

Haruna M, Gambo A M, Haruna F A, Attah M O O. Trigonella Foenum-Graecum L. Ameliorates Diclofenac-Induced Renal, Hepatic and Duodenal Damages in Albino Rats. IJT 2023; 17 (3) :27-35
URL:
http://ijt.arakmu.ac.ir/article-1-1214-en.html
1- Department of Human Anatomy, Faculty of Basic Medical Sciences, University of Maiduguri, Borno State, Nigeria
2- Department of Human Anatomy, Faculty of Basic Medical Sciences, University of Maiduguri, Borno State, Nigeria, and Faculty of Medicine, Cyprus International University, Nicosia, Cyprus , Marthaorendua@unimaid.edu.ng
Full-Text [PDF 1478 kb]
(655 Downloads)
|
Abstract (HTML) (1801 Views)
Full-Text: (611 Views)
Introduction
Diclofenac is one of the most widely used non-steroidal anti-inflammatory drugs (NSAID), particularly in low-income nations, because of its high efficacy and low cost (1). Due to its analgesic, anti-inflammatory, and antipyretic properties, diclofenac is frequently prescribed, particularly in developing nations (2). The long-term and improper use of NSAIDs have been known to have serious side effects, such as gastrointestinal damages, cardiovascular illness, neurotoxicity, nephrotoxicity, and hepatotoxicity (1, 3-6). Alternatively, many nations have long used traditional herbal remedies to manage and treat many of the above mentioned illnesses. Nowadays, pharmaceutical industry increasingly includes a significant amount of active ingredients derived from plants in their products (7). The herb Trigonella foenum-graecum L. (TFG) also known as "fenugreek seeds" is extremely popular in South and Central Asia, Africa, India, and Iran as a medicinal plant (8, 9). Also, it is one of the most commonly used medicinal plants in folk treatment (10).
Traditional medicine has employed fenugreek to treat a many illnesses, since the seeds and leaves are known to have a variety of medicinal properties (11, 12). The plant and aerial parts have long been valued as the sources of protein for humans and animals, as well as in traditional medicine, for the treatment of a variety of ailments (9, 10). Interestingly, fenugreek has a wide range of therapeutic effects, such as pain relief, anti-diabetic, anti-atherosclerotic, anti-inflammatory, and antispasmodic properties. It also prevents GI gas formation and serves as laxative, anti-cancer, improving sexual desire, cardiotonic and antihypertensive. Further, it is popular in the treatment of high blood triglycerides, stimulation of breast milk secretion and enhancing oxytocin function (10-13).
Levofloxacin and similar brands have frequently been prescribed for the treatment of renal and hepatic toxicities. However, these drugs have many side effects, such as headaches, dizziness, difficulty breathing, abdominal pain, and coughs among others (14, 15). In addition, people in low-income nations, especially those in rural areas with poor healthcare systems and lack of drugs availability, cannot easily afford to obtain these medications.
Aim of the Study: The current study was planned to assess the efficacy of TFG to prevent diclofenac-induced damages to the duodenal, renal and hepatic tissues compared to the use of Levofloxacin. Based on our preliminary literature review, TFG may be used to protect against liver and kidney damages. We investigated the effect of TFG extract on the histology of the rats’ liver, kidneys and intestinal tissues.
Materials and Methods
Plant Authentication, Extraction and Storage: The TFG samples were purchased from a local herb store. The seeds were identified and authenticated by a taxonomist from the Department of Biological Science, University of Maiduguri, Nigeria. The plant samples were deposited and registered at the herbarium of the Department of Human Anatomy with a registration voucher number: UM/HAH/2021/003. The plant extraction was carried out as described below. A 200g portion of the dried seeds was mixed in one liter of distilled water in a beaker and heated for one hour at 40°C. It was allowed to cool down at room temperature and strained through a filter cloth. The filtrate was then evaporated until the volume was reduced to 400ml. The extract was then kept in a refrigerator until it was used in further experiments.
Animal Husbandry: Thirty five Albino rats, weighing 112-252 grams, were used in this study and kept in the animal house in well aerated standard cages (7 rats/cage), and were fed standard laboratory diet and tap water ad libitum. The cages were kept at room temperature, optimal humidity, and automatic alternating 12 hours of light and dark cycles, which were a suitable environmental condition for rats. Before starting the experiments, the rats were allowed to acclimatize for seven days to get adapted to the animal house conditions.
Experimental Design: A simple random sampling method was used to divide the rats into five groups, designated as I, II, III, IV and V, comprising of seven rats per group. The study protocol was followed strictly in compliance with the established “Guide for the care and use of laboratory animals in research and teaching” as detailed in NIH publications and in accordance with the ethical requirements of the University of Maiduguri, Nigeria. The aqueous extract of Fenugreek seeds was administered to the rats via the orogastric route for a period of seven days, as described in the treatment protocol below and in Table 1.
