BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijt.arakmu.ac.ir/article-1-805-en.html


 , Reza Kheirandish2
, Reza Kheirandish2 

 , Sepideh Heidari3
, Sepideh Heidari3 
 , Mansour Mirtadzadini4
, Mansour Mirtadzadini4 
 , Amir Asadi3
, Amir Asadi3 
 , Navid Hassanabadi2
, Navid Hassanabadi2 
 , Fariba Sharififar *5
, Fariba Sharififar *5 


2- School of Veterinary Medicine, Shahid Bahonar University of Kerman, Kerman, Iran.
3- Herbal and Traditional Medicine Research Center, Kerman University of Medical Sciences, Kerman, Iran.
4- Department of Biology, Faculty of Sciences, Shahid Bahonar University of Kerman, Kerman, Iran.
5- Herbal and Traditional Medicine Research Center, Kerman University of Medical Sciences, Kerman, Iran. ,
Introduction
Medicinal plants have been widely used for therapeutic purposes especially in developing countries mostly because of accessibility, effectiveness and high acceptance by the populations [1]. Most people who use medicinal plants to meet their needs are unaware of their adverse side effects and mistakenly believe that all medicinal plants are completely free of toxicity. Also due to the antioxidant properties of medicinal plants [2, 3], people in various nations have found these natural sources useful in the treatment or prevention of many diseases. However, there is evidence to suggest that severe adverse effects for medicinal plants exist. Examples include hypertension due to Ephedra, hepatotoxicity due to pyrrolizidine alkaloids, coltsfoot plant, comfrey, and acute renal failure due to the use of chamomile, aloe and Glycyrrhizaglabra, leading to excess cortisol release [4, 5]. Thus, further studies are warranted on the toxicity of medicinal plants to safeguard against the toxic risks [6]. Liver and kidneys are the organs mostly prone to injury from many drugs due to their specific physiological and anatomical structure, and their vital role in metabolism and detoxification processes [7].
Seeds from Glycine max L. (G. max), known as soybeans from Leguminosae family, are rich sources of various nutrients and phytochemicals. This plant offers health promotion effects and decreases the risk of cancer and osteoporosis, while protecting against cardiovascular disease [8]. Soybean-based diets reduce the blood levels of total cholesterol, LDL cholesterol and triglycerides in humans [9]. Also, consuming soybean is believed to improve learning impairment [10, 11]. Further, Salvia rhytidea Benth (S. rhytidea) is one of the Salvia species which has been widely available in Kerman province in southeastern Iran. The branches and leaves of this plant are known to have antifungal, anti-cholinesterase, anti-leishmania and anti-herpes properties [12-15]. The efficacy of the plant on the serum glucose and alpha amylase inhibition supports its anti-diabetic role in traditional medicine in Iran [16, 17]. Also, various species of Salvia have been used for memory enhancement in traditional medicine in Europe [18].
We have previously reported the antioxidant and anti-cholinesterase properties of the methanolic extracts of G. max and S. rhytidea [13, 19]. As an extension to that work, we conducted this study to further investigate the toxicity of the extracts of these plants on the liver, kidneys and blood parameters of mice. Interestingly, our preliminary findings suggest that the extracts did not induce functional or pathological alterations in the mice liver and kidneys.
Materials and Methods
Plant materials: The S. rhytidea aerial parts were collected from Bidkhoon area in Kerman province and soybean was grown in the botanical gardens of the Faculty of Pharmacy, Kerman University of Medical Sciences. The plants were air-dried in the shade and kept in a dark and cold room until the experiments were conducted. The materials from both plants were authenticated by an expert in the Department of Pharmcognosy, where a certificate was issued for the two plant samples, KF1249 and KF1251, respectively.
Extraction process: A sample of S. rhytidea and soybean (200 g) each were extracted with 70% methanol by percolation method. The extracts were evaporated under vacuum and dried in an oven at 40°C. The extracts were used combined with 10% dimethylsulfoxide (DMSO), dissolved in normal saline (v/v) and 0. 5% CMC (w/v), respectively.
