Introduction
Epilepsy is a chronic disorder of the Central Nervous System (CNS) that is associated with relapsing seizures [
1]. The severity of the seizures varies from mild episodes of involuntary movements, affecting a limited part of the body, to generalized seizures that affect all of the body [
1,
2]. The prevalence of epilepsy is 40-70 per 100000 persons in developed countries and 100-190 per 100000 persons in developing nations [
3]. At the turn of 19th century, pharmacologic treatment of epilepsy became popular, when phenobarbital was discovered by Alfred Hartmann in 1912. Numerous antiepileptic drugs were discovered over the following decades, including carbamazepine, valproate and phenytoin [
4]. Over 60% of people with epilepsy need anticonvulant medications [
5]. These drugs have side effects, such as drowsiness, ataxia and cognitive disorders [
6,
7]. Despite using the current medications, 30% of the patients still suffer from seizures [
8]. Therefore, there is a need for the development of new anticonvulsant medications with low side effects and high efficacy [
5,
6]. Clinical trials have shown that adding antioxidants, such as melatonin, to antiepileptic drugs reduces neurological disorders caused by epilepsy [
9]. Ketogenic diets reduce mitochondrial Reactive oxygen species (ROS) and reactive nitrogen species (RNS) due to alteration in energy sources, such as using fat-derived ketone bodies [
10].
Seizure is an abnormal electrical discharge of action potentials from the CNS neurons that presents as pathologic episodes of electrical activity in the brain. The most prevalent neurological disorders associated with epilepsy are migraine, stroke, Alzheimer’s disease, and seizure [
11,
12]. Studies have shown that seizure damages the neurons by producing free radicals and promoting oxidative stress [
13,
14]. Status epilepticus may cause mitochondrial respiratory chain dysfunction and result in energy failure. The consequences can heighten the severity of oxidative stress and cause neuronal death in the hippocampus [
15]. Therefore, administration of antioxidant agents may reduce the risk of injuries in the brain caused by seizures [
16]. One way to induce seizure in rodents is using high doses of Pilocarpine [
17], which damages the gamma-aminobutyric acid (GABA)-ergic neurons in the hippocampus via developing oxidative stress and eventually inducing status epilepticus [
18-
21].
Pioglitazone, a PPAR-γ nuclear receptor agonist, increases the sensitivity of insulin receptors and is being used in type-2 diabetes [
22]. Studies have shown that pioglitazone has antioxidant effects and protects neurons from the injuries of oxidative stress [
19,
23]. Pioglitazone, due to its radical scavenging property, potentiates antioxidant defence system [
24]. Therefore, in the current study, we assessed the antioxidant and anticonvulsant effects of pioglitazone on pilocarpine-induced seizures in mice.
Material and Methods
Animals: Twenty-eight mature male mice, 4-6 weeks old, were obtained from Qazvin University of Medical Sciences’ animal housing, weighing 20±2 grams. The mice had free access to food and water during all stages of the study. They were kept in separate cages under 12 hours of light/dark cycle at 22-24ºC room temperature. This study was approved by the Research Ethics Committee of Qazvin University of Medical Sciences (Ethics Code: IR.QUMS.REC.1398.004). Also, this study was conducted in accordance with the international, national, and institutional protocols and guidelines for conducting experiments on animals [
25].
Medication preparation: Pioglitazone hydrochloride (E6910), ketamine hydrochloride (K113), xylazine hydrochloride (X1251) and pilocarpine hydrochloride (P6503) were purchased from Sigma Aldrich (St. Louise, USA). In this study, pioglitazone (80 mg/kg) was suspended in carboxymethyl cellulos (CMC) 0.1% (w/v) and administered to the animals orally [
26]. A single dose of pilocarpine (400 mg/kg) was injected Intraperitoneally (IP) to induce seizure [
27]. Also, the animals were anesthetized with Ketamine or Xylazine IP, at a dose of 100 or 10 mg/kg, respectively.
Study groups: The animals were divided into four groups of seven mice each as outlined below:
Control group: Normal Saline (vehicle for pilocarpine) was injected IP, four hours after the oral administration of CMC 0.1% (pioglitazone vehicle).
