Introduction
In many conditions, especially testicular malignancies, cytotoxic chemotherapy has improved the survival rates. However, the treatment is associated with severe morbidities. The most common long-term side effects of chemotherapy include testicular dysfunction. Oxaliplatin (OXP), is a “1,2-diaminocyclohexane platinum” compound, and is used widely in the treatment of various neoplasias, including colorectal cancer. The anticarcinogenic efficiency of OXP is attributed to its ability to generate DNA adducts, which inhibit the proliferation of neoplastic cells [
1,
2,
3]. The immunological mechanism of OXP-treatment for colorectal cancer cells is due to an immune response, thus enhancing its antineoplastic activity. In addition, the decreased level of reduced glutathione is the principal cause of OXP cytotoxicity [
4,
5,
6,
7]. Testicular toxicity and neuropathy are among the toxic effects that occur as a result of OXP treatment [
8,
9].
The severe side effects of OXP necessitate investigations into alternative therapies including the use of natural herbs to ameliorate these effects, especially those rich in antioxidant, anti-inflammatory, and anticancer properties [
10,
11]. Costus roots (Saussurea costus), is a member of the Asteraceae plant family. Earlier researchers have attributed its therapeutic activities to the presence of flavonoids, steroids, terpenes, alkaloids, sesquiterpenes, costunolide, dehydrocostus lactone, cynaropicrin, and chlorogenic acid. Hence, the reason for its widely use in traditional medicine world-wide [
12,
13,
14].
The current study aimed to investigate the inhibitory effect of the ethanolic extract of costus against Oxaliplatin-induced testicular toxicity in male albino rats, the promising results of which are presented in this article.
Materials and Methods
Extraction and in vitro Assessments: Costus (Saussurea costus) roots were purchased from Imtinan Company, Egypt. We then authenticated the roots by a botanical scientist from the Zoology Department, Faculty of Science, Al-Azhar University in Assuit, Egypt (taxonomic serial #: TSN-780691). The dried roots were then powdered and used to prepare the ethanolic extract according to a method published previously [
15]. The total phenolic content of the roots extract was determined according to a previous method [
16]. Lastly, the radical scavenging and reducing activities of the roots extract were determined, using established biochemical methods [
17,
18].
Animals and experimental design
Fourty male albino rats, weighing 150-200g, were purchased from the experimental animal house of the National Research Center (Cairo, Egypt). The animals were cared for and treated based on the guidelines established by the Ethics Committee of the National Research Center (Code: FWA 00014747). The animals were divided into four groups of 10 rats each. The first group (negative control) were fed standard diet; the second group were orally administered 50 mg/kg/day of the roots extract for six weeks; the third group (positive control) received 10 mg/kg/week of OXP via IP injections for six weeks; and the fourth group (experimental) were given OXP concurrently with a daily oral dose of the extract for six weeks.
Blood and tissue sampling
At the completion of the treatments, the animals were sacrificed and samples from the sera were taken from the rats and stored at -80°C until the analyses as described later. Tissue samples of the testes from each rat were taken and divided into three parts. One part used for the estimation of oxidative stress markers, another part for the estimation of DNA fragmentation, and third part was kept in 10% formalin-saline buffer for the subsequent histopathological examinations.
Biochemical determinations
The activities of serum ALT and AST were determined, using appropriate kits obtained from Germany. The levels of serum urea and creatinine were estimated, using biodiagnostic kits obtained from Egypt. The levels of serum TNF-α, IL-1β, CD4, total and free testosterone were also estimated, using ELISA kits obtained from China. Lastly, the levels of GSH, NO, SOD and CAT activities in the testis were estimated, using biodiagnostic kits from Egypt, while the level of malondialdehyde (MDA) in the testis tissue samples was estimated biochemically based on a previously published method [
19].
DNA fragmentation
The percentages of DNA fragmentation were determined according to a previously published method [
20].
Histopathology examinations
Samples of the testis tissue from all rat groups were stained with hemotoxiline and eoasin (H&E), and examined under light microscopy [
21].