Total Flavonoid Content: The Total Flavonoid Contents (TFC) of the plants were determined as established by previous studies [20, 21]. Biefly, genistin and rutin were used as standards for the TFC determination, using thin layer chromatography fingerprinting. Five serial concentrations were prepared from rutin and genistein separately (25, 50, 100, 200 or 300 ppm). A volume of 2ml of the stock solution was mixed with 2 mL of 2% aluminum chloride and was incubated for 30 min at room temperature. The absorbance of the solutions was read against the blank at 247nm and 381.3 nm for rutin and genistein concentrations, respectively, by a spectrophotometer (Perkin, Elmer, Germany). The total flavonoid content of the plant was calculated from the slope of calibration curve and expressed as gram of rutin or genistein equivalents in 100 g of dried plant extract. All experiments were carried out in triplicate.
Acute toxicity: Eleven groups of six animals each, weighing 25±3g each were treated with varying doses of the extract of S. rhytidea or soybean at doses of 250, 500, 1000 or 2000 mg/kg. The animals were observed for 24 hours and the morbidity and mortality were monitored for up to 24 hours [22].
Toxicity studies: A total of 66 adult male NMARI mice weighing 25-30 grams each were randomly divided into the control or experimental groups. They were kept at the animal house in plastic cages under standard laboratory conditions of 12 hours of light and dark cycles at a room temperature of 25±2°C with free access to food and water. The experimental protocol was approved by the university committee for animal care. Animals were acclimatized for a week before the experiments began.
Experimental groups: The animals in groups 1 to 11 received plant extract (IP for 7 consecutive days) as shown in Table 1. The study protocol was approved by the Ethics Committee of Kerman University of Medical Sciences (Registration ID: IR. KMU. REC.1391.533).
Sample collection and pathological studies: The animals were fasted after the last extract administration and sacrificed under ether anesthesia. The blood samples were collected by cardiac puncture and allowed to stand for one hour, then centrifuged at 4000 rpm for 10 min. The serum samples were separated, transferred to micro-tubes and used for blood biochemical assays. For pathological examinations, the liver and left kidney were removed and immediately washed in normal saline, dried with filter paper and weighed accurately. The organs were fixed in 10% formalin for the histopathological examinations.
Statistical analyses: We performed statistical analyses of the data (ANOVA), using SPSS software, v. 15. The statistical differences at a P≤0. 05 were considered significant. The data were expressed as Means±SD (SE).
Results
The estimated total flavonoid contents of S. rhytidea and soybean extracts were 1. 23% (rutin equivalent flavonoid/g of dried S. rhytidea extract) and 1. 16% (genistin equivalent flavonoid/ g of dried soybean extract), respectively.
Acute toxicity studies: No mortality was recorded up to 2 g/kg for both extracts in any experimental group of rats. Injection of higher doses in this study was not feasible due to the extract solubility problem.
Changes in body and organ weights: No significant changes was observed in body weight and that of the liver and left kidney of the animals treated with S. rhytidea or soybean extract compared to those of the control group (data no shown; P>0. 05).
Blood parameters: The data representing liver enzymes, such as ALT, AST, and ALP showed no significant differences in the mice administered with S. rhytidea (doses: 100, 200 or 400 mg/kg) compared to those for the control group. Varying doses of soybean extract administered IP caused no significant changes in the above parameters (P>0. 05). The creatinine and BUN values in mice treated with S. rhytidea or soybean were not significantly different from those of the control group (P>0. 05) (Table 2).