Treatment Protocol: Diclofenac (50mg/kg) was administered orally to the rats in groups II, III, IV and V to induce toxicity to the liver and kidneys. This regimen was continued in these groups for a period of three days. Levofloxacin at 500mg/kg was administered as the treatment to rats in group V, which served as the positive control group. The TFG extract and treatment was administered to rats in groups III and IV orally through an orogastric tube. Twenty four hours after the last treatment, all animals were sacrificed and the organs of interest removed for histopathological examinations. The brief treatment protocol is also presented in the Table 1.
Animal Sacrifice: For euthanasia, Ketamine hydrochloride (100mg/kg) was administered to each rat via intramuscular route into the left thigh. A laparascopic procedure was performed with a midline incision to expose the rats’ liver, kidneys and abdominal organs. The liver and kidneys were harvested, rinsed in normal saline (0.9%), weighed and fixed in 10% formalin for later histological examinations.
Histological Analyses: The harvested liver, kidneys and duodenal tissues were rinsed in 0.9% saline, then fixed in 10% neutral buffered formalin and were dehydrated in graded alcohol (Sigma- Aldrich, USA). The dehydration process was carried out by immersing the issue specimens in graded series of ethanol solutions of increasing concentrations until absolute alcohol was used. Xylene (Veckridge Chemicals, New Jersey) was the reagent used to clear the alcohol present in the tissue samples after the dehydration process and to prepare them for the next stage of tissue preparation. The tissues were then infiltrated and embedded with paraffin in tissue blocks which were clamped onto a microtome for sectioning (Leica RM2125 rotary microtome; Wetzlar, Germany). Sections of 5μm thickness were made and stained with Hematoxylin and Eosin (Abbey Color, Philadelphia). The sections were then photographed, using an Amscope light microscope (MBJX- ISCOPE, Los Angeles) which was fitted with a digital camera (M500, 64X, version 3.7) and observed at 40X and 00X magnifications. Ample photomicrographs were taken from the histological sections of liver, kidneys and duodenum at 40X, 100X and 400X magnifications.
Statistical Analyses: The obtained data were presented as means ±standard error of the means. One-way analysis of variance (ANOVA) was also used to calculate the total variations in the data sets. A P<0.05 value was set as the statistical significance level.
Results
The Effect of TFG on the Physical Appearance of Rats: Rats in group I showed no weakness or hair loss, and generally gained weight during the course of the study. Rats in group II became very weak and sluggish compared to those in other groups, and their weakness level was compared to the rats in groups III and IV. The rats in groups II to V that received diclofenac showed loss of appetite and inactivity, which affected their weights during the period of administration of the extract and treatment.
The Effect of TFG on Rats’ Weights: The weight of the rats in all groups that were administered diclofenac declined during the course of the study. The rats weight in the control group; however, increased by 2.7g on average. The other groups showed a decrease in the body weight, however, the group that were treated with 1000mg/kg TFG showed a slight increase in weight during the study compared to the group that received TFG at 500mg/kg. There was also a decrease in the body weights of the rats (group V) that received Levofloxacin as the treatment (Figure 1).
The Effect of TFG on the Liver and Kidneys Indices: The kidneys and liver indices were determined as the ratio of the organs’ weights to the weight of the body, multiplied by 100. As shown in Figure 2, the liver indices in groups III and IV that received TFG extract was higher than those in the control and treatment groups (II & V). The kidney indices showed an increase in group II (negative control) and group IV that received 1000 mg/kg TFG extract (Figure 3).
Histological Observations
Effect of TFG on the Duodenum: The histological features of the duodenum in all groups are shown in Figures 4A-E and the intestinal villi are shown in Figures 5A-E. The normal histology of the rats’ duodenum in the untreated group revealed that the entire thickness of the intestinal layers was preserved, demonstrating the intestinal villi as finger-like projections extended into the luminal space. The lamina propria extended into the villi, intestinal glands were seen close to the submucosal layer, and opened into the lumen through crypts of Lieberkuhn. The submucosal layer extended though the length of the duodenum, containing blood vessels and connective tissue. The muscular layer was thick and external to the submucosa (Figure 4A).
Figure 4B shows the histology of the duodenum in group II. The duodenal sections from this group showed abnormal histological structures in the mucosal lining. Sloughing off of the surface epithelial cells was evident with necrosis of the functional cells of the intestinal glands, causing the villi in this group to be shorter than those in the control group. The nuclei of the glandular lining epithelia showed nuclear pyknosis, erosion in the epithelial layer and causing the intestinal crypts of Lieberkuhn to open up directly into the luminal space. Peyer’s patches were also observed in the submucosal space in group II. Also, the micrographs of the duodenum in group III rats showed shorter villi and structures similar to those of the rats in group II (Figure 4C).
The villi in the duodenum of rats in group IV were preserved well. The intestinal crypts opened up into the spaces among these glands and numerous intestinal glands were located in the submucosa, which were more robust in this group than those of all other groups. The muscular layer in this group was also thicker compared to those of other groups (Figure 4D). The intestinal villi in group V were preserved and contained a core of lamina propria. The intestinal glands were abundant in the in the submucosa, and the muscular layer was similar to those seen in other groups (Figure 4E).