Statistical analyses
The data from all rat groups were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests at the significance level of P≤0.05 and expressed as the Means ±Standard Errors. For this purpose, we used the statistical analysis system (SAS) program software (SAS Institute, Cary, NC, USA) [
22].
Results
Results of the estimated levels of total phenolic content, scavening activity and reducing power (
Figures 1 &
2) revealed that the roots extract contained high amounts of phenolic compounds and radical scavenging activity, which are the essential antioxidant mechanisms required for its protective effects.
.jpg)
.jpg)
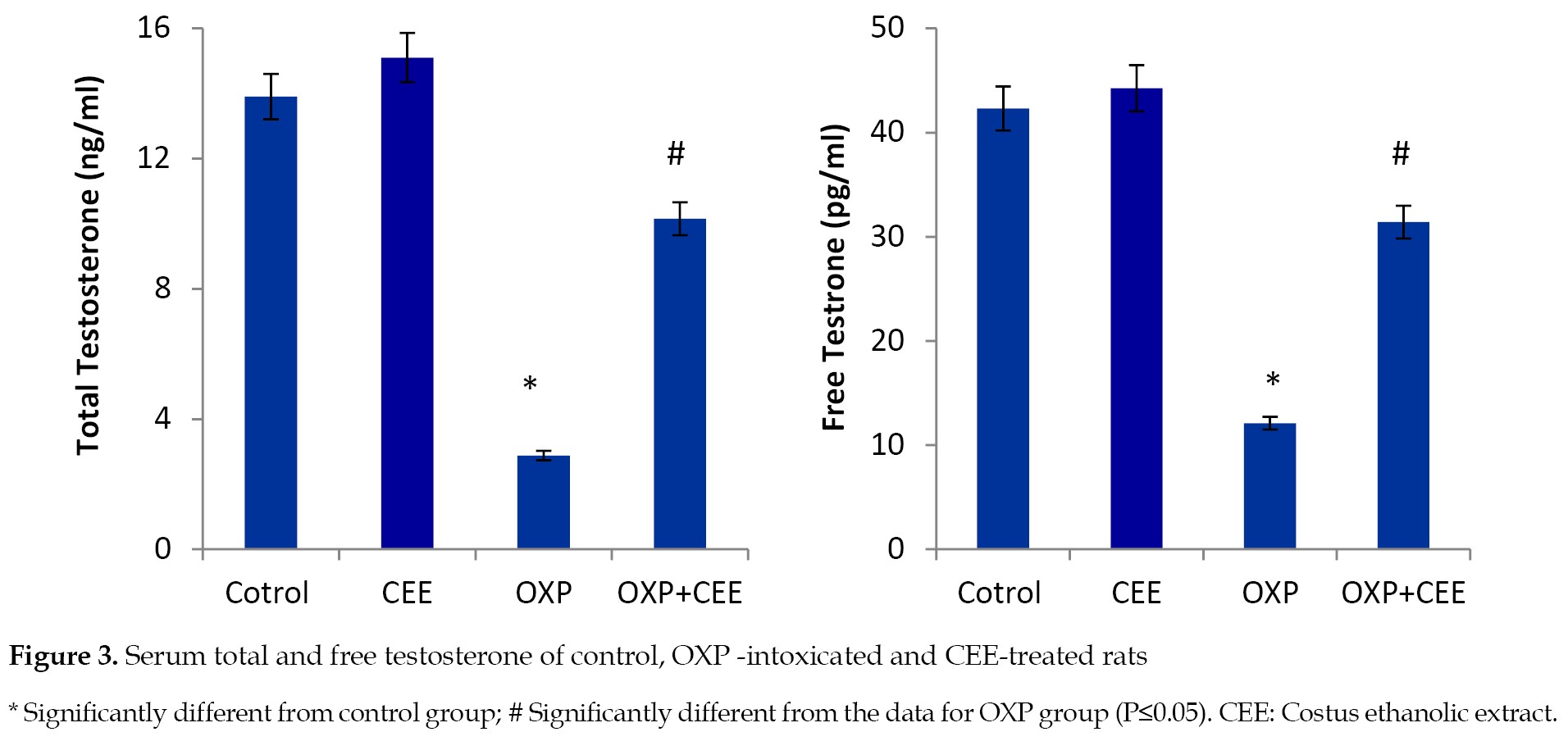
The administration of OXP resulted in a significant decline in the serum levels of total and free testosterone, compared to those of the control group. Interestingly, the co-administration of OXP and the roots extract improved the serum levels of total and free testosterone compared to that observed in the OXP-treated rats (
Figure 3).
