Introduction
Diabetes mellitus is a typical non-communicable disease that has sprouted strong research interest globally. It occurs due to defective macromolecule metabolism or pancreatic β-cells toxicity, leading to low insulin release, resistance or insensitivity, resulting in hyperglycaemia characterised by significant elevation of blood glucose above the clinically acceptable level [
1,
2]. A major chemical model that has shown profound positive result as pancreatic β-cytotoxic agent is Alloxan; however, streptozotocin can also serve as a toxic alternative. Alloxan’s hyperglycaemic property is achieved by either the inhibition of glucokinase activity via abolishing insulin secretion or through superoxide, hydroxyl radicals and hydrogen peroxide formation via autoxidation of dialuric acid, leading to β-cells toxicity and necrosis [
3]. The resultant effects of this chronic hyperglycaemia are followed by such pathologies as cardiopathy, nephropathy, hepatopathy, retinopathy, neuropathy and importantly low life expectancy of the patients [
4,
5]. Subsequently, painful inflammation, organ transplantation, and losses of humans and capital to the nation are inevitable [
6,
7].
Many anti-diabetic therapies have been developed and are currently in use, such as intraperitoneal insulin administration, sulfonylureas and pancreas transplantation [
8,
9]. Others pharmacological treatments include dipeptidyl peptidase inhibitors (DPPIs), adenosine monophosphate dependent protein kinase (AMPK) stimulations, glucagon-like peptide-1 (GLP-1) modulation, and ATP gated K+ channel blockage. These therapeutic models function by improving insulin secretion and sensitivity, or inhibiting glycolytic pathways [
10]. However, they have their adverse side effects, ranging from mild to severe β-cell apoptosis, pancreatitis, and thyroid and pancreatic cancers [
11,
12,
13]. In spite of successes recorded in the management of diabetes, scientists are still concerned about the its increased mortality rate. Therefore, it is necessity to search for newer treatment models with little or no side effects. One such effective approach is the use of medicinal or traditional plants, containing phytochemicals with tissue regenerative and antioxidant properties [
14].
Several African plants have shown potentials as medicinal agents in addition to Calotropis procera (C. procera). The plant, Aiton. This plant is a xerophytic shrub, growing wildly in both tropical and semitropical areas, and belongs to the Asclepiadaceae family. The English nomenclatures for this plant include: Giant milkweed, Dead Sea apple, Sodom apple, Swallow-wort, auricular tree, Sodom’s milkweed, rooster tree, rubber-bush and small crown flower [
15,
16]. Despite the different titles for this plant in other countries; however, in Nigeria, the Igbos, Yorubas and Hausas tribes refer to it as Otosi, Bom-Ubomu and Tumfafiya, respectively [
17,
18,
19]. Scientific reports have documented various ethno-medicinal activities of the plant’s latex, leaf and flower extracts. The known activities include anti-hyperglycaemic, gastroprotective, antibacterial, anticancer, anti-inflammatory, anti-anglogenic, nephroprotective, anti-tumor, anticonvulsant, anti-fertility, analgesic/antipyretic effects [
20,
21]. However, our literature search provided few research articles on the medicinal properties of the extract of this plant’s roots, such as anti-hyperglycaemic, anti-hyperlipidemic, and in vitro antioxidant activity. Also, it is used as a flatus reliever in dyspepsia and jaundice treatment, and as an antibacterial agent [
16,
22,
23]. Nevertheless, the hepatoprotective effect of the extract in cases of alloxan toxicity is not available in the published literature.
Aim of the study: Considering the above facts, this study was undertaken to evaluate the anti-diabetic activity and hepatoprotective potential of the aqueous-methanol extract of the C. procera roots in Wistar rats with respect to pancreas toxicity induced by alloxan. The findings of this study are likely to offer experimental or clinical benefits to toxicology.
Materials and Methods
Plant material authentication: The plant samples (C. procera roots) were obtained from Nigeria Police Academy (NPA), and authenticated by Dr Aminu Jabbi - a taxonomist with the Department of Biological Science. The authentication was further documented by the university’s herbarium (Registered: NPAH 111).
Plant material preparation: After air-drying the fresh roots of C. procera for 14 days, they were ground into powder. A cold maceration method was used to extract 372.76 grams of the powdered root in 1500 ml of water-methanol solution, and left at room temperature for 48 hours. The obtained filtrate (800 ml) was then concentrated by evaporation at a normal ambient temperature of 28°C, and the solid root extract was recovered (6.56 g).