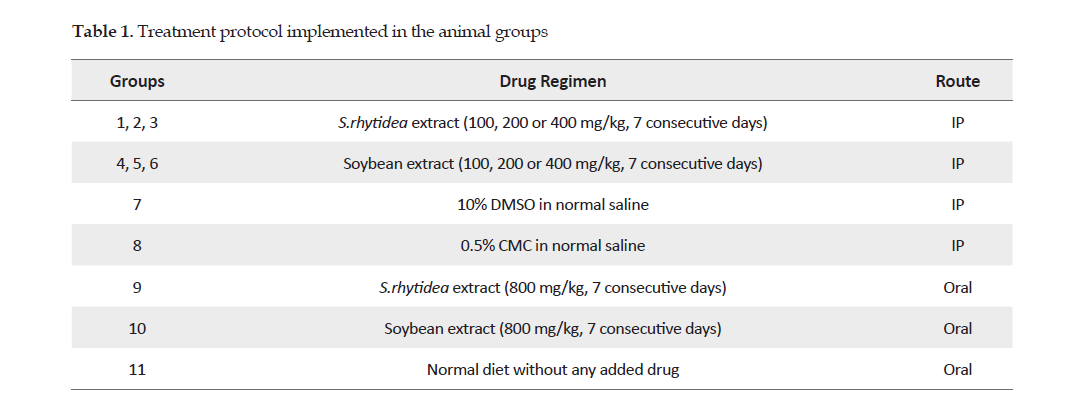
Microscopic examinations: The microscopic examinations of the liver and kidney samples revealed no pathological and tissue lesions in mice treated with S. rhytidea extract at 100 or 200 mg/kg. However, some mild cellular lesions were observed, such as small vacuoles in the cytoplasm of hepatocytes. Also, infiltration of lymphocytes and plasma cells was observed within the portal and pre-portal areas plus thickening of liver capsule at the extract dose of 400 mg/kg. Similarly, the oral dose of 800 mg/kg of S. rhytidea caused vacuolization in the hepatocytes’ cytoplasm, especially around the central vein. There was mild infiltration of mononuclear inflammatory cells into the hepatic parenchyma (Figure 1).
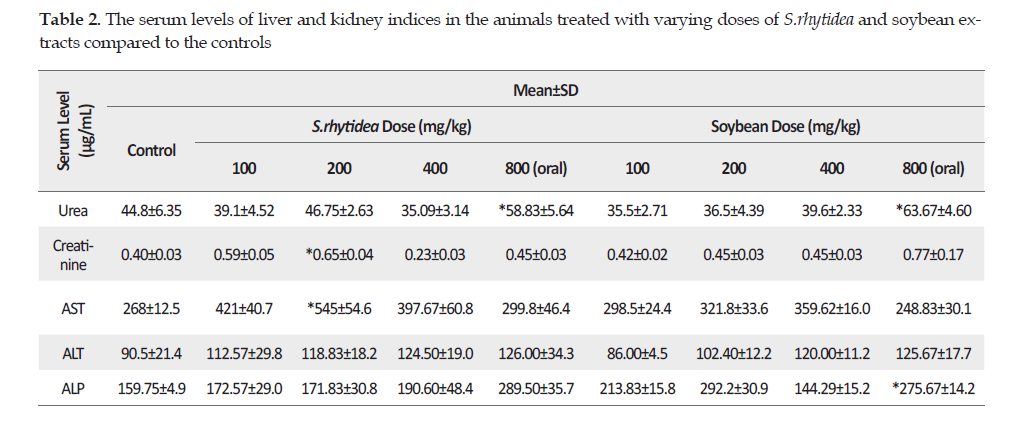
The histological examinations of the kidney tissue samples from the mice treated with S. rhytidea extract revealed no pathological alterations at any of the doses of 100, 200 or 400 mg/kg (IP). However, the animals were affected by the oral dose of this extract at 800 mg/kg, causing hyperemia and degeneration of proximal kidney lobules. Also, mild glomerular atrophy and dilation of urinary spaces were observed (Figure 2).