Figures 5A-E present higher magnifications of the villi present in the cell lining the intestinal tract. In group I, the cells lining the villi were short columnar epithelia, with rounded nuclei located close to the base of the cells. The cells in the epithelial layer were continuous and arranged adjacent to each other. The lamina propria was cellular and several cells were located along with connective tissue components (Figure 5A). The continuous layer of epithelial cells was disrupted, as shown in the micrographs representing group II. Anucleated columnar cells located adjacent to each other, with gaps between them (Figure 5B). The lining epithelia in the duodenum of group III had numerous anucleated cells interspersed in between the columnar epithelial cells of the villi. The lamina propria appeared to have fewer cells compared to those in other groups (Figure 5C). The lamina propria in group IV was very cellular and the columnar lining cells were preserved well in this group (Figure 5D). The columnar epithelial cells of the duodenum in group V also had numerous goblet cells interspersed in between them, and the lamina propria extended among the lining cells (Figure 5E).
Effect of TFG on the Liver: Figures 6A-E represent the liver tissues from all groups. In the control group I, the hepatocyte cords radiated towards the central veins and the sinusoids appeared as spaces in between the hepatocyte cords (Figure 6A). The hepatocytes section from the negative control rats (diclofenac-treated) showed massive histopathological distortions with cytoplasmic vacuolations noted in the hepatocytes in most peripheral regions of the liver lobules. The hepatic cords were disorganized and the spoke-like arrangements radiating towards the central vein were not as evident as that in group I, and the sinusoidal spaces were the widest in this group (Figure 6B). These arrangements were restored in the liver of rats in group III as the hepatic cords radiated towards hepatic veins, as seen in Figure 6C. The