Pioglitazone group: Normal Saline (vehicle for pilocarpine) was injected IP, four hours after the oral administration of pioglitazone (80 mg/kg).
Pilocarpine group: Pilocarpine was injected IP at 400 mg/kg, four hours after the oral administration of CMC 0.1%.
Treatment group: Pilocarpine was injected IP at 400 mg/kg four hours after the oral administration of pioglitazone (80 mg/kg, PO).
Assessment of seizure: The convulsive behavior of the animals was observed via video for 60 min after the pilocarpine injection. The intensity of the convulsions was recorded based on modified Racine’s scale [
28], as outlined below:
Stage 0: No response.
Stage 1: Hyperactivity, restlessness, and vibrissae twitching.
Stage 2: Head nodding and clonus, and myoclonic jerks.
Stage 3: Unilateral or bilateral limb clonus.
Stage 4: Clonic seizures of the forelimbs.
Stage 5: Generalized tonic–clonic seizures and falling.
Sample preparations: Animals were decapitated by guillotine under deep anaesthesia with high doses of ketamine after assessment of the seizure severity. The brain was instantly pulled out from the cranium and the hippocampus was separated, blotted dry, weighed and homogenized in KCl solution (1.5%). For biochemical assay, the homogenates were centrifuged to obtain Post-Mitochondrial Fluid (PMF) of hippocampus. Bovine serum albumin was used as the standard to assay the amounts of proteins in the hippocampus [
29].
Biochemical tests
Lipid peroxidation assay: The Malondialdehyde (MDA) level was measured to evaluate lipid peroxidation in the mice’s hippocampus, using the Thiobarbituric Acid (TBA) method. A 0.5 ml of hippocampus sample was transferred to a centrifuge tube, to which 3ml phosphoric acid 3% and 1 ml TBA 0.6% were added. The mixture was heated for 45 min in a boiling water bath. After cooling down, 5 ml n-butanol was added to the mixture and centrifuged at 20000 rpm for 20 min. Finally, the absorption of the organic layer (n-butanol) was read at 535 nm on a spectrophotometer (Bioquest®, UK). The standard curve was drawn, using 1, 1, 3, 3-tetramethoxypropane as the standard. The data were presented as the MDA nanomoles present in one gram of the hippocampus [
30].
Catalase activity: We used Claiborne method to evaluate the Catalase (CAT) activity. Based on this method, a combination of 0.05 mL PMF, 1.0 mL H2O2 (0.019 M) and 1.95 mL phosphate buffer (0.1 M, pH 7.4) was used as the reaction solution. Subsequently, the absorbance was read at 240 nm, and the results were expressed as nmol H2O2 consumed/min per mg protein [
31].
Superoxide dismutase activity: The Superoxide Dismutase (SOD) activity was quantified based on the ability of this enzyme to inhibit the reduction of Nitroblue-tetrazolium (NBT). A 0.1 mL of the sample was added to the reagent mixture, consisting of 0.067 M KHPO4 (pH 7.8), 0.1M EDTA, 0.3 mM NaCN and 1.5 mM NBT. After adding riboflavin at 0.12 mM to the samples, the reaction mixture was incubated for 10 min. The absorbance was then read at 560 nm spectrophotometrically. The data were quantified as the unit of enzyme per mg of protein. One unit was the amount of enzyme required to produce a 50% inhibition [
32].
Glutathione reductase activity: The Carlberg and Manevrick method was used to assess the Glutathione Reductase (GR) activity. A combination of 1.65 mL phosphate buffer (0.1 M, pH 7.6), 0.1 mL EDTA (0.5 mM), 0.05 mL GSH (1 mM), 0.1 mL NADPH (0.1 mM), and 0.1 mL of 10% PMS was used as the reagent mixture. The consumption of NADPH was considered as a marker to measure the enzyme activity. The absorbance was read at 340 nm and the data were expressed as nmol NADPH oxidized/min per mg of protein [
33].
Statistical analyses: We used GraphPad prism 8 for the data analyses. All data were shown as the Means±SEM in groups of seven items. We also used one-way Analysis of Variance (ANOVA) to compare the differences between the means. A P value less than 0.05 was considered as statistically significant.
