Introduction
Ethanol is a psychoactive substance that is widely used after caffeine [
1]. Chronic alcohol use presents a major public health hazard that is associated with various diseases and toxicities, especially among young people. Although the liver breaks down the majority of ingested ethanol, it has deleterious effects on the brain and other organs [
2]. Ethanol is known to disrupt the oxidative balance, through a process termed neuroexcitotoxicity, which leads to the development of oxidative stress. The processes affect the cells as a whole, and the synthesis of proteins, lipids, and DNA, thereby provoking neurotoxicity and neurodegeneration. Further, ethanol-induced oxidative stress leads to various medical disorders, such as hepatic, cardiovascular, and other alcohol-induced neurotoxic damages [
3].
Alcohol induces numerous intoxication symptoms in the Central Nervous System (CNS), such as impaired brain activity, behavioral changes, and poor motor coordination [
4]. It exerts its effects on the synthesis, release, and signaling of neurotransmitters, such as serotonin, glutamate, gamma-Aminobutyric acid (GABA) [
3], endocannabinoids and the relevant receptors [
5]. Cerebellar damage ultimately leads to disorders in fine motor coordination, equilibrium, posture, and learning [
6]. Though its damage does not abolish these behaviors, it reduces their accuracy and flexibility.
Riboceine, a patented molecule, has been shown to effectively deliver cysteine molecules into cells, enabling them to produce optimal amounts of glutamate [
7]. It contains ribose and cysteine that are found to naturally occur in the human bodies. Riboceine is one of the synthetic antioxidants that help cells produce glutathione on demand. The active ingredient of riboceine is D-ribose-L-cysteine. This study was designed to investigate the possible ameliorative effects of riboceine on the cerebellum of rats following chronic ethanol-induced neurotoxicity.
Materials and Methods
Care and management of animals: Twenty-four male Wistar rats, weighing between 120-170 grams, were procured from the Animal House of the Department of Anatomy, Adeleke University in Ede, Nigeria, and were housed in the animal house of Osun State University, Osogbo, Nigeria. They were acclimatized for seven days under standard laboratory conditions with natural light and dark cycle, and were fed rat pellets, with free access to drinking water ad libitum. All experimental procedures were consistent with the guidelines for care and use of laboratory animals as approved by the UNIOSUN Health Research Ethics Committee (URC Publication No.: 7:218-2-201-11031-7, 2018).
Treatment and solutions: Ethanol (22.5% w/v) was obtained from the Department of Biochemistry, College of Health Sciences, Osun State University, Osogbo, Nigeria. Riboceine was obtained from Max international, Inc. (B00VZA45H58, Lagos, Nigeria) and was dissolved in distilled water. These solutions were freshly prepared each day prior to the administration to the rats.
Animal grouping and treatment: The rats were randomly divided into four groups (A-D) with six rats in each. Rats in group A were administered distilled water (0.5 ml/kg) for 60 days. Rats in group B were administered one mL/kg ethanol (22.5% w/v) for 60 days. Rat in group C were administered one mL/kg ethanol (22.5% w/v) combined with riboceine (30 mg/kg) simultaneously for 60 days. Rats in group D were given one mL/kg ethanol (22.5% w/v) combined with 70 mg/kg riboceine simultaneously for 60 days. All compound administrations were carefully done orally through a gavage tubing.
Rotarod test: Twenty-four hours after the completion of the treatments, the rotarod test was performed to evaluate the rats’ motor coordination [
8]. The duration spent on the apparatus was scored automatically with infrared sensors in a Rotamex-5 rotarod (Columbus Inst; Columbus, Ohio). The rats were initially trained before the testing phase to keep themselves on the rotating rod. During the training, the rats were placed on the rotating rod at 4-40 rotations per minute (rpm) for 2 minutes. Twenty-four hours after the training, the testing phase commenced, and each rat from the groups was placed on the rotating rod with the acceleration set at 4-40 rpm for 300 seconds. This phase consisted of three trials, separated by a 15-min interval between pairs of trials. The time spent on each rod was record for each group before the animals fell off.