liver tissue in group IV also showed hepatocytes cords radiating towards the central veins accompanied by vascular congestion of the lumen (Figure 6D). Again, the arrangements were distorted in group V. The sinusoidal spaces were discontinuous and did not run towards the central vein as did in other groups (Figure 6E).
Effect of TFG on the Renal Tissue: The effect of TFG extract on the renal tissue is represented in Figures 7A-E and 8A-E. The glomerular tufts were located in the renal capsules, which were suspended in the Bowman’s space, and the renal tubules surrounded the glomeruli in the renal parenchyma. These tubules were lined by simple cuboidal epithelia, resting on a basement membrane (Figure 7A). The kidney tissues in all groups showed similar structures as those described for the control group at lower magnifications (Figures 7B-E).
At a higher magnification, the flattened epithelial cells formed the glomerular capsules, which were observed in the group I rats, surrounded the cellular glomeruli. The cuboidal epithelia lining the renal tubules were also observed with centralized and rounded nuclei (Figure 8A). The glomerular tufts in group II had fewer cells than those in group I. Some red blood cells were observed in the Bowman’s capsules. Also, the cuboidal epithelia in the renal tubules appeared distorted and the cellular arrangements were not as apparent as that of the control group (Figure 8B).
Table 1. Treatment Protocols in Experimental Animals.
| Group |
Treatment |
| Group I |
Control (equivalent ml of distilled water) |
| Group II |
Negative Control (50mg/kg Diclofenac) |
| Group III |
Low Dosage (500mg/kg TFG + 50mg/kg Diclofenac) |
| Group IV |
High Dosage (1000mg/kg TFG + 50mg/kg Diclofenac) |
| Group V |
Positive Control (50mg/kg Diclofenac + 500mg/kg Levofloxacin) |
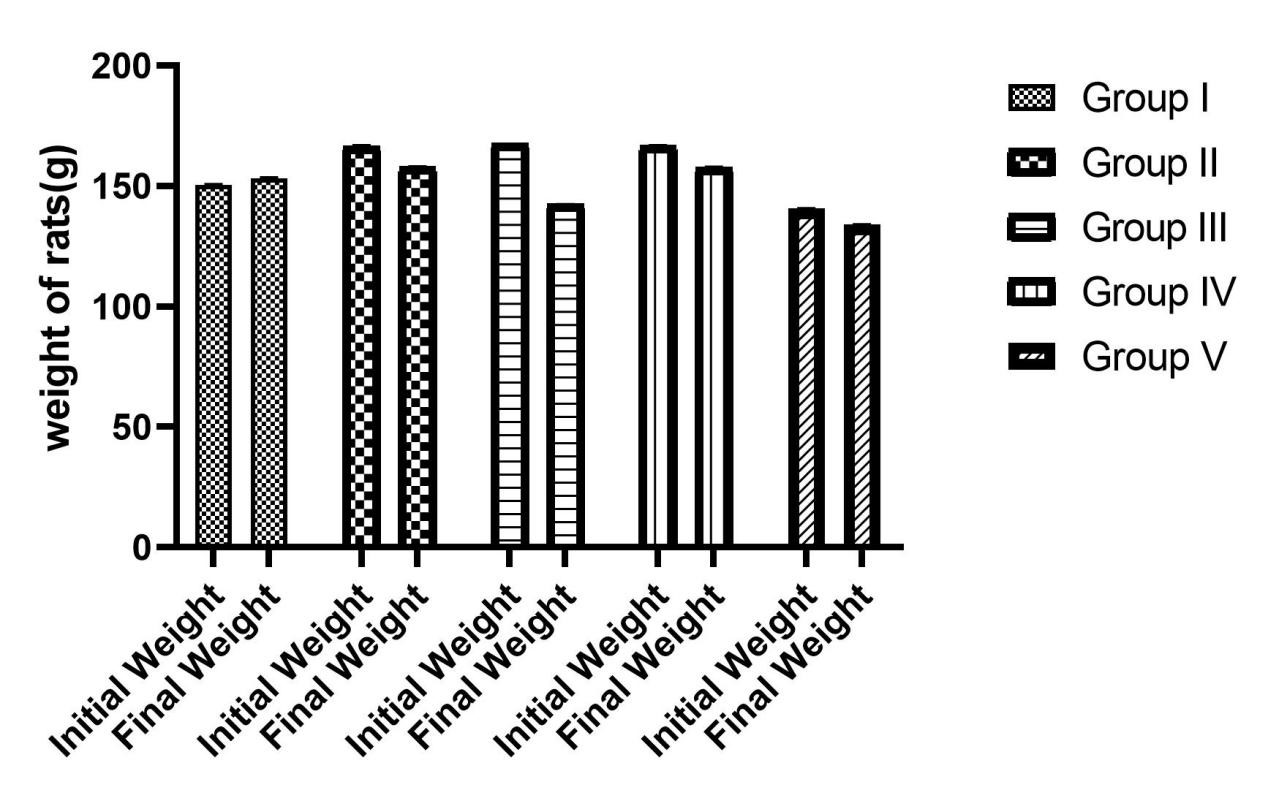
Figure 1. Weight Changes in the Rats at the Start and End of the Experimental Study.
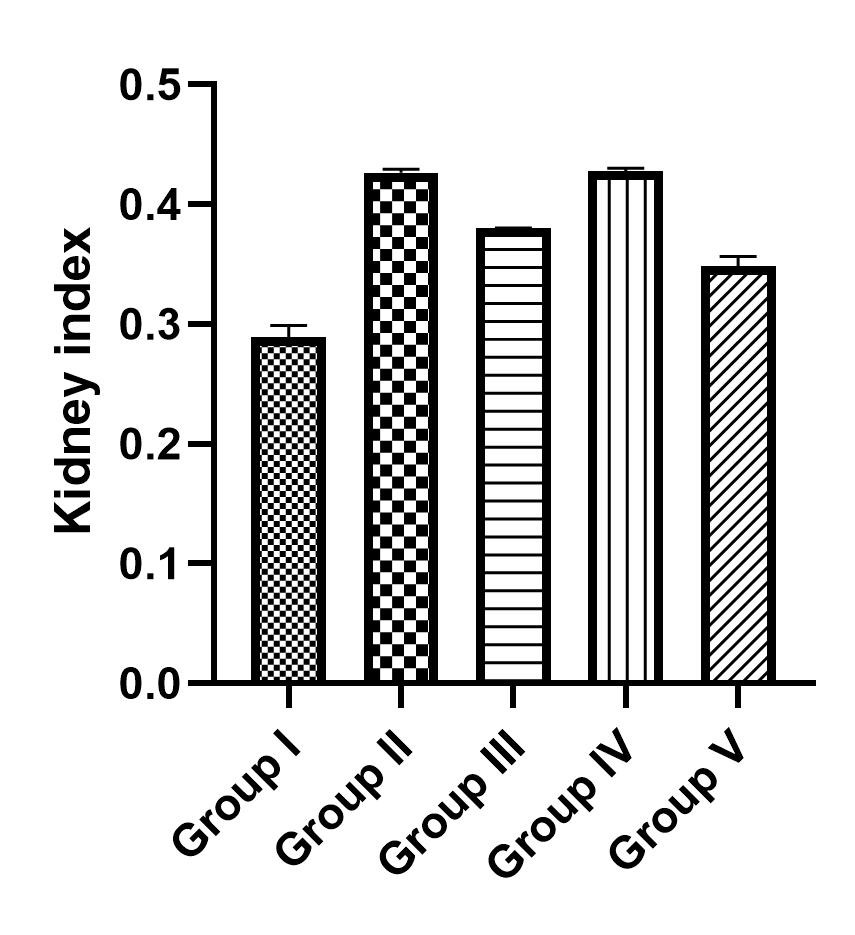
Figure 2. The liver index in groups I-V.