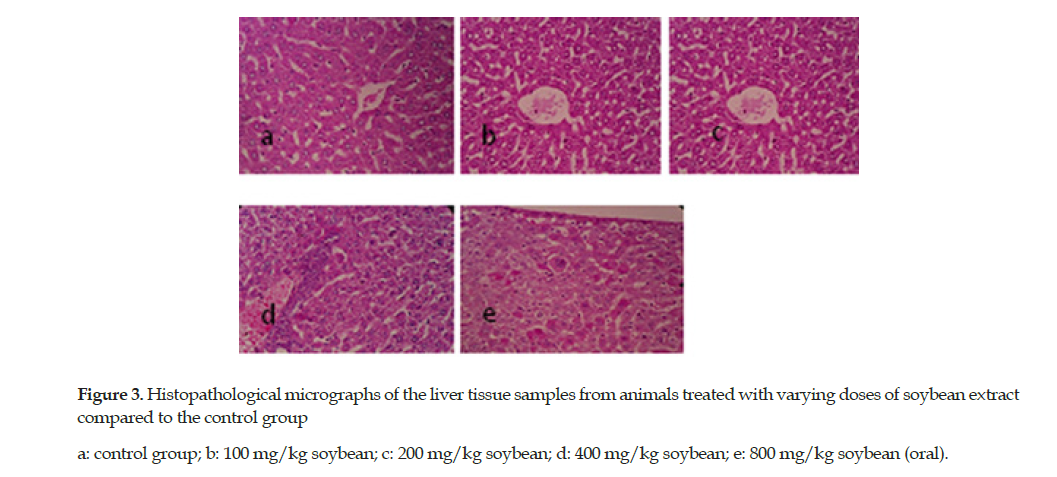
The soybean extract at a dose of 400 mg/kg exhibited vacuolization of hepatocytes and mild infiltration of mononuclear inflammatory cells within the portal vein area. This extract caused mild degeneration of hepatocytes and focal infiltration of mononuclear inflammatory cells at an oral dose of 800 mg/kg. The histological features of liver appeared normal at the extract doses of 100 and 200 mg/kg (Figure 3).
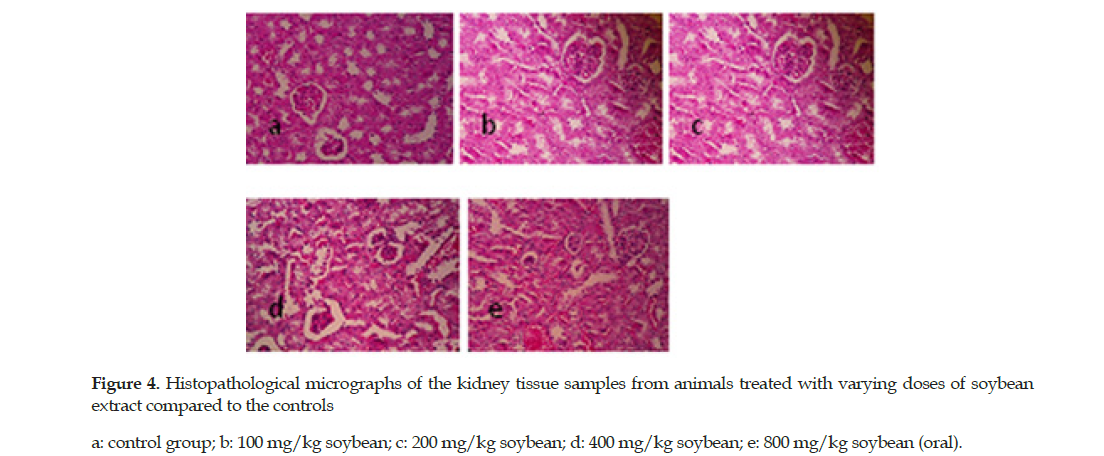
The soybean extract also caused mild degenerative changes in proximal lobules and dilation of urinary spaces at 400 mg/kg (IP) and 800 mg/kg (oral). No microscopic lesions in the tissue samples were detected in the mice treated with the extract dose of 100 or 200 mg/kg (Figure 4).