The treatment with OXP significantly elevated the serum AST and ALT activities, and the urea and creatinine levels compared to those documented for the control group (
Table 1).
.jpg)
Further, co-treatment with OXP and the root extract caused a significant decline in the elevated levels of liver enzymes and kidney function indices.
The injection of OXP into the rats induced a marked deterioration of the testis oxidative stress status, as evident by the significant increase in the MDA and NO levels from the testis tissue samples coupled with a significant reduction in the SOD, CAT and GSH levels (
Table 2).
.jpg)
The concurrent administration of OXP and the root extract led to a significant decline in the MDA and NO levels from the testis tissue samples combined with marked rises in the GSH, SOD and CAT levels as compared with the data noted for the OXP-treated group. There was a significant increase in TNF-α, IL1-β and testis DNA fragmentation level coupled with a significant decrease in CD4 cells in the OXP-treated group, compared with those obtained for the control group. Interestingly, the co-administration of OXP and the root extract brought the inflammatory cytokines and DNA fragmentation in the testis tissue samples to within normal values. Also, the combined formulation significantly decreased the TNF-α, IL1-β and DNA fragmentation but significantly increased the CD4 cells level compared with those noted for the OXP-treated animals (
Figure 4).
.jpg)
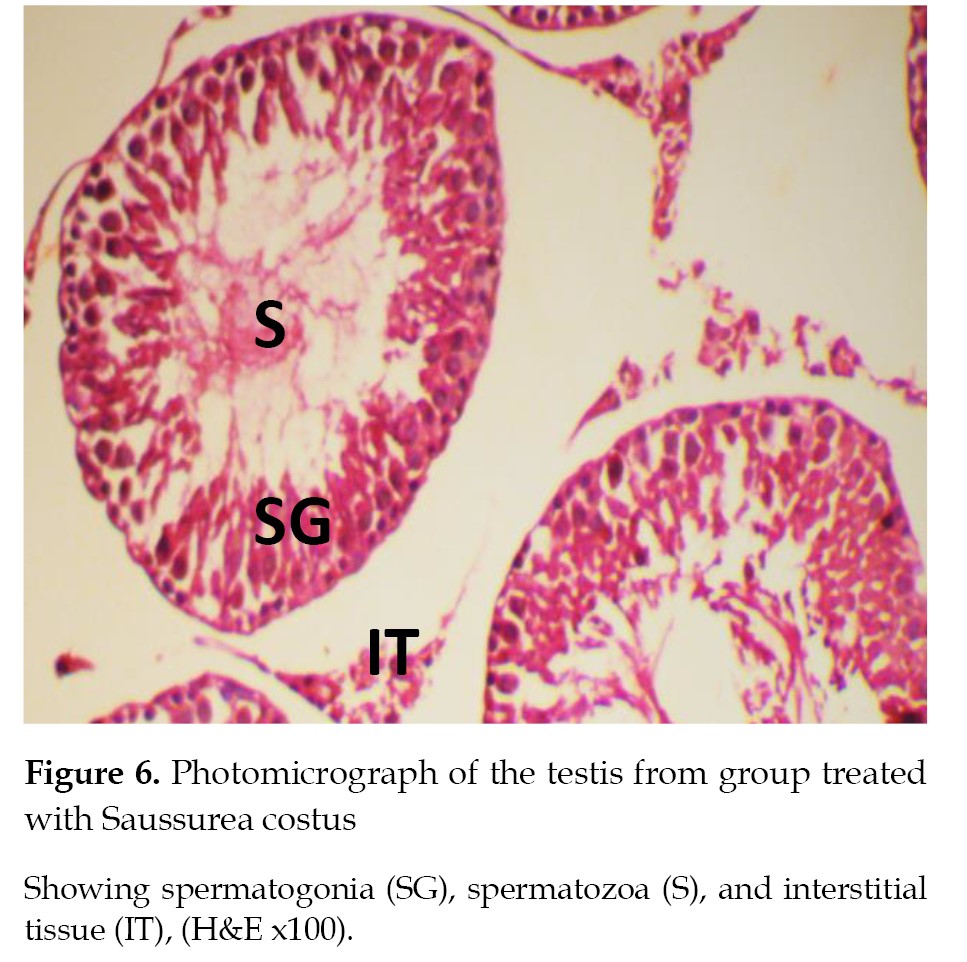
Histopathological findings
The results of histopathological examinations of the sections from the testis tissue samples for the rat groups are described and illustrated in
Figures 5-
8.