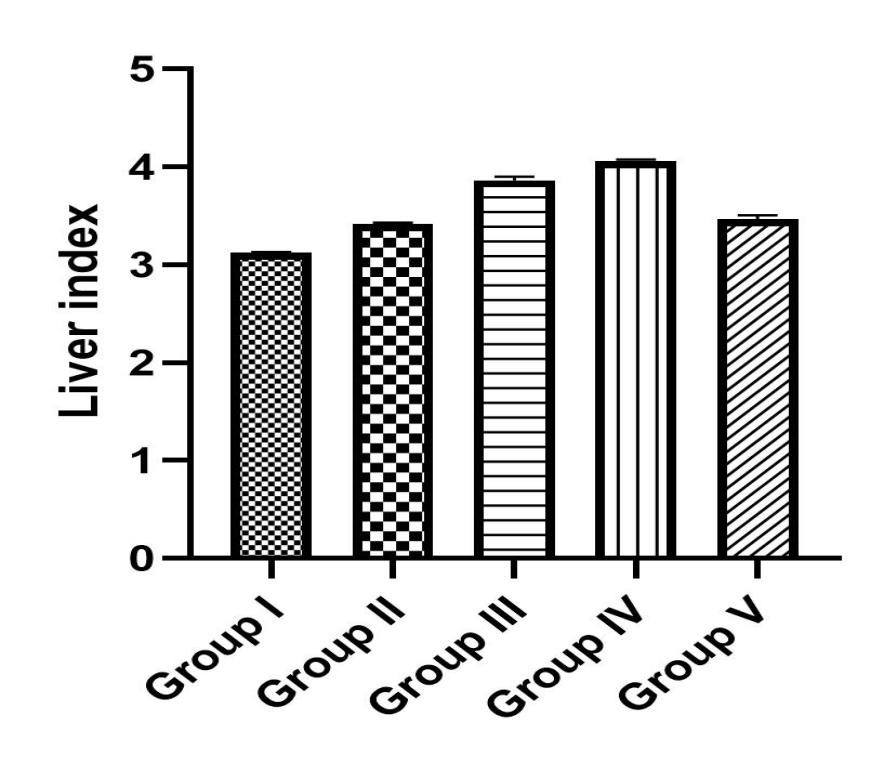
Figure 3. The kidney index in groups I-V.
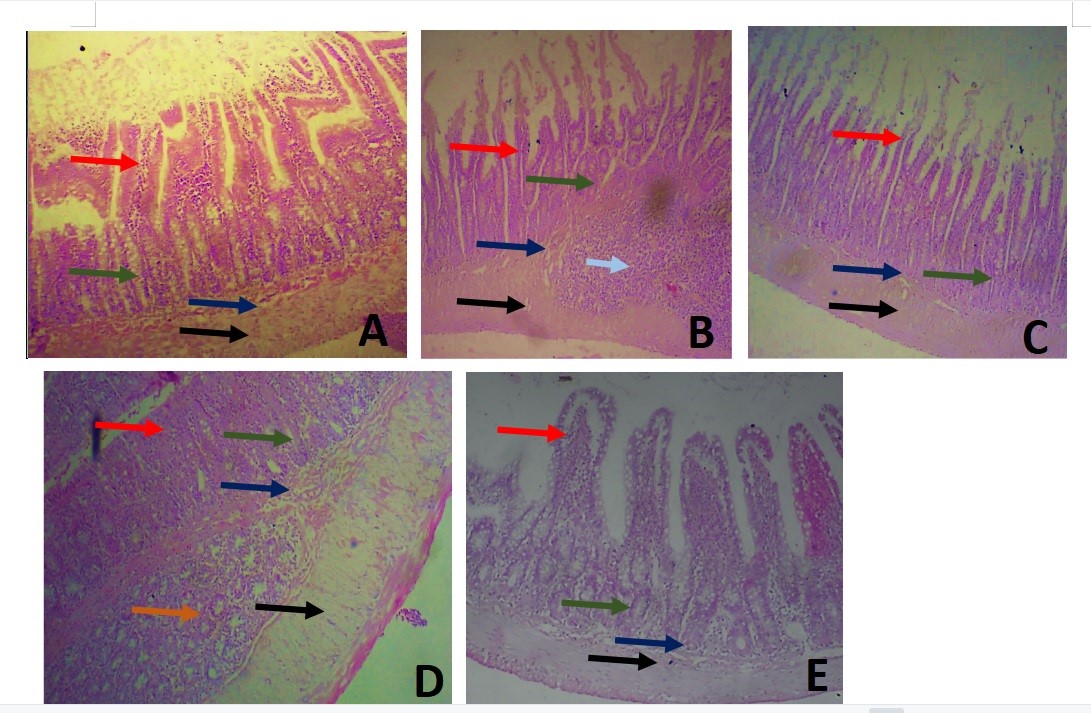
Figure 4. Microscopic image of the duodenum at a magnification of 40X showing the histology of the duodenum of rats in treated and control groups. A) Showing the normal histology of intestinal villi (red arrow) (B) this was distorted in group II which was the negative control group; intestinal glands were found in all groups (green arrow) with intestinal glands of Brunner (orange arrow). Peyer’s patches (pale blue arrow) was also observed in this group (C) Group III represents the rats that received 500mg/kg TFG showing eroded villi in group IV (D) represents the group that received 1000mg/kg TFG and (E) represents the duodenum of rats treated with Levofloxaxin. The submucosa in all groups (navy arrow) contained blood vessels and connective tissue. The muscular layer in all groups was robust and arranged below the submucous layer (black arrow). H&E stained.