Animal Treatment: Twenty male Wistar rats weighing 100-105 g and aged 3-4 months were acquired from Department of Pharmacology, University of Lagos, Nigeria. They were kept in the laboratory to acclimatize for 14 days under typical conditions (photo cycle: 12-hour dark/light cycle, temperature of 22°C and humidity, 55%). Also, they were fed commercially formulated rat food and potable water. The animal treatment procedure was based on a previously described method [
24].
Induction of Diabetes in Animals: For the purpose of inducing diabetes in the animals, an established method was employed [
25]. Alloxan monohydrate was purchased from the Sigma-Aldrich (Catalogue #: 45-A7413-10G; Germany), was dissolved in physiological saline, and a dose of 150 mg/kg body weight was given once to the animals after 12hours of overnight fasting. After 72 hours of post administration, type-1 diabetes mellitus was induced in the experimental animals. In comparison with the normal group (85 mg/dl), the diabetic rats showed a substantially elevated blood glucose levels (>250 mg/dl) as monitored by a glucometer (ACCU-CHEK model; Roche, UK). Some abnormal signs, such as excessive urination, polydipsia, weight loss and tiredness were observed in the experimental animals.
Experimental design: The rats were divided into the following groups:
● Group 1: Six rats received only rat food and drinking water (normal group).
● Group 2: Seven rats received 150 mg/kg alloxan monohydrate once intraperitoneally (Diabetic untreated group).
● Group 3: Seven rats received 150 mg/kg alloxan monohydrate once intraperitoneally and then given 200 mg/kg aqueous-methanol C. procera roots extract daily for 12 consecutive days (Diabetic treated group).
Quantitative Analyses of the Plant Extract (AMRECP): An established protocol, as described by an earlier study [
26], was used for the determination of alkaloid content of the extract. The flavonoid content was quantified based on another published method [
27]. Further, the saponin and terpenoid contents of the extract were determined by separate methods [
10,
28]. Finally, the plant extract’s cardiac glycoside content was measured based on an earlier technique [
29] while the reducing sugars were quantified according to a previously described method [
30].
AMRECP’s antioxidant activity
DPPH (2,2-diphenyl,1-picrylhydrazine) Assay: To conduct this assay, the method of a former study [
10] was applied. Concentrations ranging from 25 to 100 mg/ml of the plant extract were prepared for this assay. Then 5 ml DPPH-methanol solution (0.004%, w/v) was added and vortexed. The solution was left in the dark for 30 minutes and the absorbance was read at 517 nm. The blank contained methanol, 80% (v/v). The comparison was made with ascorbic acid as the control, and the percentage of scavenging activity in each test tube was determined based on
Equation 1:
.jpg)
Nitric Oxide Antioxidant (NOA) Assay: For the purpose of this assay, a previously described method [
31] was followed. A test tube was set up containing 5 ml sodium nitroprusside (SNP; 5mM) dissolved in phosphate buffer at pH 7.3 and incubated at 25°C for 3 hours and then exposed to oxygen interaction. Equal volumes of the resultant solution and Griess reagent (1.0 ml each) were mixed thoroughly. The Griess reagent consisted of a mixture of 0.1% naphthylethylene diamine dihydrochloride and 1% sulphanilamide in 5% phosphoric acid. The absorbance of the purple-coloured (azo dye) solution was read at 546 nm. The inhibition percentage was evaluated based on
Equation 2:
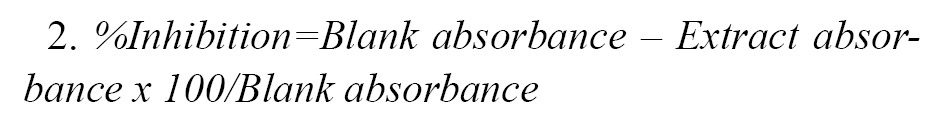
Ferric Reducing Antioxidant Power Assay: The ferric reducing antioxidant power (FRAP) assay was performed as described by an earlier study [
32] and varying concentrations between 25 and 100 mg/ml were prepared. A 2 ml aliquot of the extract was then mixed with a 2 ml of 0.2 M phosphate buffer, a 2 ml potassium ferricyanide (10 mg/ml) was added, and incubated at 50°C for 20 minutes. The mixture was centrifuged at 3000 rpm followed by adding 2 ml trichloroacetic acid (100 mg/l) and stayed for 10 minutes. A 2 ml aliquot of distilled water was added to the supernatant (2 ml), followed by adding 0.8 ml of 0.1% (w/v) newly prepared ferric chloride. The resultant mixture was incubated for 10 minutes, and the absorbance was read at 700 nm. A calibrated curve showing the different concentrations of ferric sulphate was plotted, from which the FRAP values were determined.