.jpg)
.jpg)
.jpg)
Discussion
Testicular toxicity is one of the effects resulting from the administration of chemotherapy drug, Oxaliplatin (OXP). The side effects of this drug include dysfunctional sperms and germ cell apoptosis in both animals and humans [
23]. There are herbal formulations that can ameliorate the OXP toxicity. The effect of these herbal agents is attributed to their flavonoid contents, a group of polyphenolic compounds known for their scavenging, hydrolytic, antioxidant and anti-inflammatory properties [
24]. In the present study, the administration of OXP to rats resulted in testicular dysfunction, mediated through lipid peroxidation and generation of excess ROS. These humoral changes denatured proteins, and promoted apoptosis, as evident by low serum testosterone and significant histological damages. In addition, there was a sharp decline in the serum levels of SOD, CAT and GSH, and increases in the serum levels of MDA and NO. These data were consistent with those reported by a number of earlier studies [
23,
25,
26,
27,
28,
29].
A previous study has shown that the Costus root extract improves the testicular damages caused by OXP likely by lowering the oxidative stress and testosterone levels in adult male rats. Further, the extract has been able to reduce lipid peroxidation and attenuate the deviations in the antioxidant defense markers, as compared to that seen in the OXP-treated rat group. These results imply that the beneficial antioxidant effect of Costus extract may be linked to its active ingredients, such as flavonoids, anthraquinone, and terpenes [
30]. Further, the OXP-treatment in our study caused a significant elevation in TNF-a, IL1-β and testis DNA damages with a significant reduction in the CD4 cells number. These findings are in agreement with those reported by an earlier study [
31].
In contrast, the co-administration of OXP with the Costus extract reduced the serum levels of pro-inflammatory cytokines (TNF-a & IL1-β) and testis DNA damages while increasing the CD4 cell numbers compared to those noted for the OXP-treated group. However, these results were not consistent with those reported by some studies previously [
32,
33,
34,
35,
36]. Our results indicated that the administration of the Costus extract alone to rats did not stimulate testis DNA fragmentation. The antioxidant property of the extract may reflect its protective effect against the toxicity induced by the OXP treatment. In addition, our study revealed that the OXP injection into rats resulted in a significant decline in serum CD4 cells level compared to that noted for the normal controls, which was consistent with the results shown by another study previously [
31]. It is already known that CD4+T-helper cells produce cytokines that modulate the functions of immune cells [
37].
Our study results further demonstrated that the administration of OXP caused significant interstitial tissue degeneration, as evident by the formation of cellular vacuoles and tissue hemorrhage, similar to those reported by a previous study [
38]. The OXP-induced degeneration of testicular tissue samples might be due to the accumulation of residual cytoplasm after dissociation of spermatogenesis and fluid retention on the lumens of some testicular tubes. Sertoli cell atrophy is a direct manifestation of decreased testosterone levels and Leydig cell atrophy caused by oxaliplatin treatment. The rise in the apoptosis rate of germ cells and consequent infertility were attributed to Sertoli cells atrophy [
38]. The Costus extract combined with OXP treatment led to the amplified oxidative stress, which was confirmed by the antioxidant profile achieved in the experimental rat group in the current study. Finally, the Costus extract has the potential to restore the normal architecture of testicular seminiferous tubules and interstitial spaces [
38].