Discussion
Prior evidence: Evaluation of the acute toxicity and determination of LD50 are the primary steps in the toxicological evaluation of unknown compounds, especially those derived from medicinal plants. The S. rhytidea and soybean extracts induced no mortality up to 2 g/kg of the mice’s body weight. The evaluation of higher doses of the extracts was not feasible due to the solubility issue. We have previously reported the acute toxicity of other plant extracts and essential oils along with their safety margins [22-24]. We believe that the beneficial aspects are largely attributable to the antioxidant effects [13, 25-28].
Liver and kidneys are vital organs of the body, playing important roles in the metabolic and detoxification processes. Among other vital functions, liver neutralizes toxins, and kidneys are responsible for maintaining the body’s homeostasis through excretion of toxic substances [29]. Oxidative stresses induce hepatic damages by inhibiting various cellular functions and promoting free radical generation. These can lead to various hepatic disorders, such as alcoholic liver diseases, cirrhosis and chronic hepatitis. In addition, there is evidence to suggest that in chronic kidney disease, the renal energy loss and uremia are associated with abnormal oxidative stresses, causing inflammatory processes and renal damages [30, 31].
Safety of the extracts: In this study, two plant extracts from S. rhytidea and soybean were tested in mice, but caused no mortality up to 2 g/kg of the animals. None of the oral or IP doses of S. rhytidea and soybean extracts at the tested doses induced significant changes in the serum enzymes’ levels compared to those for the control group (Table 2). Increases in the ALT and AST values, indicative of disturbance in the integrity of cell membranes, would represent damages to the hepatocytes [32, 33]. The value of ALP was not significantly different in animals treated with varying doses of S. rhytidea and soybean extracts. However, as observed through microscopic examinations, S. rhytidea extract at doses of 400 mg/kg (IP) and 800 mg/kg (oral) caused small cellular lesions, such as vacuolization of the cytoplasm in the hepatocytes and inflammatory cells infiltration in the liver tissue. Soybean also caused mild degenerative changes in the hepatocytes at doses of 400 mg/kg (IP) and 800 mg/kg (oral). These findings indicated that the toxic effects of soybean extract were mostly structural rather than functional. These changes were not considerable but might become more pronounced with the chronic use of either extract, the study of which was not the focus of this study.
Effects on liver function: The serum levels of ALT and AST did not change significantly, ruling out major hepatocellular damages. The kidney parameters, such as BUN and creatinine, also exhibited no significant alterations in the mice treated with either S. rhytidea or soybean extract compared to those observed for the control group. Increases in creatinine is indicative of impaired renal function [34], which did not develop following the administration of the extracts in this study. Results from our microscopic examinations demonstrated that the soybean extract at dose of 400 mg/kg (IP) and 800 mg/kg (oral) caused only mild degeneration of proximal lobules and dilation of urinary spaces in the kidneys. Obviously, a panel of biomarkers was needed to do more accurate assessments of liver and kidney safety margines. However, there are several similar reports for other species of S. rhytidea and soybean. For instance, in a study on human subjects, normal drinking water was replaced with Salvia officinalis tea for 14 days and the results indicated no pathological changes in the liver enzymes of the participants compared to those of the control group [35]. In another study, three doses of S. officinalis increased the serum levels of abumin, creatinine and proteins but significantly decreased the liver enzymes levels [36]. Also in a clinical trial, soy supplementation significantly caused a decrease in the ALT values compared to the controls (casein group), consisting of patients with chronic hepatitis C [37].
Effects on kidney function: There are controversies about the efficacy of the soybean protein in patients with chronic kidney disease. In a study based on soy protein, decreases were observed in the kidney cyst volumes and the fluid contents. The serum creatinine levels were normalized by the plant too [38]. In another study, Salvia miltiorrhiza (danshen), a different species of Salvia, the serum urea nitrogen level, malondialdehyde and creatinine were significantly reduced in iron-overload animals that received this plant [39]. This plant also increased the activity of both superoxide dismutase and glutathione peroxidase. The pathogical changes in the kidneys were ameliorated by S. miltiorrhiza based on the histopathological findings [39]. To our knowledge, this is the first report on both the hepatotoxicity and nephrotoxicity of these two extracts. Elucidation of the significance of the findings reported in the current study awaits future research.