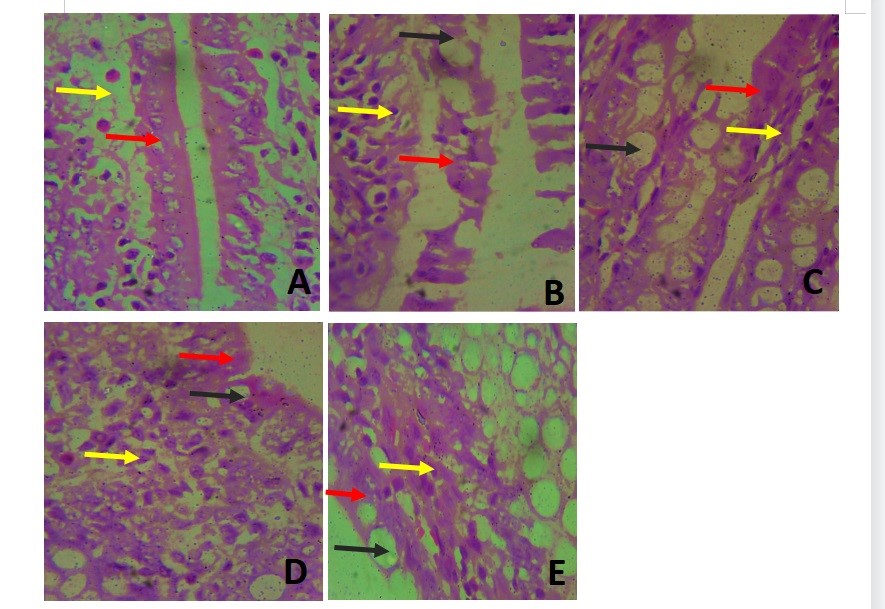
Figure 5. Photomicrograph of the intestinal villi at 400X. The lining epithelial cells (red arrow) had several goblet cells (black arrow) interspersed between them in groups II-V. The connective tissue of the lamina propria (yellow arrow) was very cellular in groups II, IV and V (Figures B, D & E).
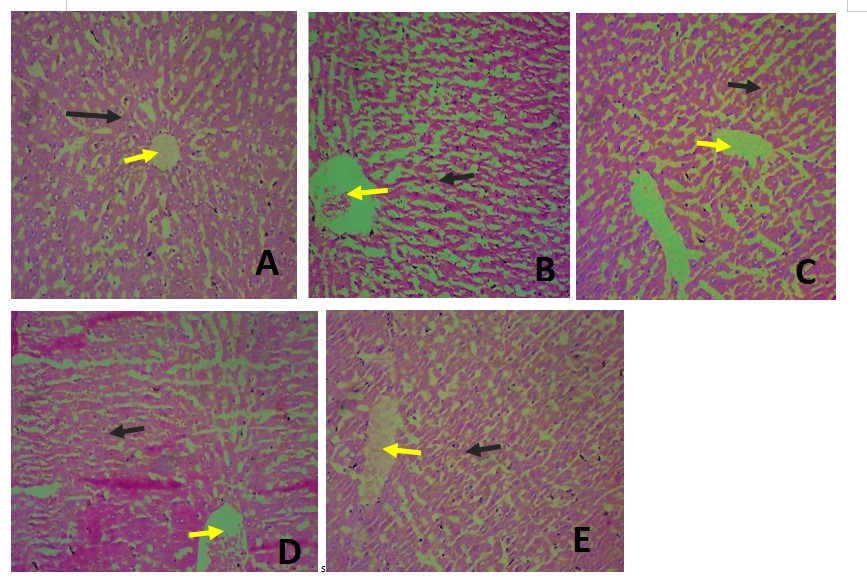
Figure 6. Photomicrograph showing the histology of the liver in all groups at a magnification of 40X.
