1. Silva M, Lidon F. Food preservatives - An overview on applications and side effects. Emirates Journal of Food and Agriculture. 2016;28(6). [
DOI:10.9755/ejfa.2016-04-351]
2. Noorafshan A, Asadi-Golshan R, Monjezi S, Karbalay-Doust S. Sodium metabisulphite, a preservative agent, decreases the heart capillary volume and length, and curcumin, the main component of Curcuma longa, cannot protect it. Folia Biol (Praha). 2014;60(6):275-80.
3. Sadowska B, Sztormowska M, Gawinowska M, Chelminska M. Sodium metabisulfite hypersensitivity in urticaria. Our Dermatology Online. 2021;12(2):106-12. [
DOI:10.7241/ourd.20212.2]
4. Lavoie JC, Lachance C, Chessex P. Antiperoxide activity of sodium metabisulfite. A double-edged sword. Biochem Pharmacol. 1994;47(5):871-6. [
DOI:10.1016/0006-2952(94)90487-1] [
PMID]
5. Mishra L, Yadav B. Assessing the Cytotoxic and Genotoxic Effects of Sodium Metabisulphite: A Food Preservative. Vegetos. 2011;24(1):32-7.
6. Kadi FZE, Bénali AI, Bénali M, Belbraouet S. Effect of Sodium Metabisulphite on Blood Metabolic Status of Wistar Rats. Food and Nutrition Sciences. 2014;05(15):1529-37. [
DOI:10.4236/fns.2014.515165]
7. Ercan S, Ozturk N, Celik-Ozenci C, Gungor NE, Yargicoglu P. Sodium metabisulfite induces lipid peroxidation and apoptosis in rat gastric tissue. Toxicol Ind Health. 2010;26(7):425-31. [
DOI:10.1177/0748233710369665] [
PMID]
8. Shekarforoush S, Ebrahimi P, Fathabad AA, Farzanfar E. Effect of Sodium Metabisulfite on Oxidative Stress and Lipid Peroxidation Biomarkers. Current Nutrition & Food Science. 2020;16(1):114-7. [
DOI:10.2174/1573401314666181024130333]
9. Naureen I, Saleem A, Nawaz A, Ghaffar I, Maqbool I. Study The Harmful Effects Of Sodium Metabisulfite On Liver, Testes, And Lipid Peroxidation In Male Mice, (Mus Musculus). International Journal of Engineering Applied Sciences and Technology. 2021;6(5). [
DOI:10.33564/IJEAST.2021.v06i05.004]
10. Gibson EK, Mahdy HM. Anatomy, Abdomen and Pelvis, Ovary: StatPearls Publishing; 2023.
11. Zangos S, Marquart F. Female Reproductive System. In: Vogl T, Reith W, Rummeny E, editors. Diagnostic and Interventional Radiology2016. p. 929-55. [
DOI:10.1007/978-3-662-44037-7_32]
12. Gasner A, P AA. Physiology, Uterus. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Aatsha P A declares no relevant financial relationships with ineligible companies.2024.
13. Bancroft JD, Layton C. The hematoxylins and eosin. Bancroft's Theory and Practice of Histological Techniques. 8th ed2019. p. 126-38. [
DOI:10.1016/B978-0-7020-6864-5.00010-4]
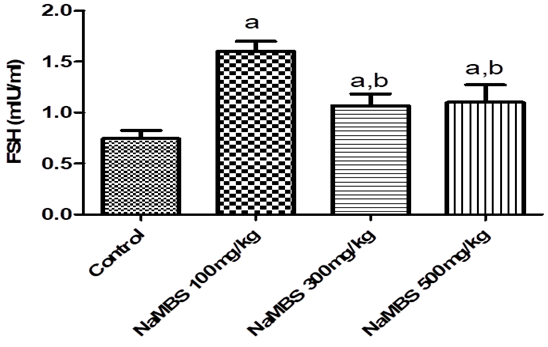
14. Padmanabhan V, Karsch FJ, Lee JS. Hypothalamic, pituitary and gonadal regulation of FSH. Reprod Suppl. 2002;59:67-82.
15. Das N, Kumar TR. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J Mol Endocrinol. 2018;60(3):R131-R55. [
DOI:10.1530/JME-17-0308] [
PMID] [
]
16. Chainy GBN, Sahoo DK. Hormones and oxidative stress: an overview. Free Radic Res. 2020;54(1):1-26. [
DOI:10.1080/10715762.2019.1702656] [
PMID]
17. Shen M, Jiang Y, Guan Z, Cao Y, Sun SC, Liu H. FSH protects mouse granulosa cells from oxidative damage by repressing mitophagy. Sci Rep. 2016;6:38090. [
DOI:10.1038/srep38090] [
PMID] [
]
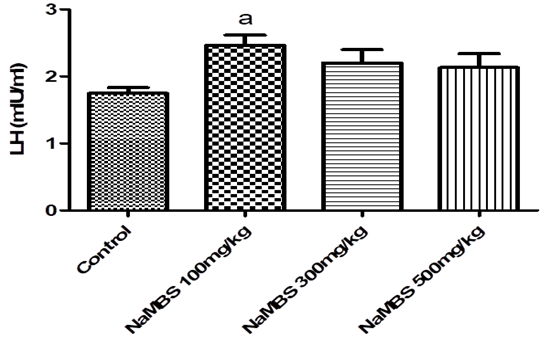
18. Nedresky D, Singh G. Physiology, Luteinizing Hormone. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Gurdeep Singh declares no relevant financial relationships with ineligible companies.2024.