Animal sacrifice and tissue processing: After completion of the behavioral testing, the animals were anesthetized, using ketamine at 2 mL/kg body weight, then sacrificed humanely. The animals were perfused transcardially with 4% phosphate buffered paraformaldehyde (PFA-PBS). Subsequently, their brains were excised by a brain forceps and post-fixed in the same fixative for 24 hours and subsequently were processed for histological and histochemical examinations. Rats for enzymatic assays were sacrificed by separating the head from the neck to avoid the interference of ketamine with biochemical redox. Brains were then excised, placed on a pre-chilled metal for the isolation of the cerebellum and rinsed in 0.25M sucrose three times for five minutes each, then placed in 30% sucrose and stored at 4°C.
Biochemical analysis of oxidative stress enzymes: The cerebellar cortex was isolated on a pre-chilled metal plate and homogenized in cold 20 mM Tris-HCl buffer at pH 7.4. The homogenate was centrifuged at 12,000 rpm for 10 minutes at 4oC to remove the nuclei and debris. The supernatants were separated, aliquoted, and assayed immediately for the quantification of Superoxide Dismutase (SOD), Catalase (CAT), Glutathione (GSH), and Malondialdehyde (MDA) activities in the cerebellar samples spectrophotometrically. The activities of these enzymes in the cerebellum were measured, using the assay kits for SOD (k335-100), Catalase (k773-100), Glutathione (K261-100), and Lipid peroxidation (MDA; K739-100), respectively. All colorimetric quantification kits of these enzymes were purchased from CliniSciences SAS (Nanterre, France) carried out according to manufacturer’s directions, and the absorbance read for each enzyme.
Histological tissue processing and staining: The histological demonstration, as modified by Drury and Wallington [
9,
10], was carried out using routine Hematoxylin and Eosin staining to characterize the general histoarchitectural profile of the cerebellar samples and Cresyl violet for the demonstration of Nissl’s substances in the cerebellar cortices of the rats.
Immunochemical GFAP (Glial Fibrillary Acidic Protein) staining procedure: This immunohistochemical procedure was used to express astrocytic filament activation in the cerebellum. Sections were treated with 0.01M citrate buffer (pH 6.0) for 10 minutes to unmask antigen and incubated in 0.3% hydrogen peroxide for 30 minutes to abolish the endogenous peroxidase activity, before blocking with 5% horse serum for 2 hours. Sections were incubated with the primary antibody (1:500 monoclonal mouse anti-GFAP) at room temperature for 18-20 hours, washed and incubated with avidin-biotinylated secondary antibodies (ABC kit, 1:200; Cambridge, UK) and then with avidin-biotin complex. Finally, sections were developed with 0.05% diaminobenzidine. Slides were stained with Mayer’s hematoxylin, and then dehydrated, cleared, mounted, and examined under light microscopy.
Statistical analyses: GraphPad Prism v. 7.0 was used to analyze the data which were expressed as Mean±SEM and differences among the groups were analyzed by one-way ANOVA. Tukey’s post hoc test was used to adjust for multiple comparisons and a P-value at <0.05 for each analysis was considered to be statistically significant.
Results
Neurobehavioral evaluation (rotarod test): In the rotarod test, the time it took for each rat to fall off the rod rotating at 4-40 rpm was recorded. The results, as shown in
Figure 1, indicated that rats in the control group held on and stayed statistically significant longer time on the rotating rod (mean, 180.0±10.00 sec) as compared to the ethanol group (mean, 75.0±9.80 sec). The riboceine treated rats performed well on the rotarod test with a mean time of 149.50±12.0 and 160.0±9.80 seconds, respectively, which were significantly longer than the mean time for the ethanol treated rats (P<0.05).
