: 1808228]
12. Huang X, Choi Y, Im H, Yarimaga O, Yoon E, Kim H. Aspartate Aminotransferase (AST/GOT) and Alanine Aminotransferase (ALT/GPT) Detection Techniques. Sensor (Switzerland). 2006; 6(7):756-82. [doi: 10.3390/s6070756]
13. González-Estrada E, Villaseño JAAPR. Shapiro-Wilk test for multivariate skew-normality. Computational Statistics. 2022; 37:1985-2001. [doi: 10.1007/s00180-021-01188-y]
14. Wang Y, Tang M, Wang P, Liu BTR. The Levene test based-leakage assessment. Integration. 2022; 87:182-93. [doi: 10.1016/j.vlsi.2022.06.013]
15. Sahota T, Danhof M, Della Pasqua O. Pharmacology-based toxicity assessment: towards quantitative risk prediction in humans. Mutagenesis. 2016; 31(3):359-74. [doi: 10.1093/mutage/gev081] [pmid: 26970519]
16. Salahudeen MS, Nishtala PS. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society. 2017; 25(2):165-75. [doi: 10.1016/j.jsps.2016.07.002] [pmid: 28344466]
17. Venkatesh SK, Torbenson MS. Liver fibrosis quantification. Abdominal radiology. 2022; 47(3):1032-52. [doi: 10.1007/s00261-021-03396-y] [pmid: 35022806]
18. Hayes A. Principles and methods of toxicology. 5th ed. New York: CRC Press2007.
19. Yi J, Ye X, Wang D, He K, Yang Y, Liu X, et al. Safety evaluation of main alkaloids from Rhizoma Coptidis. Journal of ethnopharmacology. 2013; 145(1):303-10. [doi: 10.1016/j.jep.2012.10.062] [pmid: 23159469]
20. Xu XL, Yang LJ, Jiang JG. Renal toxic ingredients and their toxicology from traditional Chinese medicine. Expert opinion on drug metabolism & toxicology. 2016; 12(2):149-59. [doi: 10.1517/17425255.2016.1132306] [pmid: 26670420]
21. Wicaksono M, Rimbawan R, K. Evaluation of Liver and Kidney Functions of Sprague-Dawley Female Rats (Rattus norvegicus) on Jamu Galohgor Administration with Multilevel Doses.Institut Pertanian Bogor2010.
22. Sata Y, Head GA, Denton K, May CN, Schlaich MP. Role of the Sympathetic Nervous System and Its Modulation in Renal Hypertension. Frontiers in medicine. 2018; 5:82. [doi: 10.3389/fmed.2018.00082] [pmid: 29651418]
23. Wahyuningrum R, Utami PI, Dhiani BA, Kumalasari M, Kusumawardani RS. Screening of Potential Free Radicals Scavenger and Antibacterial Activities of Purwoceng (Pimpinella alpina Molk). Tropical life sciences research. 2016; 27(supp1):161-6. [doi: 10.21315/tlsr2016.27.3.22] [pmid: 27965755]
24. Purwita A, Indah N, Trimulyono G. Use of Srikaya Leaf Extract (Annona Squamosa) as In Vitro Control of Fusarium Oxysporum Fungus. Lentera Bio Berk Ilm Biol. 2013; 2(2):179-83.
25. Jabeen E, Janjua N, Ahmed S, Ali T, Murtaza I, Ashraf Z. Antioxidant Activity and Hepatotoxicity of Flavonoids and Their Metal Complexes Through Co-Administration of β-Cyclodextrin. Chemistry Select. 2019; 4(32):9420-32. [doi: 10.1002/slct.201902124]
26. Kyselova Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdisciplinary toxicology. 2011; 4(4):173-83. [doi: 10.2478/v10102-011-0027-5] [pmid: 22319251]
27. Neuman MG, Cohen L, Opris M, Nanau RM, Hyunjin J. Hepatotoxicity of Pyrrolizidine Alkaloids. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2015; 18(4):825-43. [doi: 10.18433/j3bg7j] [pmid: 26626258]
28. Kifayatullah M, Mustafa M, Senguptha P, Sarker M, Das A, Das S. Evaluation of The Acute and Sub-acute Toxicity of The Ethanolic Extract of Pericampylus glaucus (Lam.) in Merr. in BALB/c Mice. Journal of Acute Disease. 2015; 4(4):309-15. [doi: 10.1016/j.joad.2015.06.010]
29. Chen Y, Dong H, Thompson DC, Shertzer HG, Nebert DW, Vasiliou V. Glutathione defense mechanism in liver injury: insights from animal models. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013; 60:38-44. [doi: 10.1016/j.fct.2013.07.008] [pmid: 23856494]
30. Chukwuma ER, Goodness Chiamaka J. Ameliorative Effect of the Flavonoid Rich Fraction of Monodora myristica (Gaertn) Dunel Seed Extract against Carbon Tetrachloride-Induced Hepatotoxicity and Oxidative Stress in Rats. Biochemistry and Pharmacology. 2017; 6(3). [doi: 10.4172/2167-0501.1000232]
Type of Study:
Research |
Subject:
General













