Conclusions
This study has clearly demonstrated that the Costus root extract is likely to offer therapeutic effect against OXP-induced testicular damage, as evident by the attenuation of oxidative stress and the subsequent histopathological alterations. Our results indicate that the extract is a potentially effective agent for concurrent use with OXP chemotherapy to ameliorate its side effects. The extract significantly reduced the toxic damages to the rats’ testicular tissue and the adverse effects of the resultant oxidative stress and testosterone levels, and increased the pro-inflammatory cytokines, cellular apoptosis and testicular histopathology.
Ethical Considerations
Compliance with ethical guidelines
The experimental animals received standard care consistent with the institutional criteria for the use of experimental animals, as approved by the Ethics Committee of National Research Center (Code: FWA 00014747). Also, the study protocol was reviewed and approved by the same Ethics Committee.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization: K.G. Abdel-Wahhab, M. Al-Ashry and H.F. Gomaa; Methodology, Investigation, Writing – original draft: All authors; Supervision and Writing – review & editing H.F. Gomaa, K.G. Abdel-Wahhab and M. Al-Ashry.
Conflict of interest
The authors declared no conflicts interests.
Acknowledgements
The authors would like to thank the colleagues at Medical Physiology Laboratory and staff at the animal house of the National Research Center, Cairo, Egypt.
References
- Kohsaka T, Minagawa I, Morimoto M, Yoshida T, Sasanami T, Yoneda Y, et al. Efficacy of relaxin for cisplatin-induced testicular dysfunction and epididymal spermatotoxicity. Basic Clin Androl. 2020; 30(3):3. [PMID] [PMCID]
- Kalemci S, Tanrıverdi O, Şimşek A, Aksun S, Celik OI, Barutca S, et al. Evaluation of oxaliplatin-induced pulmonary toxicity in rats. Contemp Oncol (Pozn). 2019; 23(3):151-6. [DOI:10.5114/wo.2019.89242] [PMID] [PMCID]
- Ibrahim A, Hirschfeld S, Cohen MH, Griebel DJ, Williams GA, Pazdur R. FDA drug approval summaries: Oxaliplatin. Oncologist. 2004; 9(1):8-12. [DOI:10.1634/theoncologist.9-1-8] [PMID]
- Rabik CA, Dolan ME. Molecular mechanism of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007; 33():9-23. [DOI:10.1016/j.ctrv.2006.09.006] [PMID] [PMCID]
- Raghavan D. Testicular cancer. Maintaining the high cure rate. Oncology (Williston Park). 2003; 17(2):218-28. [PMID]
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009; 8(1):10-6. [PMID] [PMCID]
- Obinna VC, Kagbo HD. Effect of methanolic leaf extract of costus lucanuscianus on male reproductive parameters in albino rats. Saudi J Med Pharm Sci. 2018; 4(1B):102-8. [DOI:10.21276/sjmps.2018.4.1.13]
- Branca JJV, Carrino D, Gulisano M, Ghelardini C, Di Cesare Mannelli L, Pacini A. Oxaliplatin-induced neuropathy: Genetic and epigenetic profile to better understand how to ameliorate this side effect. Front Mol Biosci. 2021; 8:643824. [PMID]
- Soladoye MO, Amusa NA, Raji-Esan SO, Chukwuma EC, Taiwo AA. Ethnobotanical survey of anti-cancer plants in Ogun State, Nigeria. Ann Biol Res. 2010; 1(4):261 73. https://www.researchgate.net/profile/Emmanuel-Chukwuma-2/publication/281560670_-Nigeria.pdf
- Huang KM, Leblanc AF, Uddin ME, Kim JY, Chen M, Eisenmann ED, et al. Neuronal uptake transporters contribute to oxaliplatin neurotoxicity in mice. J Clin Invest. 2020; 130(9):4601-6. [DOI:10.1172/JCI136796] [PMID] [PMCID]