Limitations of the study: This preliminary study was limited to using a small number of animals due to the ethical consideration and to minimize animal suffering. Also, we could not test a panel of biomarkers due to limited laboratory facilities. The biomarkers panel would enable us to present a more accurate assessment of the bio-safety aspects of S. rhytidea and soybean extracts in relation to the liver and kidney functions.
Conclusions
The findings of this study suggest that there were no functional disorders caused in the liver and kidneys, and no signs of pathological and structural changes observed in the animals treated with either S. rhytidea or soybean extract. These effects may be attributed to the antioxidant properties of the extracts. However, this assertion needs be verified by future studies to accurately characterize the probable toxicity on the liver and kidneys, which may be the case particularly with the chronic use of the extracts.
Ethical Considerations
Compliance with ethical guidelines
The protocol of this study was approved by the University’s Committee on Research Ethics and Standards.
Funding
This paper was extracted from the Pharm. D. thesis of Dr. Sepideh Heidari, conducted at the Herbal and Traditional Medicine Research Center, Kerman University of Medical Sciences, Kerman, Iran.
Author's contributions
All authors were equally contributed in preparing this article.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors wish to thank Vice Chancellor for Research of Kerman University of Medical Sciences for funding this study (Grant #: 90/244).
References
1.Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002; 20(12):522-31. [DOI:10. 1016/S0167-7799(02)02080-2]
2.Mandegary A, Soodi M, Sharififar F, Ahmadi S. Anticholinesterase, antioxidant, and neuroprotective effects of Tripleurospermum disciforme and Dracocephalum multicaule. J Ayurveda Integr Med. 2014; 5(3):162-6. [DOI:10. 4103/0975-9476. 140474] [PMID] [PMCID]
3.Abdel-Wahhab MA, Aly SE. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 2005; 25(3):218-23. [DOI:10. 1002/jat. 1057] [PMID]
4.Asif M. A brief study of toxic effects of some medicinal herbs on kidney. Adv Biomed Res. 2012; 1:44. [DOI:10. 4103/2277-9175. 100144] [PMID] [PMCID]
5.Calo LA, Zaghetto F, Pagnin E, et al. Effect of aldosterone and glycyrrhetinic acid on the protein expression of PAI-1 and p22 (phox) in human mononuclear leukocytes. J Clin Endocrinol Metab. 2004; 89(4):1973-6. [DOI:10. 1210/jc. 2003-031545] [PMID]
6.Rates S. Plants as source of drugs. Toxicon. 2001; 39(5):603-13. [DOI:10. 1016/S0041-0101(00)00154-9]
7.Vega-Villa KR, Takemoto JK, Yanez JA, et al. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008; 60(8):929-38. [DOI:10. 1016/j. addr. 2007. 11. 007] [PMID]
8.Suthar AC, Banavalikar MM, Biyani MK. Pharmacological activities of Genistein, an isoflavone from soy (Glycine max): Part II -- Anti-cholesterol activity, effects on osteoporosis & menopausal symptoms. Indian J Exp Biol. 2001; 39(6):520-5.
9.Taku K, Umegaki K, Sato Y, et al. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2007; 85(4):1148-56. [DOI:10. 1093/ajcn/85. 4. 1148] [PMID]
10.Khodamoradi M, Asadi-Shekaari M, Esmaeili-Mahani S, et al. Effects of hydroalcoholic extract of soy on learning, memory and synaptic plasticity deficits induced by seizure in ovariectomized rats. Basic Clin Neurosci. 2017; 8(5):395-403. [DOI:10. 18869/nirp. bcn. 8. 5. 395] [PMID] [PMCID]
11.Mandegary A, Sharififar F, Salari S. Anticholinesterase effect of soybean extract (Glycine max) in the brain of amnesic mice and its protective effects against beta-amyloid induced cytotoxicity in pc12 cells. Unique j. ayurvedic herb. med. 2014; 2(6):61-5.