A) The liver tissue showed a normal arrangement of cord-like hepatocytes (black arrow) radiating towards a central vein (yellow arrow). B) showed the liver in the negative control group which showed a distortion of the spoke-like arrangement. (C) the group treated with 500mg/kg TFG had clear central vein whereas the central vein was filled with blood in group IV (Fig. 6D) and V (Fig.6E)
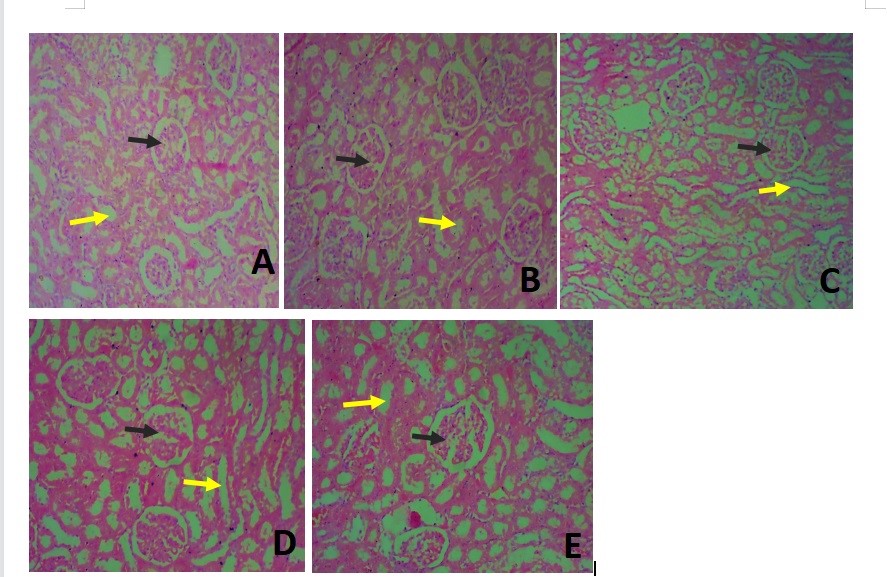
Figure 7. Photomicrograph of the kidney at a magnification of 40X. (A) A shows the normal histology of the kidney showing the glomerulus (black arrow) in all groups and surrounded by renal tubules (yellow arrow). These appeared distorted in group II and dilated in groups III and IV (Figures 6C and 6D). The kidney in group V appeared histologically normal at this magnification (6E).
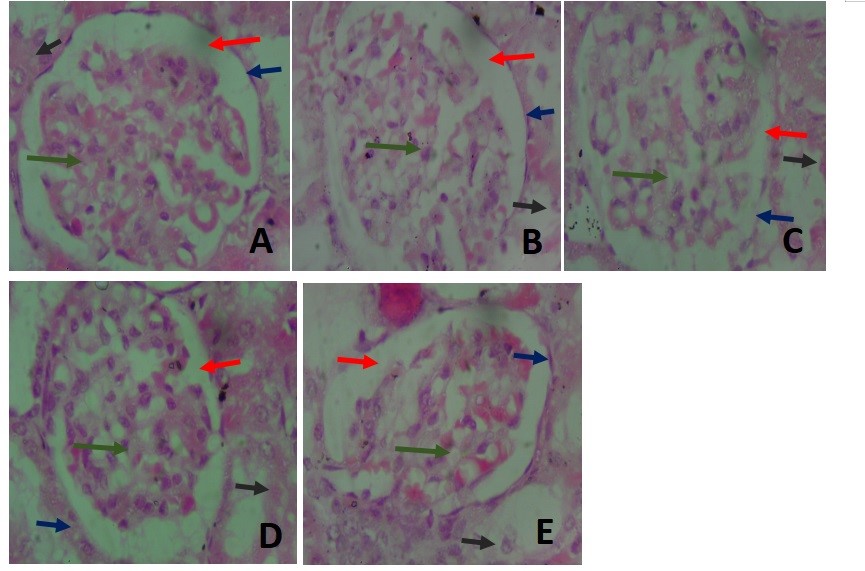
Figure 8. Photomicrograph of the kidney at a magnification of X400. (A) shows the histo-architecture of the glomerulus and renal tubules at a higher magnification showing the capillary tuft of the glomerulus in the control group(green arrow). The glomerulus was very cellular in groups I and IV (Figure 8A and 8D). The glomerular space had traces of blood in the kidney of rats in group V (Figure 8E). The capsular space (red arrow) was widest in group V (Figure 8E) and had blood elements in group II (Figure 8B). The renal capsule (blue arrow) was continuous in all groups but appeared thickest in group IV (Figure 8D). The cells in the renal tubules (black arrow) appeared deteriorated in group III (Figure 8B).
The renal glomeruli in group III showed similar cellular arrangements as those in group II, with the renal tubules distorted (Figure 8C). The renal tissue in group IV rats showed cellular arrangements most similar to those of the control group. The renal tubules also had cuboidal cells with centralized, rounded nuclei. The Bowman’s capsules appeared thickest in this group as compared to those in other groups (Figure 8D). Also, the glomeruli in group V showed red blood cells within their spaces, with the urinary spaces being widest as compared to those seen in other groups. The cuboidal cells lining the renal tubules were well preserved, centrally located, and had round nuclei (Figure 8E).
Discussion
The use of herbs and medicinal plants as a source of medication is a widely spread practice in many parts of the world. Natural products are used as therapeutic agents for the treatment of various diseases due to their minimal side effects (3). As a Leguminosae family member, TFG is well-known for its therapeutic and nutritional properties as has been discussed well by earlier studies. It has been reported that TFG exhibits protective effect against sodium nitrate-induced hepatotoxicity (16) and thioacetamine-induced nephrotoxicity (17). However, TFG’s effect on diclofenac induced hepatorenal damages, and gastrointestinal disturbances in rats have not been investigated. Therefore, this study aimed to evaluate the potential ameliorative effect of TFG on the histopathological changes in diclofenac-induced hepatorenal and intestinal damages in rats.