- Tousson E, Hafez E, Zaki S, Gad A. The cardioprotective effects of L-carnitine on rat cardiac injury, apoptosis, and oxidative stress caused by amethopterin. Environ Sci Pollut Res Int. 2016; 23(20):20600-8. [DOI:10.1007/s11356-016-7220-1] [PMID]
- Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem Toxicol. 2014; 72:138-46. [DOI:10.1016/j.fct.2014.06.029] [PMID]
- Krishnakumar N, Kanna U, Parthiban KT, Preethi Shree M. Growth performance of thorn less bamboos (Bambusa balcooa Roxb. and Bambusa vulgaris Schrader ex J. C. Wendland). Int J Curr Microbiol App Sci. 2017; 6(4):32-9. https://www.ijcmas.com/6-4-2017/N.%20Krishnakumar,%20et%20al.pdf
- Kim EJ, Lim SS, Park SY, Shin HK, Kim JS, Park JH. Apoptosis of DU145 human prostate cancer cells induced by dehydrocostus lactone isolated from the root of Saussurea lappa. Food Chem Toxicol. 2008; 46(12):3651-8. [DOI:10.1016/j.fct.2008.08.038] [PMID]
- Jayaprakasha GK, Rao LJ. Phenolic constituents from lichen parmotrema Stuppeum (NYI.) Hale and their antioxidant activity. Z Naturforsch C J Biosci. 2000; 55(11-12):1018-22. [DOI:10.1515/znc-2000-11-1227] [PMID]
- Nogala-Kalucka M, Korczak J, Dratwia M, Lampart-Szczapa E, Siger A, Buchowski M. Changes in antioxidant activity and free radical scavenging potential of rosemary extract and tocopherols in isolated rapeseed oil triacylglycerols during accelerated tests. Food Chem. 2005; 93(2):227-35. [DOI:10.1016/j.foodchem.2004.09.021]
- Sethiya NK, Trivedi A, Mishra S. The total antioxidant content and radical scavenging investigation on 17 phytochemicals from dietary plant sources used globally as functional. Food Biomed Prev Nutr. 2014; 4(3):439-44. [DOI:10.1016/j.bionut.2014.03.007]
- Ruiz-Larnea MB, Leal AM, Liza M, Lacort M , de Groot H. Antioxidant effects of estradiol and 2 hydroxyestradiol on iron induced lipid peroxidation of rat liver microsome. Steriod. 1994; 59(6):383-8. [DOI:10.1016/0039-128X(94)90006-X]
- Perandones CE, Illera VA, Peckham D, Stunz LL, Ashman RF. Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol. 1993; 151(7):3521-9. [PMID]
- Drury RAB, Wallington EA. Preparation and fixation of tissues. In: Montgomerie Carleton H, Drury RAB, Wallington EA, editors. Carleton’s histological technique. Oxford: Oxford University Press; 1980. https://www.google.com/books/edition/Carleton_s_Histological_Technique/4-dqAAAAMAAJ?hl=en&gbpv=0
- Steel RGD, Torrie GH. Principles and procedures of statistics: A biometrical approach. New York: McGraw Hill; 1980. https://www.google.com/books/edition/Principles_=0
- Sastry MS, Motghare M, Gotmare V, Dighade S. Cytotoxic effects of a chemotherapeutic drug oxaliplatin on rat testis and plasma testosterone concentration. Int J Secur Netw. 2011; 2(2):359-67. https://www.semanticscholar.org/paper/CYTOTOXIC-EFFECTS-OF-A-CHEMOTHER-77
- Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci Technol. 2007; 40(2):344-52. [DOI:10.1016/j.lwt.2005.09.011]
- Kianifard D,Mousavi SM. Assessment of the leydig cells population in rat testicular tissue following treatment with oxaliplatin. J. Reprod. Infertile. 2018; 19(2 Supple):122. https://www.researchgate.net/publication/337913974_Assess
- Arjumand W, Sultana S. Role of VHL gene mutation in human renal cell carcinoma. Tumour Biol. 2012; 33(1):9-16. [DOI:10.1007/s13277-011-0257-3] [PMID]