19. Lincoln GA, Fraser HM. Negative feedback regulation of pulsatile LH secretion during treatment with an LHRH antagonist in rams. J Androl. 1990;11(3):287-92. [
DOI:10.1002/j.1939-4640.1990.tb03242.x]
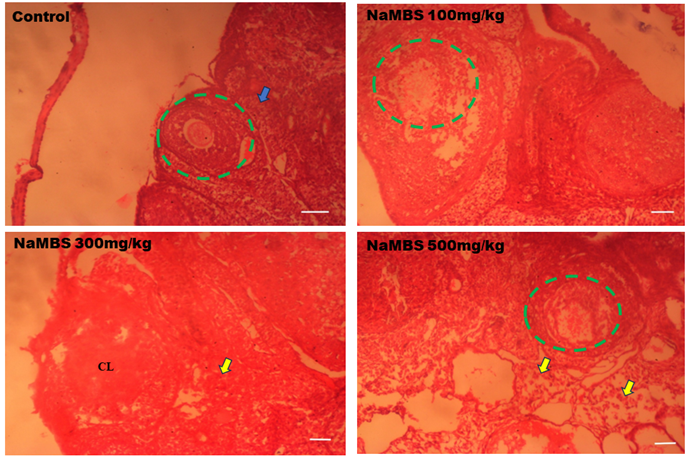
20. Rezaee N, Nematollahi Z, Shekarforous S, Hoseini E. Effect of Sodium Metabisulfite on Rat Ovary and Lipid Peroxidation. Iranian Journal of Toxicology. 2016;10(2):23-8. [
DOI:10.32598/IJT.10.2.316.1]
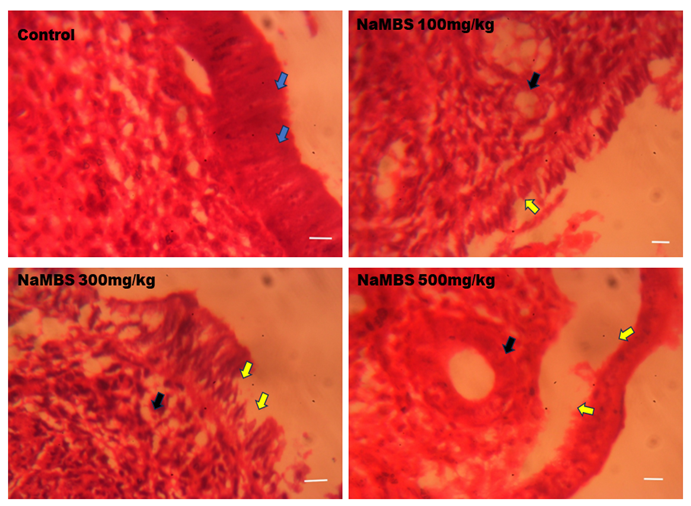
21. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18(12):1754-67. [
DOI:10.1038/nm.3012] [
PMID] [
]
22. Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22(1):104-15. [
DOI:10.1093/humupd/dmv044] [
PMID] [
]
23. Tabatabaei Shafiei M, Carvajal Gonczi CM, Rahman MS, East A, Francois J, Darlington PJ. Detecting glycogen in peripheral blood mononuclear cells with periodic acid schiff staining. J Vis Exp. 2014(94). [
DOI:10.3791/52199] [
PMID] [
]
24. Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS. Glycogen and its metabolism: some new developments and old themes. Biochem J. 2012;441(3):763-87. [
DOI:10.1042/BJ20111416] [
PMID] [
]
25. Daghlas SA, Mohiuddin SS. Biochemistry, Glycogen. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Shamim Mohiuddin declares no relevant financial relationships with ineligible companies.2024.
26. Zhang Q, Bai Y, Yang Z, Tian J, Meng Z. The molecular mechanisms of sodium metabisulfite on the expression of K ATP and L-Ca2+ channels in rat hearts. Regul Toxicol Pharmacol. 2015;72(3):440-6. [
DOI:10.1016/j.yrtph.2015.05.021] [
PMID]
27. Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. [
DOI:10.1155/2014/360438] [
PMID] [
]
28. Amedu NO, Omotoso GO. Influence of Vitexin on ataxia-like condition initiated by lead exposure in mice. Toxicology and Environmental Health Sciences. 2020;12(4):305-13. [
DOI:10.1007/s13530-020-00041-x]