In vitro AMRECP’s anti-diabetic activity
Alpha amylase assay: The alpha amylase assay (α-AM) was performed based on a previously described method [
33]. A series of 250 ml aliquots of different concentrations of the extract and acarbose (25-100 µg/ml) plus 2 U/ml α-AM (500 ml) in 100 mM phosphate buffer were incubated for 20 minutes at 37°C. To the above mixtures, we added 1% soluble starch in 100 mM phosphate buffer (250 ml) and incubated the tubes at 37°C for 60 minutes. The samples were then boiled for 10 minutes after the addition of 500 ml of dinitrosalicylic acid (DNSA). Finally, the dilution was achieved by adding 5 ml distilled water. The controls were prepared in a similar manner, except the extract was replaced with distilled water. The samples’ absorbances were read at 540 nm, and the inhibitory activity of α-AM was determined as follows (
Equation 3):

Alpha glucosidase assay: The alpha glucosidase assay (α-GLU) was performed based on an earlier method [
33]. Briefly, 250 ml aliquots of different concentrations of the extract and acarbose (25-100 mg/ml) were added to 1.0 U/ml α-GLU in 100 mM phosphate buffer (500 ml) and incubated at 37°C for 15 minutes. Further, we added 5 ml pNPG (3 mM; 4-nitrophenyl-d-glucopyronoside) dissolved in 100 mM phosphate buffer (5 ml). After incubation at 37°C for 25 minutes, we added 5% (w/v) Na2CO3 to the test tubes. Five minutes later, the entire content was diluted with 5 ml water and vortexed for 10 minutes. The same procedure was repeated for the controls, except that the extract was absent. The absorbance of the product (p-nitrophenol) was read at 405 nm and the percent inhibition of α-GLU was determined based on
Equation 4:
.jpg)
Evaluation of the oxidative stress markers: The evaluation of the oxidative stress markers of the supernatants from the liver homogenates after centrifugation at 3000 rpm was achieved based on a method described previously [
32]. This test evaluated the catalase (CAT), reduced glutathione (GSH), and superoxide dismutase (SOD) activities of the samples. The malondialdehyde (MDA) level was then evaluated by the protocol established by an earlier study [
10].
Evaluation of hepatic function indices: After 12 days of the oral administration of the extract, the animals were anaesthetized by chloroform and the blood samples collected, and were subsequently sacrificed. The blood samples were centrifuged at 3000 rpm for 5 minutes, and the supernatants were used for the evaluation of the liver enzymes’ activities of alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum total protein (STP), and albumin (ALB) levels. The standard assay kits were obtained from Randox Laboratories Limited (London, UK).
Histological examinations of the organs’ samples: The histopathological examinations of liver and pancreas tissue samples were performed based on a previously described method [
34]. Before the microscopic examinations, the excised liver and pancreas tissue samples were preserved in 10% formalin for three days. The samples were further dehydrated in different alcohol gradients (50, 70, 90, or 100%), cleared in xylene I and II solutions, and embedded in paraffin wax. Finally, 4-5μm sections of each organ samples were made by a microtome (Leica RM2125RTS, China), and stained in haematoxylin and eosin (H&E) solution.
Statistical analyses: To perform the statistical analyses, we used an SPSS software, and determined the means and standard deviations. This was followed by performing ANOVA and Tukey’s post hoc tests at a confidence level of P<0.05.
Results
AMRECP’s phytochemical constituents: According to
Figure 1, the result showed that the plant extract’s flavonoids content (38.057±0.28 mg/100 g) was the highest while the terpenoids content was the lowest (17.177±0.20 mg/100 g) compared to other phytochemical compounds quantified.
.jpg)
The results demonstrated that the plant extract was rich in flavonoids. Also, the extract consisted of triterpenoids, the details of which are outlined in
Table 1 below.
.jpg)
Plant extract’s antioxidant activity: The antioxidant characteristics of the extract in terms of median inhibition concentration (IC50) is shown in
Figure 2.
.jpg)
The extract’s IC50 from 2,2 diphenyl-1-picrylhydrazine (DPPH) assay (36.65±0.30 µg/ml) was lower compared to those of nitric oxide (NO) (69.40±0.35 µg/ml) and 106.99±0.16 µg/ml for ferric reducing antioxidant power (FRAP), respectively. The results indicate that the plant extract inhibited the activity of DPP٭ (2,2 diphenyl-1-picrylhydrazyl free radicals) more than nitric oxide free radicals (NO٭) activity and Fe3+ to Fe2+ redox reaction. Again, a significant difference existed between ascorbic acid (control) and plant extract in the various antioxidant assays used (P<0.05).