- Moretti E, Mazzi L, Terzuoli G, Bonechi C, Iacoponi F, Martini S, et al. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod Toxicol. 2012; 34(4):651-7. [DOI:10.1016/j.reprotox.2012.10.002]
- Chen CY, Li H, Yuan YN, Dai HQ, Yang B. Antioxidant activity and components of a traditional Chinese medicine formula consisting of Crataegus pinnatifida and Salvia miltiorrhiza. BMC Complement Altern Med. 2013; 13:99. [DOI:10.1186/1472-6882-13-99] [PMID] [PMCID]
- Shoar SMM, Dolatyari M, MousaviA, Khalilzadeh E, Kianifard D. Protective effectsof propolis on the alteration of leydig cells population in rat testicular tissue followingtreatment with oxaliplatin. J Reprod Inferitl. 2018; 19(2 Supple):91. https://www.researchgate.net/publication/337913745_Protective_Effects_of_Propolis_on_the_Alteration_of_Leydig
- Sari IP, Nurrochmad A, Setiawan IM, Hertiani T, Paramita AD, Annisa AY. Effects of Costus speciosus ethanolic extract on male rats: The action mechanism and the ability to impregnate. Pak J Pharm. Sci. 2018; 31(3Supplementary)):997-1001. [PMID]
- Stojanovska V, Prakash M, McQuade R, Fraser S, Apostolopoulos V, SakkalS, et al. Oxaliplatin treatment alters systemic immune responses. BioMed Res Int. 2019; 2019:1-15. [DOI:10.15258/istarules.2019.16]
- Alnahdi HS. Injury in metabolic gland induced by pyrethroid insecticide could be reduced by aqueous extract of sassura lappa. Int J Pharm Res Allied Sci. 2017; 6(2):86-97. https://ijpras.com/storage/models/article/R5PmlT9tcZG.pdf
- Atere TG, Akinloye OA. High dose of standardised extract of Costus Afer leaves potentiates cadmium reproductive toxicity in Wistar rats. Andrologia. 2019; 51(9):e13360. [DOI:10.1111/and.13360] [PMID]
- El-Rahman GIA, Behairy A, Elseddawy NM, Batiha GE, Hozzein WN, Khodeer DM, et al. Saussurea lappa ethanolic extract attenuates triamcinolone acetonide-induced pulmonary and splenic tissue damage in rats via modulation of oxidative stress, inflammation, and apoptosis antioxidants. Antioxidants (Basel). 2020; 9(5):396. [DOI:10.3390/antiox9050396] [PMID] [PMCID]
- Riccioli A, Starace D, D'Alessio A, Starace G, Padula F, De Cesaris P, et al. TNF-alpha and IFN-gamma regulate expression and function of the Fas system in the seminiferous epithelium. J Immunol. 2000; 165(2):743-9. [DOI:10.4049/jimmunol.165.2.743] [PMID]
- Oyinloye BE, Adenowo AF, Osunsanmi FO, Ogunyinka BI, Nwozo SO, Kappo AP. Aqueous extract of Monodora myristica ameliorates cadmium-induced hepatotoxicity in male rats. SpringerPlus. 2016; 5:641. [PMID]
- Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: Differentiation and functions. Clin Dev Immunol. 2012; 2012:925135. [PMID]
- Bieber AM, Marcon L, Hales BF, Robaire B. Effects of chemotherapeutic agents for testicular cancer on the male rat reproductive system, spermatozoa, and fertility. J Androl. 2006; 27(2):189-200. [DOI:10.2164/jandrol.05103] [PMID]
- Ezejiofor AN, Orisakwe OE. The protective effect of Costus afer Ker Gawl aqueous leaf extract on lead-induced reproductive changes in male albino Wistar rats. Ezejiofor AN, Orisakwe OE. 2019; 23(3):215-24. [PMID]










































 , Doaa Galal ELSahra2
, Doaa Galal ELSahra2 

 , Khaled G. Abdel-Wahhab3
, Khaled G. Abdel-Wahhab3 

 , Mahenor E. Abdelsalam4
, Mahenor E. Abdelsalam4 
 , Hagar H. Mourad3
, Hagar H. Mourad3 
 , Alaa M.H. El-Bitar1
, Alaa M.H. El-Bitar1 

 , Heba F. Gomaa *5
, Heba F. Gomaa *5 


.jpg)
.jpg)