 Effects of ethanol and Riboceine on cerebellar oxidative stress markers:
Effects of ethanol and Riboceine on cerebellar oxidative stress markers: Superoxide Dismutase activities decreased following ethanol administration. Results obtained from spectrophotometric assay, as depicted in Table 1, showed that SOD activities in the ethanol group were significantly reduced (P<0.05) compared to the control group. Furthermore, there was a significant elevation in the SOD activities in the riboceine treated rats (groups C & D) compared to the rats that received ethanol only (P<0.05). A significant decrease was observed in cerebellar catalase activity in group B as compared to that in the control group (P<0.05). There was also a significant reduction in the catalase activity of the rats that received 30 mg/kg riboceine, compared to those in the control group (P<0.05). However, in rats that received 70 mg/kg riboceine, the catalase activity was not significantly different from that of the control group. The catalase activity in rats treated with 30-70 mg/kg riboceine increased significantly, compared to those that received ethanol only (Table 1).
Increased level of cerebellar lipid peroxidation was observed in the rats treated with ethanol only, compared to those in the control group (P<0.05). The MDA activities in rats treated with riboceine declined significantly, compared to those that were given ethanol only (Table 1). As shown in Table 1, the cerebellar glutathione activity in the ethanol-treated rats decreased significantly, compared to those in the control and riboceine treated groups.
Histopathological and immunohistochemical findings
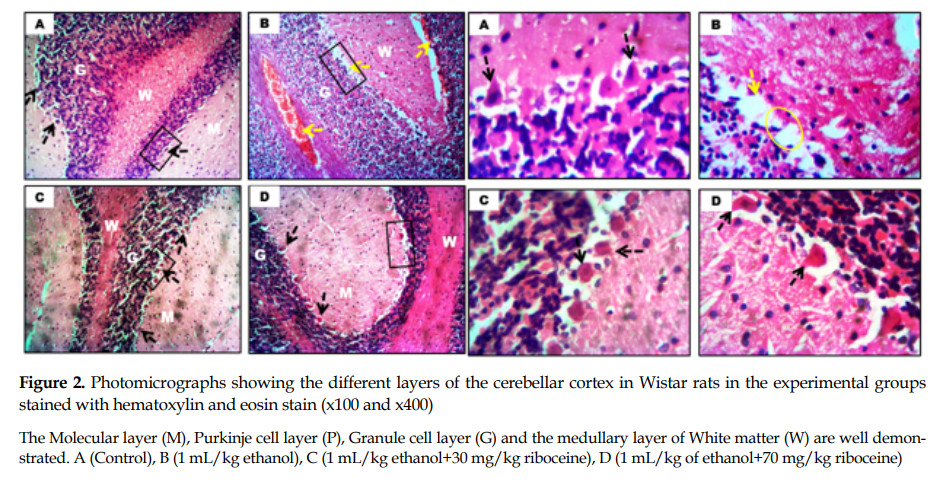
General cerebellar morphology: The ethanol-treated rats showed degenerative changes in the Purkinje cell layers, characterized by reduced number of Purkinje neurons and atrophy along this layer compared to the control rats (yellow circle,
Figure 2), which had numerous neurons along the Purkinje cell layer. Also, the rats given riboceine had similar morphological presentation as those in the control group (
Figure 2).
 Evaluation of Nissl body:
Evaluation of Nissl body: As shown in
Figure 3, the Nissl profiles as visualized by CFV stain across cerebellar sections revealed normal, densely populated and well stained Nissl proteins in the control and riboceine treated rats, compared to those given ethanol only. However, the ethanol group revealed reduced staining in the cytoplasmic Nissl proteins, especially along the Purkinje cell layer (yellow arrow,
Figure 3).
 Expression of reactive astrocytes:
Expression of reactive astrocytes: The results showed increased population of astrocytes in the ethanol-treated rats compared to the control and riboceine treated groups (
Figure 4).