Results
Effect of pioglitazone on convulsion: The results indicated that the administration of pioglitazone (80mg/kg, PO) or its vehicle (control group) did not induce seizure in the mice. Pilocarpine (400mg/kg, IP) induced seizure of stages 1-5 after injection. Comparisons between the pioglitazone and pilocarpine groups indicated that the pioglitazone administration, four hours before injecting pilocarpine, significantly increased the onset of stages 1 to 4 of seizure (P≤0.01-0.001). Also, pioglitazone prevented the development of stage 5 pilocarpine-induced seizure (
Table 1).

Effect of pioglitazone on MDA: Pilocarpine-induced seizure significantly enhanced the lipid peroxidation level in the mice hippocampus, such that the MDA level was significantly higher than that of the control group (
Figure 1; P<0.01). However, the MDA level was significantly lower in the mice treated with pioglitazone (80 mg/kg, PO; P<0.01) than that of the pilocarpine group (
Figure 1). Therefore, the administration of pioglitazone significantly decreased the lipid peroxidation level after pilocarpine-induced seizures in mice (
Figure 1).

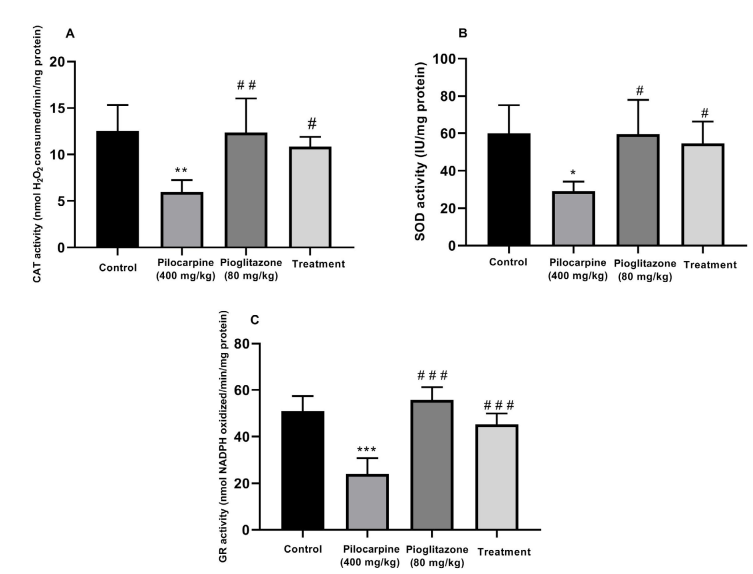
Effect of pioglitazone: The pilocarpine-induced seizure significantly decreased the CAT activity (P<0.01;
Figure 2A), SOD (P<0.05;
Figure 2B) and GR (P<0.001;
Figure 2C) in the mice hippocampus as compared with those of the control group. Also, the administration of pioglitazone (80 mg/kg, PO) four hours before pilocarpine injection (400 mg/kg, IP) significantly increased the CAT activity (P<0.05;
Figure 2A), SOD (P<0.05; Figure 2B) and GR (P<0.001; Figure 2C) as compared with those of the pilocarpine group. Lastly, the administration of pioglitazone alone did not have a significant effect on the activity of these enzymes in the mice hippocampus, as compared with those of the control group (P>0.05).
.PNG) Discussion
Discussion
This study investigated the anticonvulsant and antioxidant effects of pioglitazone on pilocarpine-induced seizure in adult mice. The seizure intensity was evaluated, based on Racine scale [
27]. Our findings demonstrated that pioglitazone administration, four hours before injecting pilocarpine, increased the onset of stages 1 to 4 of pilocarpine-induced seizure. Previous investigations have demonstrated that excessive production of free oxygen radicals have a major role in neuronal damages in pilocarpine-induced seizure in rodents. After pilocarpine-induced seizure, the overproduction of free oxygen radicals leads to peroxidation of lipids and development of MDA in the mice hippocampus. The results also showed that pilocarpine injection significantly increased the MDA level and reduced the antioxidant activity of CAT, SOD and GR enzymes in the mice hippocampus.