In vitro Anti-diabetic activity of AMRECP: The results, as presented in
Figure 3, illustrate the plant extract’s inhibitory effect on alpha amylase (α-AM) and alpha glucosidase (α-GLU) activities in relation to the IC50 value.
.jpg)
As shown in
Figure 3, the plant extract inhibited the α-AM and α-GLU activities. However, the inhibition of α-AM compared to that of α-GLU was 3.99%. As a result, the plant extract was a strong inhibitor of α-AM activity compared to that on α-GLU. However, compared to the standard drug (acarbose), the latter inhibited these carbohydrate metabolising enzymes (α-AM and α-GLU) stronger than the plant extract. Finally, the results indicate a significant interaction existed between acarbose and AMRECP (P<0.05).
Anti-diabetic activity of AMRECP: The blood glucose lowering effect in Wistar rats, as induced by alloxan, is presented in
Figure 4.
.jpg)
After 72 hours of treatment with alloxan, the animals’ blood glucose levels significantly increased compared to those in the normal group. But after 12 days of consistent oral administration with 200 mg/kg of the plant extract, the animals’ blood glucose levels dropped drastically by 64.25%. Furthermore, the results showed a significant difference (P<0.05) between diabetic untreated (DUT) and diabetic treated (DT) groups; however, a substantial difference did not exist between the DT and normal groups.
Anti-oxidative stress effect of AMRECP:
Figure 5 shows the effects of the plant extract on the markers of oxidative stress. In comparison with the DUT groups, a rise in the glutathione (GSH) levels was observed in both DT and normal groups.
.jpg)
Moreover, there was significant differences (P<0.05) among the normal, DUT and DT groups. However, no significant differences existed between the DT and DUT groups. The result also showed a significant reduction in the malondialdehyde (MDA) levels between the DT and DUT groups. The MDA levels significantly declined in the normal control group. The result further indicated that a statistically significant difference (P<0.05) existed among the three groups. However, no considerable difference existed between the DT and DUT groups. With respect to superoxide dismutase (SOD) activity, there was a minimal biological effect observed in the DT and normal groups, as compared to the DUT group. However, substantial differences were not observed among the groups. Finally, the results showed that the catalase (CAT) activity increased insignificantly (0.23 µmol) per ml/min/mg in both the DT and normal groups compared to the DUT group.
Anti-hepatotoxic persistence of AMRECP: As shown in
Figure 6, alloxan significantly increased the AST activity in DUT group; however, it declined in the DT group (P<0.05).
.jpg)
Despite the fact that the AST level was lower in the normal control group compared to DUT, no statistical difference existed between the DUT and normal groups. The ALT activity in the normal and DUT group was significantly lower, while the ALT level declined minimally in the DT group compared to that in the DUT group. In addition, statistically significant differences existed between DUT, normal and DT groups. By comparison, the ALP activity in the DT and normal groups dropped remarkably after oral treatment compared to that of the DUT group. However, no statistical significances were observed between the DUT and DT groups. Furthermore, the serum albumin (ALB) level increased insignificantly in both DT and normal groups compared that in the DUT group. Finally, the normal group’s serum total protein (STP) level increased significantly (P<0.05) compared to that in the DUT group. Also, the STP level in the DT group showed a slight increase.
Effect of AMRECP on the liver and pancreas tissues: As seen in
Figure 7 photomicrographs, there was normal hepatocyte distribution in both the DT and normal groups, while blood vessel congestion was observed in the DUT group.
.jpg)
Furthermore, normal pancreatic β-islet cells covered with exocrine acini were noticed in the DT and normal groups. However, necrotic pancreatic β-cells were observed in the DUT group (
Figure 8).
.jpg) Discussion
Discussion
This study investigated the anti-diabetic and hepatoprotective effects of the aqueous-methanol root extract of C. procera in Wistar rats, intoxicated with alloxan. Lipid peroxidation is one of the many biological reactions that occurs in the body, and generates free radicals with considerable side effects. However, the side effects could be minimized or prevented through the use of antioxidants. Evidence-based theories and experimental results have established a positive correlation between reducing power and anti-radical scavenging effects, based on low IC50 values and antioxidant activity of various compounds. In this study, the extract from C. procera roots was found with strong antioxidant property, which might be due to its significantly low IC50 values. This finding suggests that the plant extract has strong scavenging capacity against free radicals [
35,
36].