12.Salari S, Bakhshi T, Sharififar F, et al. Evaluation of antifungal activity of standardized extract of Salvia rhytidea Benth. (Lamiaceae) against various Candida isolates. J Mycol Med. 2016; 26(4):323-30. [DOI:10. 1016/j. mycmed. 2016. 06. 003] [PMID]
13.Mehrabani M, Sharififar F, Hassanzade A, et al. Anticholinesterase and antioxidant activity of the essential oil and different fractions of Salvia rhytidea Benth. Inven Impact Ethnopharmacol. 2013; 14(1):93-7.
14.Ansari M, Sharififar F, Arabzadeh AM, et al. In vitro evaluation of anti-herpes simplex-1 activity of three standardized medicinal plants from Lamiaceae. Anc Sci Life. 2014; 34(1):33-8. [DOI:10. 4103/0257-7941. 150777] [PMID] [PMCID]
15.Sharifi F, Sharifi I, Pournamdari M, et al. Antileishmanial effect of coffea arabica, salvia rhytidea and bunium persicum against leishmania major and leishmania tropica promastigotes and their cytotoxicity and antioxidant activities. European Joural of Medicinal Plants. 2018; 22(3):1-10. [DOI:10. 9734/EJMP/2018/39364]
16.Mohamadi N, Fooladi S, Ansari Ml. Effect of Salvia rhytidea Benth. extract of serum glucose, gut alphaglucosidase in healthy and streptozocin-induced diabetic rats. Ayurvedic Herb Med. 2016; 2(2):40-42.
17.Gholamhoseinian A, Fallah H, Sharififar F. The inhibitory effect of some Iranian plants extracts on the alpha glucosidase. Iran J Basic Med Sci. 2008; 11(1):1-9.
18.Tildesley NT, Kennedy DO, Perry EK, Ballard CG, Wesnes KA, Scholey AB. Positive modulation of mood and cognitive performance following administration of acute doses of Salvia lavandulaefolia essential oil to healthy young volunteers. Physiol Behav. 2005; 83(5):699-709. [DOI:10. 1016/j. physbeh. 2004. 09. 010] [PMID]
19.Sharififar F, Moshafi MH, Shafazand E, Koohpayeh A. Acetyl cholinesterase inhibitory, antioxidant and cytotoxic activity of three dietary medicinal plants. Food Chem. 2012; 130(1):20-3. [DOI:10. 1016/j. foodchem. 2011. 06. 034]
20.Pournamdari M, Mandegary A, Sharififar F, Zarei G, Zareshahi R, Asadi A, et al. Anti-inflammatory subfractions separated from acidified chloroform fraction of fenugreek seeds (Trigonella foenum-graecum L.). J Diet Suppl. 2018; 15(1):98-107. [DOI:10. 1080/19390211. 2017. 1326431] [PMID]
21.Jamshidzadeh A, Shokri Y, Ahmadi N, Mohamadi N, Sharififar F. Quercus infectoria and Terminalia chebula decrease melanin content and tyrosinase activity in B16/F10 cell lines. J Pharm Pharmacogn Res. 2017; 5(5):270-7.
22.Mandegary A, Arab-Nozari M, Ramiar H, Sharififar F. Anticonvulsant activity of the essential oil and methanolic extract of Bunium persicum (Boiss). B. Fedtsch. J Ethnopharmacol. 2012; 140(2):447-51. [DOI:10. 1016/j. jep. 2012. 01. 024] [PMID]
23.Ansari-Dogaheh M, Sharififar F, Arabzadeh M, Shakibaie M, Heidarbeigi M. Inhibitory effect of a standard extract of Zhumeria majdae Rech.f. and Wendelbo. against Herpes simplex-1 virus. Wendelbo. Against herpes simplex-1 virus. J Med Sci. 2013; 13(8):755-60. [DOI:10. 3923/jms. 2013. 755. 760]
24.Mandegary A, Sharififar F, Abdar M. Anticonvulsant effect of the essential oil and methanolic extracts of Zataria multiflora Boiss. Cent Nerv Syst Agents Med Chem. 2013; 13(2):93-7. [DOI:10. 2174/1871524911313020001] [PMID]
25.Ahmadipour A, Sharififar F, Nakhaipour F, Samanian M, Karami-Mohajeri S. Hepatoprotective effect of Zataria multiflora Boisson cisplatin-induced oxidative stress in male rat. J Med Life. 2015; 8:275-81.