 Discussion
Discussion
The present study investigated the ameliorative and neuroprotective potential of riboceine against chronically ethanol-induced cerebellar toxicity in rats. The rotarod test (Figure 1) is a very useful neurobehavioral tool for the evaluation of motor coordination in rats. Upon this test, motor impairment is reliably associated with increased number of falls at rapid rotary speeds, on consecutive sessions of the test [
11]. In a previous study, ethanol exposure has been shown to impair motor coordination and balance in experimental rats [
8]. In this study, the poor motor coordination seen in ethanol treated rats, 24 hours after the completion of treatment, adversely affected the cerebellar cortex, since this CNS component is responsible for motor coordination and balance. Based on this reason, it was evident from our findings that ethanol administration induced atrophy and neuronal loss in the cerebellum, especially in the Purkinje layer of the ethanol administered rats as seen in
Figure 2, which may have accounted for the motor impairments. Conversely, animals that received riboceine showed improved motor coordination and balance. Hence riboceine might have protected against the neurodegenerative effects of ethanol on the cerebellum [
12].
As presented in
Table 1, results from the assessment of oxidative stress markers showed ethanol-induced oxidative stress, based on the elevated levels of malondialdehyde and reduced glutathione, SOD and catalase in the cerebellar samples. Previous studies have also reported that ethanol has induced oxidative stress in various parts of the brain [
13,
14].

Ethanol is a known disruptor of the oxidative balance, by promoting oxidative stress and disrupting normal neuronal activities, thereby triggering neurodegeneration [
15]. Kolato et al. [
16] have reported that ethanol administration triggers the generation of reactive oxygen species, causing cellular damages via depletion of enzyme activities through expanding lipid peroxidation and decreasing the activity of enzymes that protect against oxidative damage in the brain.
In this study, riboceine treatment maintained the integrity of the antioxidant profile in the cerebellum. Of note, the GSH activity increased significantly even compared with the results documented for the control group. Also, the catalase and SOD levels elevated significantly in rats given riboceine. However, the increased MDA level assayed after the ethanol administration decreased significantly following treatment with Riboceine. This drug has been reported to attenuate the elevated levels of MDA and decreased glutathione and catalase levels following scopolamine-induced amnesia [
17]. Although, the body is equipped with certain antioxidant enzymes that counterbalance the effect of oxidative agents [
18], the administration of riboceine augmented the activities of endogenous antioxidants and offered protection against ROS accumulation in the cerebellum that cause oxidative damages. The elevated SOD, glutathione, and catalase activities following riboceine administration convert free radicals to non-radical products, which play crucial roles in the neurological antioxidant defense [
19]. Furthermore, the reduction in the MDA enzymatic activities, triggered by riboceine, prevented or ameliorated the process of lipid peroxidation, which was consistent with the findings reported by Oyenihi et al. [
13].
The cerebellar role is known for fine motor coordination, and motor learning [
20]. Previous studies have shown that ethanol induces deleterious effects on the microarchitecture of the cerebellum [
21,
22]. Wallauer et al. [
23] have reported that loss of Purkinje cells in the cerebellum was evident following ethanol administration. In this study, several histopathologic presentations were observed in the cerebellar cortex of rats administered with ethanol, including loss of neurons in the Purkinje cell layer. The histological observations in this study are consistent with the findings reported by Asadi et al. [
24]. They reported degenerative changes, evidenced by cortical neuronal reduction, vacuolations and loss of cellular components plus the reduced number of Purkinje cells in cerebral sections of rats treated with ethanol. The loss of Purkinje cells could adversely affect the motor activities, such as loss of movement, disturbance in the maintenance of equilibrium, and muscle tone dysregulation [
25].
Further, Kayakabe et al. [
26] reported that cerebellar Purkinje cells are GABAergic neurons that project to the vestibular and deep cerebellar nuclei, forming connections with thalamus and brainstem, and are also involved in motor control regulation. Alterations in these structures and in GABA-A receptors and their downregulation leads to reduced phasic and tonic inhibition by GABAergic actions, which accompany chronic ethanol administration followed by eventual motor impairment. In the current study, the expression of normal and densely populated Nissl proteins, well stained and outlined neurons were seen in riboceine treated groups, whereas ethanol exposure caused a severe reduction in the cytoplasmic Nissl proteins. Further, treatment with riboceine was found to attenuate the alteration of these Nissl proteins loss and general histopathology of the cerebellum following ethanol administration, thereby protecting against motor impairment resulting from chronic ethanol toxicity.
Studies have shown that astrocytes play important regulatory roles in the brain health and function, such as neurogenesis and synaptogenesis [
27,
28]. It is also known that astrocytes are activated when there is an insult to the brain. In addition, astrocytic dysfunction may play a role in the aberrant neuronal circuitry, seen in certain neurodegenerative disorders. As presented in Figure 4, our results from GFAP assays showed significant positive immune response in the rats treated with ethanol, suggestive of astrocytic activation following ethanol exposure, thus establishing the neuronal insult induced by alcohol. However, the expression of reduced reactive astrocytes in animals administered with riboceine serves as a good evidence in support of the beneficial role of riboceine against ethanol-induced neurotoxicity in the rats’ cerebellar cortex. Though the mechanism by which riboceine inhibits the up-regulation of Glial Fibrillary Acidic Protein (GFAP) is not fully understood, our findings suggest that the anti-inflammatory property of riboceine may play a significant role in this inhibitory process of astrocyte activation in the cerebellar cortex secondary to the ethanol neurotoxicity.
Conclusions
Based on our experimental findings, the chronic ethanol treatment led to impairment of motor coordination and increased oxidative stress, as evident by an increase in lipid peroxidation and depletion in the levels of superoxide dismutase, catalase, and glutathione. Further, ethanol administration induced astrogliosis and histopathological alterations in the cerebellum. Conversely, the riboceine regimen prevented oxidative neuronal damages arising from the ethanol toxicity, likely by modulating key antioxidant biomarkers in the cerebellum.
Some limitations of the study were as follows: a) we used only adult male Wistar rats in this study, b) we were unable to access the extent of synaptic remodeling and activities of dendritic spine following ethanol administration and the expression of neurobiological factors underpinning neuronal damages. Therefore, these facts should be considered in the interpretation of similar results in future studies.
There is a need to evaluate the putative role of riboceine as a therapeutic agent in alcohol-induced neurotoxicity in both male and female juvenile animals. Assessing the effects of riboceine in neurogenesis, expression of key proteins associated with brain health, and neurochemical characterization following ethanol and riboceine treatments will provide more information on the mechanisms by which riboceine confers protection on brain cells in ethanol-induced toxicity.
Ethical Considerations
Compliance with ethical guidelines
All experimental animal procedures were conducted following the guidelines for care and use of laboratory animals as approved by the UNIOSUN Health Research Ethical Committee (URC Publication No. 7:218-2-201-11031-7; 2018).
Funding
This paper was extracted from a research project of the First Author at the Department of Anatomy, Faculty of Basic Medical Sciences, Osun State University, Osogbo, Nigeria.
Author's contributions
All authors contributed fairly equally to the experiments and preparation of the drafts of this manuscript.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors express their gratitude to Dr. Atere T.G. and the Medical Biochemistry Laboratory of Osun State University for their contributions to this research project.
Refrences:










































 , Olorunfemi Samuel Tokunbo *2
, Olorunfemi Samuel Tokunbo *2 

 , Moyinoluwa Ajayi1
, Moyinoluwa Ajayi1 

 , Olawale Ayobami Abayomi3
, Olawale Ayobami Abayomi3 

 , David A. Ofusori4
, David A. Ofusori4 