Hyperglycaemia is linked to a rapid rise in the blood glucose level due to either excessive starch degradation or low glucose absorption rate into cells. These in turn may occur secondary to the defective activity of carbohydrate metabolising enzymes, such as alpha amylase (α-AM) and alpha glucosidase (α-GLU). Scientific reports have demonstrated that hyperglycaemia’s harmful effect occurs when the fasting blood glucose level is higher than postprandial plasma glucose concentration [
37]. To overcome this problem, a therapeutic method was developed via the inhibition of α-AM and α-GLU in the gastrointestinal tract, thereby significantly decreasing glucose digestion [
38,
39]. However, sometimes α-AM and α-GLU inhibition results in certain side effects, such as abdominal discomfort, flatulence and diarrhoea, which are the typical symptoms of using acarbose and other hypoglycaemic synthetic drugs [
33,
40]. Studies have suggested that some plant extracts are inhibitors of α-GLU and α-AM [
33,
41]. Conversely, the findings of the current study demonstrated that AMRECP inhibited the activity of α-AM better than that of α-GLU, which is consistent with the results of a previous research [
10]. The extract’s strong inhibition of α-AM is likely due to its strong antioxidant property or its low IC50 values. Further, a comparative study of past research on C. procera roots extract has shown that AMRECP preferably lowers blood glucose significantly [
42]. The remarkable anti-hyperglycaemic effects of this plant extract as documented in this study may be supported by the following reasons: (1) the extract’s rich content of flavonoids; (2) it’s strong inhibition of α-AM and α-GLU activities (3); its promotion of β-cells regeneration in the pancreas, thereby improving insulin secretion, as evident by the data presented in
Figure 8; and
Figure 4, the extract’s antioxidant power is derived from its ursolic and saponin oleanolic acids contents [
39,
43,
44,
45,
46].
A significant product of lipid peroxidation in biological membrane is the formation of malondialdehyde (MDA), an indicator of chronic hyperglycaemic condition that contributes to the depletion of antioxidant enzymes, such as SOD, CAT and GSH. However, after oral treatment with the plant extract, a reduction in MDA level was recorded with the corresponding increases in the GSH, SOD, and CAT activities. These findings further affirm the strong antioxidant property of AMRECP [
43]. A former study has reported that pre-treatment with alloxan causes hepatotoxicity due to increasing free radical formation, which results in hepatocellular membrane disruption and significant rises in serum AST, ALP and ALT levels. These events occur prior to reductions in the serum levels of ALB and STP [
47]. In this study, the hepatocellular disruptions are likely attributed to the extract’s ability to reduce hepatocyte membrane permeability while stimulating regenerative effects in the liver induced by ursolic acid. Based on the findings of this study it may be concluded that the C. procera roots extract can ameliorate the pathological lesions associated with hepatocellular damages caused by diabetes mellitus [
44].
Conclusions
To bring about a blood glucose reduction, various synthetic drugs are available; however, they may not necessarily address the complications associated with diabetes mellitus. The present study not only produced a phenomenal anti-hyperglycaemic effect, it also reversed the hepatocellular abnormalities in response to alloxan treatment. These positive therapeutic outcomes could be linked to the plant’s strong antioxidant activities, which were contributed by some of its phytochemical constituents, such as flavonoids, ursolic acid, lupeol, and oleanolic acid. In the final analysis, the C. procera root’s extract is highly likely to possess the ability to protect against β-cells toxicity and damages in the pancreas and liver in cases of diabetes mellitus.
Limitations of the study: For future investigation, the number of animals and funding support should be improved, as these factors limited the present study.
Recommendation for future studies: Given the present results, in-depth evaluation of the molecular indicators of hyperglycaemia and liver pathologies are recommended for future studies.
Ethical Considerations
Compliance with ethical guidelines
This study protocol was approved by Ethics Committee of Nigeria Police Academy, Wudil, Kano (NPA/ETC/031/2022).
Funding
The authors declare no financial support was received from any public, commercial or not-for-profit sectors.
Authors' contributions
Conceptualisation and supervision: Ihegboro Godwin Okwudiri; Data computation: Ononamadu Chimaobi James; Laboratory Supervision: Owolarafe Tajudeen Alowonle; Materials and Data collection: Haruna Bello and Matthew Kufre Akpan; Manuscript drafting, revision and Edition: All Authors. All authors approved the final version for submission.
Conflict of interest
The authors declare no conflict of interests with any internal or external entities in conducting this research.
Acknowledgments
The technical services provided by Sunday Adenekan, Olayinka Onifade, Maicah Chijoke and Cadet Jadeoliseh are well appreciated.
References
- American diabetes association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010; 33(1):S62-9. [DOI:10.2337/dc10-S062] [PMID] [PMCID]
- Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol. 2013; 4:37. [DOI:10.3389/fendo.2013.00037] [PMID] [PMCID]
- Lenzen S. The mechanisms of alloxan and streptozotocin-induced diabetes. Diabetologia. 2008; 51(2):216-26. [DOI:10.1007/s00125-007-0886-7] [PMID]
- Andrade FC. Measuring the impact of diabetes in life expectancy and disability-free-life expectancy among older adults in Mexico. J Gerontol Series B Psychol Soc Sci. 2009; 65B(3):381-9. [DOI:10.1093/geronb/gbp119] [PMID] [PMCID]
- Deshpande AS, Harris-Hayes M, Schoolman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008; 88(11):1254-64. [DOI:10.2522/ptj.20080020] [PMID] [PMCID]
- Ray JA, Valentine WJ, Secnik K, Oglesby AK, Cordony A, Gordois A, et al. Review of the cost of diabetes complications in Australia, Canada, France, Germany, Italy and Spain. Curr Med Rese and Opinion. 2005; 21(10):1617-29. [DOI:10.1185/030079905X65349] [PMID]
- Scully T. Diabetes and numbers: Mercury's mysteries start to unfold. Nature. 2012; 485(7398):52-3. [DOI:10.1038/485052a] [PMID]
- Abdel Aziz MT, El-Asmar MF, Rezq AM, Mahfouz SM, Wassef MA, Fouad HH, et al. The effect of a novel curcumin derivative in pancreatic regeneration in experimental type-1-diabetes in rats (long term study. Diabetol Metabol Synd. 2013; 5(1):75. [DOI:10.1186/1758-5996-5-75] [PMID] [PMCID]
- Matsumoto S, Noguchi H, Hatanaka N, Shimoda M, Kobayashi N, Jackson A, et al. Estimation of usability for islet transplantation in the United State, with the Kyoto islet isolation method. Cell Transplant. 2009; 18(5-6):549-56. [DOI:10.1177/096368970901805-610] [PMID]
- Ihegboro GO, Ononamadu CJ, Owolarafe TA, Shekwolo I. Screening for toxicological and anti-diabetic potential of n-hexane extract of tapinanthus bangwensis leaves. Toxicol Res Appl. 2020; 4:2397847320972042. [DOI:10.1177/2397847320972042]
- Del-Guerra S, Mareslli I, Lupi R, Boggi U, Mosca F, Benzi L, et al. Effects of prolonged in vitro exposure to sulfonylureas on the formation and survival of human islet. J Diabetes Complications. 2005; 19(1):60-4. [DOI:10.1016/j.jdiacomp.2004.05.001] [PMID]
- Maedler K, Carr RD, Bosco D, Zuellig RA, Berney T, Donath MY. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J Clin Endocrinol Metab. 2005; 90(1):501-6. [DOI:10.1210/jc.2004-0699] [PMID]
- Tahrani AA, Bailey CJ, Barnett AH, Del-Prato S. Management of type-2-diabetes, new and future developments in treatment. Lancett. 2011; 378(9786):182-97. [DOI:10.1016/S0140-6736(11)60207-9] [PMID]
- Xiu LM, Miura AR, Yamamoto K. Pancreatic islet regeneration by ephedrine in mice with streptozotocin-induced diabetes. Am J Chinese med. 2001; 29:493-500. [DOI:10.1142/S0192415X01000514] [PMID]
- Ravi KU. Ethnomedicinal, pharmaceutical and pesticidal uses of c. procera (aiton) (family asclepiadaceae). Intl J Green pharma. 2014; 8:135-46. [DOI:10.22377/ijgp.v8i3.375]
- Shashank K, Abhay KP, Ashufosh G. Calotropis procera root extract has the capability to combat free radicals mediated damage. ISRN Pharmacol. 2013; 2013:691372. [DOI:10.1155/2013/691372] [PMID] [PMCID]
- Ajagbonna OP, Adeniran A, Lawal RI. Ethnobotanical assessment of plants used to aid parturition, Abuja, Nigeria. Sokoto J Vet Sci. 2019; 17(1):1-9. [DOI:10.4314/sokjvs.v17i1.1]
- Linus CS. Nigerian folklore medicinal plants with potential anti-infertility activity in males: A scientific appraisal. Res J Med Plants. 2016; 10(3):201-27. [DOI:10.3923/rjmp.2016.201.227]
- West African health organisation (WAHO). West African herbal pharmacopoeia. Bobo-Dioulasso: West African Herbal Pharmacopoeia; 2020. [Link]
- Ajay KM, Ajay Y, Mruthyumjayaneda R. Ayurvedic use and pharmacological activities of c. procera, linn. Asian J Trad Med. 2011; 6(2):45-53. [Link]
- Al-Shafi AE. The constituents and pharmacological properties of c. procera-an overview. Intl J Pharm Rev Res. 2015; 5(3):259-75. [Link]
- Mainasara MM, Aliero AA, Aliero BL, Yakubu M. Phytochemical and antibacterial properties of root and leaf extracts of C. procera. Nig J Basic and Appl Sci. 2012; 20(1):1-6. [Link]
- Onu A, Aliyu A, Bilbis S, Ladan MJ, Lawal A, Saidu Y. Α-glucosidase inhibitory potential of selected anti-diabetic plants used in north-western Nigeria. J Med Plant Res. 2013; 7(2):2010-8. [DOI:10.5897/JMPR12.1005]
- NR Council. Guide for the care and use of laboratory animals. Washington: National Academies Press; 2011. [Link]
- Emordi JE, Agbaje EO, Oreagba IA, Iribhogbe OI. Antidiabetic effects of the ethanolic root extract of uvaria chamae p. beauv (annonaceae) in alloxan-induced diabetic rats: A potential alternative treatment for diabetes mellitus. Adv Pharmacol Sci. 2018; 2018:1314941. [DOI:10.1155/2018/1314941] [PMID] [PMCID]
- Gracelin DH, Britto A, Kumar BJ. Quantitative and qualitative analysis of phytochemicals in five Pteris species. Intl J Pharm Pharma. 2013; 5(1):105-7. [Link]
- Ihegboro GO, Afor, E, Alhassan AJ, Edonyabo D, Ononamadu CJ, Owolarafe TA, et al. Nutmeg toxicity: Ameliorative effect of aqueous extract of guiera senegalensis in experimental rat model. Ife J Sci. 2019; 21(2):277-85. [DOI:10.4314/ijs.v21i2.2]
- Gladis RMC, Chellaram. C. Phytochemical screening, total flavonoid, total terpenoid and anti-inflammatory activity of aqueous stem extract of salacia oblonga. J Chem Pharmaceut Sci. 2017; 10(1):550-6. [Link]
- Ghazisaeedi N, Hadjiakhoondi A, Tofighi Z, Yassa N. Determination of cardiac glycosides and total phenols in different generations of securigera securidaca suspension culture. Res J Pharmacogny. 2016; 3(2):25-31. [Link]
- Benedict’s quantitative solution. A quantitative test for reducing sugars. J Biol Chem. 2017; 103:485-7. [Link]
- Alisi CS, Onyeze GOC. Nitric oxide scavenging ability of ethylacetate fraction of methanolic leaf extracts of C. odorata (linn.). Afr J Biochem Res. 2008; 2(7):145-50. [Link]
- Ononamadu CJ, Alhassan AJ, Ibrahim A, Ihegboro GO, Imam AA, Owolarafe TA, et al. In vitro and in vivo anti-diabetic and anti-oxidant activities of methanolic leaf extracts of ocimum canum. Caspian J Intern Med. 2019; 10(2):162-75. [DOI:10.22088/cjim.10.2.162] [PMID] [PMCID]
- Kazeem MI, Adeola SA, Dansu TV. Inhibitory effects of Azadirachta indica A juss leaf extract on the activities of α-amylase and α-glucosidase. Pakistan J Biol Sci. 2013; 16(21):1358-62. [DOI:10.3923/pjbs.2013.1358.1362] [PMID]
- Loha M, Mulu A, Abay SM, Ergete W, Geleta B. Acute and subacute toxicity of methanol extract of syzygiym guineense leaves on the histology of the liver and kidney and biochemical composition of blood in rats. Evid Based Complement Alternat Med. 2019; 2019:5702159. [DOI:10.1155/2019/5702159] [PMID] [PMCID]
- Kumar S, Gupta A, Pandey AK. Calotropis procera root extract has the capability to combat free-radical mediated damage. ISRN Pharmacol. 2013; 2013:691372. [DOI:10.1155/2013/691372] [PMID] [PMCID]
- Hitesh VP, Bhautik P, Jatin DP. Comparative efficacy of phytochemical analysis and antioxidant activity of methanolic extract of c. gigantea and c. procera. Intl J Biol pharma Res. 2014; 5(2):107-13. [Link]
- Ketema EB, Kibret KT. Correlation of fasting and postprandial plasma glucose with HbA 1c in assessing glycemic control; systematic review and meta-analysis. Arch Pub Health. 2015; 73:43. [DOI:10.1186/s13690-015-0088-6] [PMID] [PMCID]
- Deshpande MC, Venkateswarlu V, Babu RK, Trivedi RK. Design and evaluation of oral bioadhesive controlled release formulation of miglitol, intended for prolonged inhibition of intestinal α-glucosidase and enhancement of plasma glycogen-like peptide-1 levels. Int J Pharma. 2009; 380(1-2):16-24. [DOI:10.1016/j.ijpharm.2009.06.024] [PMID]
- Kazeem MI, Mayaki AM, Ogungbe BF, Ojekale AB. In-vitro studies on calotropis procera leaf extracts as inhibitors of key enzymes linked to diabetes mellitus. Iran J Pharm Res. 2016; 15:37-44. [PMID] [PMCID]
- Bafna M, Sancheti S, Sancheti S, Seo SY. Antioxidant and α-glucosidase inhibitory properties of Carpessium abrotanoides. L. J Med Plant Res. 2010; 4:1547-53. [Link]
- Pinto Mda S, Kwon YI, Apostolidis E, Lajolo FM, Genovese MI, Shetty K. Potential of ginkgo biloba l. leaves in the management of hyperglycemia and hypertension using in vitro models. Bioresour Technol. 2009; 100(24):6599-609. [DOI:10.1016/j.biortech.2009.07.021] [PMID]
- VH B, Ajay SS. Anti-hyperglycaemic and anti-hyperlipidemic activities of root extract of c. procera (ait) R.Br. on streptozotocin-induced diabetic rats. Jordan J Biol Sci. 2009; 2(4):77-80. [Link]
- Ahmed MB, Gwarzo MY, Anwar S. “Antioxidative and anti-hyperglycaemic effect of c. procera in alloxan-induced diabetic rats. Academic J. 2016; 10(5):54-8. [DOI:10.5897/JMPR2014.5704]
- Alqahtani A, Hamid K, Kam A, Wong KH, Abdelhak Z, Razmovski-Naumovski C, et al. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr Med Chem. 2013; 20(7):908-31. [DOI:10.2174/0929867311320070007] [PMID]
- Luyen NT, Dang NH, Binh PTX, Hai NT, Dat NT. Hypoglycemic property of triterpenoid saponin PFS isolated from polyscias fruticosa leaves. An Acad Bras Cienc. 2018; 90(3):2881-6. [DOI:10.1590/0001-3765201820170945] [PMID]
- Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Association of dietary flavonoids with risk of type-2-diabetes and markers of insulin-resistance and systemic inflammation in women” A prospective study and cross-sectional analysis. J Am Coll Nutr. 2005; 24:376-84. [DOI:10.1080/07315724.2005.10719488] [PMID]
- Perera S, Lohsoonthorn V, Jiamjarasrangsi W, Lertmaharit S, Williams MA. Assessment between elevated liver enzymes and metabolic syndrome among Thai adults. Diabetes Metab Syndr. 2008; 2:171-8. [DOI:10.1016/j.dsx.2008.04.012] [PMID] [PMCID]










































 , Tajudeen Alowonle Owolarafe2
, Tajudeen Alowonle Owolarafe2 

 , Chimaobi James Ononamadu2
, Chimaobi James Ononamadu2 

 , Haruna Bello2
, Haruna Bello2 

 , Matthew Kufre-Akpan2
, Matthew Kufre-Akpan2 


.jpg)
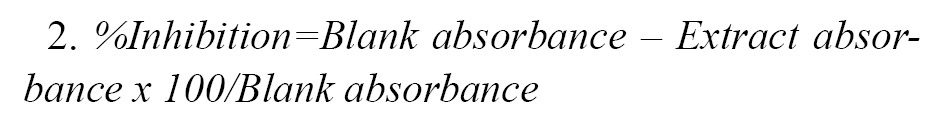

.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