Previous studies have suggested that the onset of status epilepticus may be related to the death of GABA-ergic Interneurons in the hippocampus [
19]. These interneurons have inhibitory effects on the activity of other neurons in the nervous system. The available evidence indicates that the dysfunction of GABA-ergic interneurons is implicated in various brain disorders, including epilepsy, schizophrenia and autism [
20]. Also, oxidative stress has been associated with the side effects. Previous studies have also demonstrated that oxidative stress leads to loss of GABA-ergic neurons and induction of seizure in rodents [
19,
20,
21]. Animal studies have demonstrated that seizure-induced oxidative stress mediates the destruction of hippocampal neurons after pilocarpine injection in rodents [
26,
34] Therefore, antioxidants can potentially prevent seizure or prolong its onset by protecting the inhibitory interneurons. Many studies have confirmed that antioxidants protect cortical and hippocampal neurons against seizure-induced oxidative stress [
35].
Pioglitazone, a potent PPAR-γ agonist, increases the sensitivity of insulin receptors and is widely used to control type-2 diabetes mellitus [
18]. Our findings revealed that pioglitazone increased the latency of seizure onset (stages 1-4) and prevented the stage 5 from happening after pilocarpine-induced epilepsy in mice. Consistent with our findings, previous studies have also shown that pioglitazone has anticonvulsant effects in other animal models of epilepsy [
36]. Hussein et al. showed that pioglitazone delayed the seizure latency and shortened its duration after induction of febrile seizure in rats [
37]. They also reported that pioglitazone has anti-inflammatory and anti-apoptotic effects in the rat hippocampus and reduces cognitive impairments induced by febrile seizure. Both Adabi Mohazab et al. [
38] and Okada et al. [
39] have demonstrated that pioglitazone has anticonvulsant effects in two different experimental animal models of seizure [
38,
39].
The results of the current study showed that pioglitazone decreased the lipid peroxidation and enhanced the CAT, SOD and GR activities in the mice hippocampus after pilocarpine-induced seizure. These findings are consistent with and confirm those of the past studies regarding the antioxidant effects of pioglitazone [
23,
40]. In vitro antioxidant studies have demonstrated that pioglitazone has substantial antioxidant activity as represented by the scavenging activities of hydrogen peroxide and nitric oxide [
41]. Perez-Giron et al. demonstrated that pioglitazone inhibited the expression of cyclooxygenase-2 enzyme by hindering the production of ROS in vascular cells [
42]. Pioglitazone also has antioxidant effects against oxidative stressors by inhibiting NADPH oxidase expression [
43]. Finally, previous studies have shown that pioglitazone enhances the CAT, SOD, GR and glutathione peroxidase activities against the overproduction of free oxygen radicals [
44]. Therefore, pioglitazone may be considered as a potential anticonvulsant agent. However, additional studies are warranted to investigate potential for clinical use in humans.
Conclusions
In this study, pre-treatment with pioglitazone significantly prolonged the latency of the onset of pilocarpine-induced seizure. In addition, it suppressed the status epilepticus caused by pilocarpine. Simultaneously, pioglitazone attenuated oxidative stress and potentiated antioxidant defence system in the hippocampus. Based on the findings of this study, it is suggested that pioglitazone possesses anticonvulsant activity, since it prevented the oxidative damage due to pilocarpine toxicity.
Ethical Considerations
Compliance with ethical guidelines
The study was reviewed and approved by the Ethics Committee of the Qazvin University of Medical Sciences (Code: #IR.QUMS.REC.1398.004).
Funding
This work was financially supported by the Student Research Committee of the School of Medicine, Qazvin University of Medical Sciences
Author's contributions
Conceptualization and methodology: Yazdan Naderi, Saba Rostamian; Data analysing and experiment: Saba Rostamian, Yazdan Naderi, Samaneh Keshavarz Hedayati; Writing the initial drafts of the manuscript: Sara Khosraviani, Yazdan Naderi, Ehsal Aali; Writing review and editing: Sara Khosraviani, Ehsan Aali; Supervision and visualization: Yazdan Naderi.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors wish to acknowledge the staff, administration and professors of the Department of Pharmacology and Faculty of Medicine, Qazvin University of Medical Sciences, Qazvin, Iran, for their support of this study.
Refrences:











































.PNG)