26.Ahmadipour A, Sharififar F, Pournamdari M, Bamkan AM, Hosseini A, Afrapoli FM, et al. Hepatoprotective effect of Zataria multiflora Boiss against malathion-induced oxidative stress in male rats. Orient Pharm Exp Med. 2016; 16(4):287-93. [DOI:10. 1007/s13596-016-0238-6]
27.Sharififar F, Derakhshanfar A, Dehghan-Nudeh G, Abbasi N, Abbasi R, Gharaei RR, et al. In vivo antioxidant activity of Zataria multiflora Boiss essential oil. Pak J Pharm Sci. 2011; 24(2):221-5. [PMID]
28.Yassa N, Sharififar F, Shafiee A. Otostegia persica as a source of natural antioxidants. Pharm Biol. 2005; 43(1):33-8. [DOI:10. 1080/13880200590903336]
29.Pandit A, Sachdeva T, Bafna P. Drug-induced hepatotoxicity: A review. J Appl Pharm Sci. 2012; 2(5):233-43. [DOI:10. 7324/JAPS. 2012. 2541]
30.Sikka S, Rajasekaran M, Hellstrom W. Role of oxidative stress and antioxidants in male infertility. J Androl. 1995; 16(6):464-8. [PMID]
31.Vostalova J, Galandakova A, Strebl P, et al. Oxidative stress in kidney disease patients. Vnitr Lek. 2012; 58(3):202-7.
32.Gowda S, Desai PB, Hull VV, Math AA, Vernekar SN, Kulkarni SS. A review on laboratory liver function tests. Pan Afr Med J. 2009; 3.
33.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000; 342(17):1266-71. [DOI:10. 1056/NEJM200004273421707] [PMID]
34.Couchoud C, Pozet N, Labeeuw M, Pouteil-Noble C. Screening early renal failure: Cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int. 1999; 55(5):1878-84. [DOI:10. 1046/j. 1523-1755. 1999. 00411. x] [PMID]
35.Lima CF, Andrade PB, Seabra RM, Fernandes-Ferreira M, Pereira-Wilson C. The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J Ethnopharmacol. 2005; 97(2):383-9. [DOI:10. 1016/j. jep. 2004. 11. 029] [PMID]
36.Arabi S, Arshami J, Haghparast A. Effects of Salvia officinalis L. extracton biochemical blood parameters in male rat. J Advances in Med Biomed Res. 2014; 22(94):34-43.
37.Oliveira LP, de Jesus RP, Boulhosa RS, Mendes CM, Gnoatto MC, Lemaire DC, et al. Effect of soy protein supplementation in patients with chronic hepatitis C: A randomized clinical trial. World J Gastroenterol. 2012; 18(18):2203-11. [DOI:10. 3748/wjg. v18. i18. 2203] [PMID] [PMCID]
38.Aukema HM, Gauthier J, Roy M, Jia Y, Li H, Aluko RE. Distinctive effects of plant protein sources on renal disease progression and associated cardiac hypertrophy in experimental kidney disease. Mol Nutr Food Res. 2011; 55(7):1044-51. [DOI:10. 1002/mnfr. 201000558] [PMID]
39.Guan S, Ma J, Zhang Y, Gao Y, Zhang Y, Zhang X, et al. Danshen (Salvia miltiorrhiza) injection suppresses kidney injury induced by iron overload in mice. PLoS One. 2013; 8(9):e74318. [DOI:10. 1371/journal. pone. 0074318] [PMID] [PMCID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |