

1- Faculty of Pharmacy, Hasanuddin University, Indonesia
2- Department of Pharmacy, Faculty of Pharmacy, Hasanuddin University, Makassar, Indonesia. , yulia.yusrini@unhas.ac.id
3- Department of Pharmaceutical Science and Technology, Faculty of Pharmacy, Hasanuddin University, Makassar, Indonesia.
Full-Text [PDF 1346 kb]
(325 Downloads)
|
Abstract (HTML) (766 Views)
Full-Text: (85 Views)
Introduction
Physalis angulata Linn is an endemic plant grown in both tropical and subtropical regions of the world. P. angulata leaves (PAL) contain several chemical compounds, including alkaloids, glycosides, flavonoids, tannins, and phenolics. The PAL extract has pharmacological and antioxidant properties against numerous conditions, such as cancer, inflammation, leishmaniosis, malaria, diabetes, asthma, and bacterial infections (1, 2). Similar to other traditional medications, the PAL extract toxicity data has not been explored extensively although this is crucial to confirm the safety of medicinal plants and their extracts. Therefore, comprehensive tests are required to assess their toxicity and adverse effects in animals and humans (3).
A logical way to ascertain the toxicity of medicinal plants is through conducting sub-acute toxicity studies. In such studies, the substance in question is administered daily at gradually increasing doses to several groups of experimental animals over a long enough periods, such as 28-30 days. These tests are performed to collect information on the effects of repeated oral exposures. The tests can also provide information regarding the appropriate concentrations of the compounds under study for chronic studies, i.e., the dose-response relationship and/or the signs and symptoms that have not been previously reported (4).
Preclinical toxicity studies usually involve both male and female animals. However, if the study exclusively uses one animal species, typically female animals serve as the best candidates. Many reports have shown that there is little difference in sensitivity between sexes, but when differences do exist, female rats tend to be slightly more sensitive in response to such studies. In this context, female animals that are considered for the study should be nulliparous and non-pregnant (4).
The majority of toxicity studies investigate the effects of substances on the kidneys and liver as these organs are more susceptible to toxicants than others (4). Kidneys are essential in maintaining body homeostasis through the excretion of urea, electrolytes, other endogenous substances, and environmental toxicants (5). The liver functions as an important gland in the biochemical processing of nutrients, excretion of waste metabolites, and controlling the flow and safety of substances absorbed from the gut before entering the systemic circulation (6).
However, studies concerning the toxicity of PAL extract are scarce. Previously, an acute toxicity study in 2011 conducted by Rathore (7) showed that a single administration of PAL extract caused no mortality in experimental animals at 2000 mg/kg. This study has not been followed up by a subacute toxicity test, using a repetitive administration of the extract over 14 to 28 days.
Aim of the Study: the current study aimed at investigating the subacute toxicity of the PAL extract on the functions and histological structures of the kidneys and liver in female Wistar rats.
Materials and Methods
Preparation of Samples and Chemicals: The P. angulata leaves (PAL) were obtained from the Biringkassi village, Indonesia. They were washed in clean water and dried under sunlight for 24 hours. The chemicals, such as ethanol (70%), sodium carboxymethyl cellulose (Na-CMC), and sodium chloride (NaCl 0.9%) solution were purchased from local distributors in Makassar, Indonesia.
Sample Extraction: Six hundred grams of dried PAL were extracted by maceration method, using 70% ethanol as the solvent, over 24 hours at room temperature with occasional stirring. The solution was filtered and the PAL material was macerated three times until a clear solution was obtained. The extract was then evaporated, using a rotary evaporator to concentrate the extract devoid of the solvent. The extract samples were stored in a desiccator and the yield was determined using the following equation:
% yield of extract = thick extract weightsample weight x 100%
Determination of Total Flavonoid Content: The total flavonoid content was determined using UV-Vis spectrophotometry with quercetin as the standard comparison. The maximum wavelength measurement was performed at 400-800 nm range, and a standard curve of quercetin was plotted, at 432.50 nm. The total flavonoid content was deduced by measuring the absorbance of the sample at the same wavelength.
Preparation of Animals: Twenty female Wistar rats, weighing 200-250 grams, were obtained and acclimatized for one week prior to starting the study. The animal care and study protocols were based on the institutional guidelines. The study protocols were reviewed and granted an ethical clearance (Registration No.: 8572/UN4.14.1/TP.01.02/2022).
Subacute Toxicity Testing: Following acclimatization, the rats were selected randomly and divided into four groups of five each. The control group was untreated and received 0.5%
Na-CMC as a placebo via oral gavage. The other three groups received oral doses of the PAL extract suspended in Na-CMC at 100, 500, or 1000 mg/kg of the rats’ body weight. The rats were observed daily for symptoms of toxicity, such as agility, stretching, diarrhea, mortality, and changes in body weight. The following serum biochemical markers were assessed: aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, and creatinine. The biomarker levels were assessed before and after the 28-day period of the assigned treatments in each group.
Determination of Organs’ Weights: At the end of the experiments, the rats were anesthetized using ether via inhalation. The liver and kidneys from each rat were harvested, cleansed in 0.9% NaCl solution, and dried with absorbent papers. Each organ was immediately weighed to obtain the absolute weight, then the relative organ weights were calculated based on the following formula:
Relative organ weight = absolute organ weightbody weight x 100%
Serum Biochemical Analyses: The blood samples were collected in vacutainer tubes and centrifuged at 3000 rpm for 20 minutes to obtain the sera. The ALT, AST, urea, and creatinine levels were measured according to the standard laboratory instructions. The blood serum (100μL) was mixed with the buffer (100μL) to homogeneity and incubated at 37°C for five minutes. Subsequently, 250µL of the kit substrate was added to the samples, mixed to homogeneity, and incubated at 37°C for one minute. The serum biomarker levels of ALT, AST, urea, and creatinine were read at 340 nm, using a Humalyzer 3500 spectrophotometer (Human, Germany) (8).
Histopathological Analyses: The kidneys and liver harvested from each rat were fixed in 10% formalin, washed, and cut to 1-cm thick sections. The specimens were stored in embedding cassettes and processed in a tissue processor (Diapath, Italy) for varying time periods at each stage. The specimens were then embedded in paraffin wax blocks and sectioned by a microtome at 4-5µm, and stretched by floating out at 40°C. The sections were then placed on glass slides and dried on an electric hotplate for two hours. Next, the sections were stained with hematoxylin and eosin. Then, one or two drops of xylene were placed on each slide and covered with a glass coverslip. The slides were allowed to stand until they dried completely. The histopathological examinations of the slides were performed under a light microscope (Magnus, India), and photomicrographs were taken at 40x magnification for further analyses.
Statistical Analyses: The data were tested for normality, using Shapiro-Wilk’s method and one-way ANOVA analyses. A Tukey’s HSD post hoc test was also used to determine the differences among the groups, with the statistical significance set at P<0.05.
Results
Extract Yield and Total Flavonoid Content: In this study, a total of 92.88 g of the PAL extract was obtained with a 15.48% percent yield. The total flavonoid content was 22.47 mg of quercetin in each gram of the PAL extract.
Clinical Symptoms: Daily observations were made to assess the symptoms of toxicity that occurred during the subacute toxicity tests for the PAL extract. Agitation, stretching, diarrhea, mortality, and changes in body weights of the rats were the metrics reported in this study. See Table 1. The results showed the only clinical sign observed in rats was diarrhea, which occurred in the groups treated with the PAL extract at 500 and 1000 mg/kg. A steady gain in body weight was documented in the control rats and those treated with the PAL extract at 100 mg/kg. Unlike these two groups, insignificant weight losses were observed in the rat groups treated with 500 and 1000 PAL extract after 7 days of administration (Figure 1).
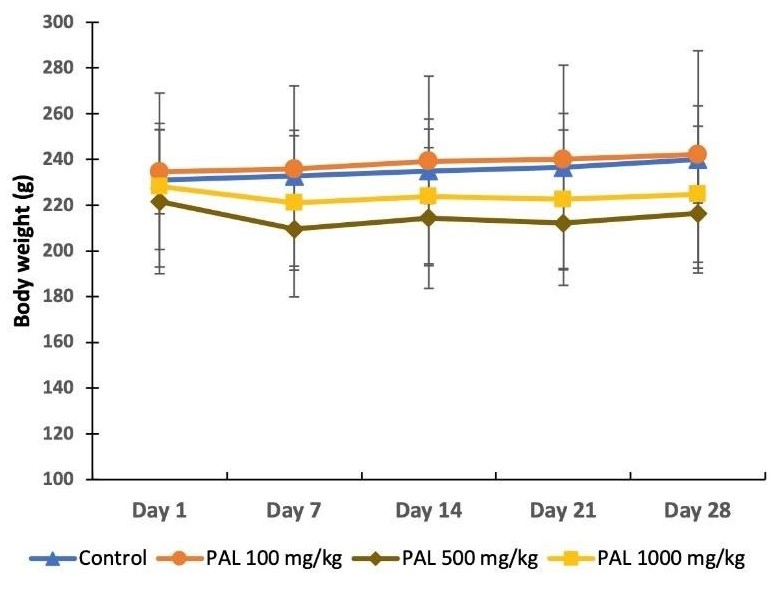
Figure 1. The body weights of rats during 28 days of the study. Key: PAL = P. angulata leaf extract.
Relative Organs Weights: The relative weights of the liver and kidneys in the control group were 3.54 ± 0.30% and 0.36 ± 0.04%, respectively. Statistical analysis indicated that there were no significant differences in the relative weight of the organs between the control and the rat groups treated with the PAL extract.
Serum Biomarkers: Figure 2 shows the levels of AST and ALT before and after the 28-day treatment period. There were no significant differences in the AST and ALT levels among all of the study groups prior to the treatments. Following the 28-day treatment, the control group had the AST and ALT levels at 107.3 ± 9.67 U/L and 60.74 ± 17.6 U/L, respectively. Meanwhile, all of the PAL-treated groups had slightly higher AST levels post treatments, i.e., approximately 40% higher than that of the controls. However, the increases in AST levels in the PAL-treated groups were not statistically significant compared to that of the controls. A slight elevation of the ALT levels was also observed in the groups that received the PAL extract at 500 and 1000 mg/kg, but this elevation did not reach the statistically significance compared to the controls.
Figure 3 demonstrates the urea and creatinine levels before and after the treatments. Similar to the liver biomarkers, the levels of serum urea and creatinine were not significantly different before and after the treatments. In the control group, the serum urea and creatinine levels were 57.1 ± 4.69 mg/dl and 0.328 ± 0.10 mg/dl, respectively. All of the PAL-treated groups had similar levels of serum urea and creatinine compared to those of the controls.
Histopathological Analyses: Figures 4 and 5 display photomicrographs of the liver and kidneys histopathology, using H&E staining under light microscopy at 40x magnification. The liver microscopic images demonstrated normal structures and organelles in the hepatocytes, sinusoids and portal veins (Figure 4, panel-1). In all of the PAL extract-treated animals, no significant liver injuries were detected. Although the hepatocytes nuclei were intact, a higher number of lymphocytes and Kupffer cells were identified in the sinusoids compared to the control group, especially in rats treated with the PAL extract at 1000 mg/kg (Figure 4, panels 2, 3, 4). However, in the renal tissue micrographs, no histopathological alterations were observed in the control, PAL 100 and PAL 500 mg/kg groups (Figure 5, panels 1, 2, 3). Nonetheless, at 1000 mg/kg, the PAL extract treatment caused mild alterations in the renal histology, as evident by the dilation of Bowman’s capsules and hemorrhages in the tubular areas (Figure 5, panel 4).
Table 1. The Clinical Signs Found during 28 Days of the Treatments.
| Study Groups / Day |
Agitate |
Stretching |
Diarrhea |
Mortality |
| Control |
|
|
|
|
| 1 |
Normal |
- |
- |
- |
| 7 |
Normal |
- |
- |
- |
| 14 |
Normal |
- |
- |
- |
| 21 |
Normal |
- |
- |
- |
| 28 |
Normal |
- |
- |
- |
| PAL Extract 100 mg/kg BW |
|
|
|
|
| 1 |
Normal |
- |
- |
- |
| 7 |
Normal |
- |
- |
- |
| 14 |
Normal |
- |
- |
- |
| 21 |
Normal |
- |
- |
- |
| 28 |
Normal |
- |
- |
- |
| PAL Extract 500 mg/kg BW |
|
|
|
|
| 1 |
Normal |
- |
- |
- |
| 7 |
Normal |
- |
- |
- |
| 14 |
Normal |
- |
- |
- |
| 21 |
Normal |
- |
√ |
- |
| 28 |
Normal |
- |
- |
- |
| PAL Extract 1000 mg/kg BW |
|
|
|
|
| 1 |
Normal |
- |
- |
- |
| 7 |
Normal |
- |
- |
- |
| 14 |
Normal |
- |
√ |
- |
| 21 |
Normal |
- |
√ |
- |
| 28 |
Normal |
- |
- |
- |
Key: PAL = P. angulata leaf extract; - = not observed; √ = observed
Table 2. Relative Weights of Rats’ Liver and Kidneys after 28 Days of Treatment.
| Study Groups |
Liver Weight (%) |
Kidneys Weight (%) |
| Control |
3.54 ± 0.30 |
0.36 ± 0.04 |
| PAL 100 mg/Kg |
3.55 ± 0.33 |
0.35 ± 0.04 |
| PAL 500 mg/Kg |
3.76 ± 0.24 |
0.37 ± 0.02 |
| PAL 1000 mg/Kg |
3.85 ± 0.13 |
0.36 ± 0.02 |
Key: PAL = P. angulata leaf extract

Figure 2. The comparison of liver biomarkers’ levels before and after the 28-day treatment in the control and extract-treated (PAL) groups.

Figure 3. The comparison of the kidneys biomarkers’ levels before and after the 28-day treatment in the control and extract-treated groups (PAL).

Figure 4. Representative micrographs (1-4) of the histological structures of the rat liver.
Liver Cells - A: Hepatocytes; B: Sinusoids; C: Kupffer cells; D: Lymphocytes.
Panels - 1: Control; 2: PAL extract, 100 mg/kg; 3: PAL extract, 500 mg/kg; 4: PAL extract, 1000 mg/kg.

Figure 5. Representative micrographs (1-4) of the histological structures of the rats’ kidneys.
Tissue Structures - A: Glomeruli; B: Bowman’s capsules; C: Hemorrhage; D: Dilation of Bowman’s capsule.
Panels - 1: Control; 2: PAL extract, 100 mg/kg; 3: PAL extract, 500 mg/kg; 4: PAL extract, 1000 mg/kg.
Discussion
Medicinal plants are believed to be the “backbone” in traditional healthcare systems worldwide (9). They have been used not only for the prevention but also in the treatment of many chronic diseases (10). In this context, the use of P. angulata leaves (PAL) is popular in traditional medicine. However, the toxicity of this plant’s materials has not been studied adequately, especially for their sub-acute toxicity, although several reports have been published on its acute toxicity to date. In this context, two studies have demonstrated that the methanolic and ethanolic extracts of PAL did not cause death after single administrations at 2000 and 5000 mg/kg, respectively (7, 11). However, this is insufficient to prove the safety of the extract. Results from toxicity studies are convincing since they justify the state of adverse effects due to interactions between the toxicants and the treated tissues and cells (12). Results from sub-acute toxicity studies provide rigorous data, since the extract is administered daily over 28 days or longer. In these studies, the liver and kidneys become the primary target organs since they would most likely be affected by the metabolites generated by the toxicants (13).
The Extract’s Major Components: In the current study, the extraction of PAL leaves yielded 92.88g (15.4%) of a thick substance. The yield of an extract can be influenced by several factors, including the type of solvent and its concentration (14). Flavonoids are the key compounds that contribute to pharmacological properties of various extracts. These compounds are hydroxylated phenolic substances, known to be synthesized by the plants in response to microbial infections (15). The study findings demonstrated that the PAL extract was rich in flavonoids, as detected in each gram of the material.
Clinical and Physical Effects: In the subacute toxicity test, daily observations of the effects were made. The results revealed clinical symptoms of diarrhea and insignificant fluctuating weight changes occurred in the rat groups that received the extract at 500 and 1000 mg/kg. Diarrhea can arise due to the stimulation of the parasympathetic nerves, promoting digestive tract activity, i.e., increases in the intestinal peristalsis and gastric juice secretion. Body weight changes may also be a good indicator of the adverse effects of drugs and chemicals, and is considered significant if body weight loss is greater than 10% compared to the initial weight (16). In the current study, there were no major body weight losses, i.e., 3-5% of the initial weight. However, the weight loss may be clinically important since it happened in the groups that received a high dosage of the extract.
Effects on the Liver Biomarkers: Serum biomarkers are the parameters of interest in toxicity studies, which provide information regarding the overall health status, mechanism of toxicity, metabolism, and adverse effects on the target organs (16). In this context, AST and ALT are biomarkers that indicate if there was any adverse effect on the liver function. The enzyme AST is found in the liver, brain, pancreas, kidneys, lung, cardiac and skeletal muscles, leukocytes, and red blood cells. On the other hand, ALT is the major enzyme found in liver cells, and it is specifically used to diagnose the liver function disorders. This enzyme is also found in skeletal muscles at low concentrations (17). Based on the statistical analyses, there were no significant differences between the serum’s AST or ALT levels among the treatment groups. Although statistically insignificant, there were elevations in the AST and ALT levels in the rat groups treated with the PAL extract at 500 and 1000 mg/kg. Indeed, the mean AST level in the groups treated with the PAL extract at 500 and 1000 mg/kg was 40% higher than that of the control group. This may serve as a precaution when using higher doses of the PAL extract in animal models over long periods of time. In this regard, one study has shown that a 30-day administration of PAL extract at the doses of 250, 500, and 1000 mg/kg was safe as judged by the liver function tests; however, it caused mild damages to the liver structures in the rats (18).
Effects on the Kidneys Biomarkers: Creatinine and urea are biomarkers that are used to detect renal damages. In this regard, both blood urea and creatinine are closely related. When creatinine increases, it is usually accompanied by a rise in the serum urea. However, creatinine is believed to be a more specific indicator of renal disease than urea (19). Based on statistical analyses, there were no significant differences among the treatment groups with respect to the serum creatinine or urea levels. This finding suggests that there was no renal dysfunction caused by the administration of the PAL extract. A previous study has shown that the kidneys seemed to be more versatile to toxicants compared to the liver in certain cases (20). This may also be the case with PAL extracts.
Histopathological Alterations: In addition to alterations in the serum biomarkers, we also examined the toxic effects on liver and kidneys. Our observations from the kidneys and liver histological examinations did not support significant degenerations in the organs of the three treatment groups. However, mild histological changes were observed in the liver and the kidneys of the rats that received the PAL extract at 1000 mg/kg. The histological changes included a higher number of Kupffer cells and lymphocytes in the sinusoids, indicating an inflammatory response might have been triggered by the administration of the PAL extract at its highest dose. Similarly, an abnormal dilation of Bowman’s capsule in the glomeruli of the rats that were given the PAL extract at 1000 mg/kg was also detected along with mild hemorrhages in the tubular areas. This strongly suggests that the PAL extract at 1000 mg/kg caused an inflammatory reaction but not severe enough to lead to disorders in the liver and renal functions.
Effects on Organ Weights: The relative organ weight is fundamental to ascertain whether the organ was exposed to the toxic injuries or was not affected by metabolic reactions caused by the toxicant [16]. The results of this study revealed that there were no significant differences in the relative weight of the organs among the treatment groups.
Conclusions
The administration of P. angulata leaves extract for 28 days in rats did not cause significant alterations in the liver and renal functions. However, there were clinical symptoms of diarrhea, slight weight loss, and mild histological alterations in the liver and kidneys. These were triggered by the PAL extract at the highest dose of 1000 mg/kg. Therefore, cautions should be exercised when using the PAL leaves extract at high doses in humans, especially for a long period, to avoid the potential adverse effects.
Ethical Considerations
All experimental protocols involving animals have been granted institutional ethical clearance by the Ethics Committee, Faculty of Public Health, Hasanuddin University (Registered number: 8572/UN4.14.1/TP.01.02/2022).
Conflict of Interests
The authors declare no conflict of interests with any internal or external entities in conducting this study.
Funding
This study was funded by the authors only.
Authors' Contributions
All authors have reviewed and approved the final version of the manuscript prior to submission. Individual authors’ contributions to this study were as follows:
Lestiariani conducted the experiment and collected data for serum and histological analysis; was involved in statistical analysis; and wrote the first draft of the manuscript.
Yulia Y. Djabir designed the study, revised the final manuscript before submission to this journal, and participated in all data analysis and interpretations.
Abdul Rahim was involved in the data analysis and interpretations, and contributed the manuscript revision before submission for publication.
Acknowledgement
This study was partly extracted from the Master’s degree thesis project data conducted by the first author.
References
1. Ramakrishna Pillai J, Wali AF, Menezes GA, Rehman MU, Wani TA, Arafah A, et al. Chemical Composition Analysis, Cytotoxic, Antimicrobial and Antioxidant Activities of Physalis angulata L.: A Comparative Study of Leaves and Fruit. Molecules. 2022; 27(5). [doi: 10.3390/molecules27051480] [pmid: 35268579]
2. Arruda JCC, Rocha NC, Santos EG, Ferreira LGB, Bello ML, Penido C, et al. Physalin pool from Physalis angulata L. leaves and physalin D inhibit P2X7 receptor function in vitro and acute lung injury in vivo. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2021; 142:112006. [doi: 10.1016/j.biopha.2021.112006] [pmid: 34392085]
3. Nguenang GS, Ntyam ASM, Kuete V. Acute and Subacute Toxicity Profiles of the Methanol Extract of Lycopersicon esculentum L. Leaves (Tomato), a Botanical with Promising In Vitro Anticancer Potential. Evidence-based complementary and alternative medicine : eCAM. 2020; 2020:8935897. [doi: 10.1155/2020/8935897] [pmid: 32215048]
4. OECD. Repeated Dose 28-Day Oral Toxicity Study in Rodents. Organizion Economic Cooperation Development Guide Test Chemical. 2008; 407(1):1-13.
5. Dipiro JT, Yee GC, Posey LM, Haines ST, Nolin TD, Ellingrod V. Pharmacotherapy A Pathophysiologic Approach Eleventh Edition. New York: McGraw Hill2020.
6. Ozougwu JC. Physiology of the liver. Internationa Journal of Research Pharmacy Bioscience. 2017; 4(8):13-24.
7. Rathore C, Dutt K, Sahu S, Deb L. Antiasthmatic activity of the methanolic extract of Physalis angulata Linn. Journal of Medical Plantet Researchs. 2011; 5(22):5351-5.
8. Fiqardina A, Djabir YY, Santoso A, Salsabil NS, Ismail I. The nephroprotective effect of clove oil (Oleum caryophylli) against levofloxacin toxicity in rats. Iranian Journal of Toxicology. 2022; 16(1):27-34.
9. Ahvazi M, Khalighi-Sigaroodi F, Charkhchiyan MM, Mojab F, Mozaffarian VA, Zakeri H. Introduction of medicinal plants species with the most traditional usage in Alamut region. Iranian Journal of Pharmatic Research. 2012; 11(1):185-94.
10. Adewale OB, Onasanya A, Anadozie SO, Abu MF, Akintan IA, Ogbole CJ, et al. Evaluation of acute and subacute toxicity of aqueous extract of Crassocephalum rubens leaves in rats. Journal of ethnopharmacology. 2016; 188:153-8. [doi: 10.1016/j.jep.2016.05.003] [pmid: 27154407]
11. Sukandar EY, Sheba SH. Acute and sub-chronic toxicity studies of a combination of Physalisangulata L. (Cecendet) extract and methylprednisolone on animals. International Journal of Integration Health Science. 2013; 7(1):48-55.
12. Das N, Goshwami D, Hasan S, Raihan SZ. Evaluation of acute and subacute toxicity induced by methanol extract of Terminalia citrina leaves in Sprague Dawley rats. Journal of Acute Disease. 2015; 4(4):316-21. [doi: 10.1016/j.joad.2015.05.001]
13. Jothy SL, Zakaria Z, Chen Y, Lau YL, Latha LY, Sasidharan S. Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules. 2011; 16(6):5268-82. [doi: 10.3390/molecules16065268] [pmid: 21701437]
14. Susanti RF, Kurnia K, Vania A, Reynaldo IJ. Total phenol, flavonoid and antioxidant activity of Physalisangulata leaves extract by subcritical water extraction. Modern Applied Sciences. 2015; 9(7):190-8.
15. Chen XG, Zhang QY, Wang Y, Liu DJ, Zhang N. Model test of anchoring effect on zonal disintegration in deep surrounding rock masses. TheScientificWorldJournal. 2013; 2013:935148. [doi: 10.1155/2013/935148] [pmid: 23997683]
16. Everds NE. Evaluation of clinical pathology data: correlating changes with other study data. Toxicologic pathology. 2015; 43(1):90-7. [doi: 10.1177/0192623314555340] [pmid: 25361750]
17. Lim AK. Abnormal liver function tests associated with severe rhabdomyolysis. World journal of gastroenterology. 2020; 26(10):1020-8. [doi: 10.3748/wjg.v26.i10.1020] [pmid: 32205993]
18. Admaja S. Acute and subchronic toxicity test of ciplukan herbal extract (Physalis angulata L.) on biochemical parameters and liver histopathology in Wistar strain rats (Rattus norvegicus). M.Sc. Thesis, Setia Budi University. Surakarta, Indonesia.2018.
19. Zhang G, Guo X. Limiting the testing of urea: Urea along with every plasma creatinine test? Journal of Clinical Laboratory Analysis. 2017; 31:1-6.
20. Djabir YY, Arsyad A, Usmar U, Wahyudin E, Arwi H, Rupang IS. The stages of development of liver and renal injuries in rats induced by fixed dose combination of anti-tuberculosis regimen. Fabad Journal of Pharmaceutical Sciences. 2020; 45(1):29-35.
Type of Study:
Research |
Subject:
General













