In the present study, TFG caused slight gains in the rats’ body weights even though there was a reduction in the rats’ weight immediately after diclofenac administration. This observation is similar to that reported by an earlier study (17) where treatment with TFG extract mildly reduced the body weights in the test and supplementary groups as compared to the control group. The liver samples’ weights in the present study were increased in groups that received TFG compared to those in the control group. This was contrary to the findings reported by an earlier study (16). That study observed that treatment with fenugreek seeds extract decreased the liver weight of the test and supplementary groups as compared to the test and control groups.
The administration of diclofenac at 50 mg/kg resulted in hepatic cellular damages characterized by cytoplasmic vacuolations detected in the hepatocytes and distorted hepatic cords. Treatment with TFG restored the arrangement of hepatocytes; however, there were still some damages found in the liver tissue at the completion of the present study. Another study (16) has shown that treatment with TFG mitigated focal and moderate hepatocytic damages, interhepatocytic hemorrhages and vascular congestions in the rats’ liver tissue due to NaNO2 toxicity. Also, a former study (15) has confirmed the hepatoprotective effect of TFG in thioacetamide-induced liver damages.
In the current study, the kidneys of the rats treated with TFG showed that the extract at 500 and 1000mg/kg preserved the kidneys histological features. In a previous study (18) it has been found that the renal structures of rats administered 2.5% fenugreek extract also showed preserved cortex, glomeruli, renal tubules, and cortical blood vessels with normal interstitial tissues. These findings are similar to those of the present study. The authors of that study also reported mild ischemic changes in the glomeruli at the TFG concentrations of 5% and 7.5% whereas in the current study, there was only slight hemorrhage in the glomeruli and into the urinary spaces at a TFG concentration of 1000mg/kg. This indicates that at higher concentrations, TFG may cause bleeding in the renal tissue.
A former study (16) has reported that exogenously administered TFG may alleviate the hepatotoxicity and nephrotoxicity effects of NaNO2, as it reduced renal intra-tubular damages in the treated groups. Also, another study (18) has reported that renal histology in diabetic rats showed mild diffuse mesangial expansions as compared to normal glomeruli, while the rats treated with 5% TFG in their feed showed significant reductions in the mesangial expansion scores. In the present study, TFG treatment ameliorated the damages detected in the intestinal mucosa following administration of diclofenac. Some studies have shown similar results consistent with the protective effect of TFG on gastrointestinal tissue (13, 18-20). These studies have shown that oral administration of TFG increased the rate of healing of digestive wounds created by phenylbutazone and azepein in rats as compared with those in control groups.
Conclusions
The findings of the current study provided experimental evidence that TFG can result in promising therapeutic effects by significantly preventing the toxicity of diclofenac in Albino rats and its induced damages in the duodenum, liver and kidneys compared to similar therapeutic effects of levofloxacin. However, further studies are warranted to explore the therapeutic and medicinal properties of TFG extract, and the mechanisms of action.
Conflict of Interests
The authors declare no conflict of interests with any entity in conducting this study.
Funding
This research project was funded solely by the authors and did not receive any funding from any internal or external sources.
Acknowledgement
The authors acknowledge the contributions of Prof. S.H. Garba, Dr. J.V. Zirahei, Dr. N.I. Dibal, Dr. H.B. Ishaya, Dr. L.L. Mshelia, Dr. M.S. Chiroma of the University of Maiduguri for their valuable advice and assistance. The authors are also grateful to the staff of the Histology Laboratory, University of Maiduguri, for their generous assistance during the course of conducting experiments to complete this research.
Ethical Considerations
Compliance with ethical guidelines: Ethical Considerations: This study was conducted in accordance with the guidelines of University of Maiduguri Research and Ethics Committee, the ARRIVE guidelines (reporting of in vivo experiment), and the National Institutes of Health (NIH) guide for the CARE and use of laboratory animals (NIH Publications No. 8023, revised 1978). It was approved by the Ethics Committee of the Department of Human Anatomy, University of Maiduguri (Code #: UM/HA/UGP 19.20‑117).
Authors' Contributions
Study conception and design: MH, AMG, FAH and MOOA. Data collection and processing, performing experiments: MH, AMG, FAH. Analysis and interpretation of the results: MH, AMH, FAH and MOOA. Writing the manuscript: MOOA, MH, AMH and FAH. Critical revision and editing of the manuscript: MOOA. Final approval of the manuscript prior to submission for publication: MH, AMG, MOOA and FAH. Study supervision: MOOA. Funding administration: MH, AMH and FAH.
References
1. Anwar MM, Laila IMI. Mitigative effect of caffeine against diclofenac-induced hepato-renal damage and chromosomal aberrations in male albino rats. BMC complementary medicine and therapies. 2022; 22(1):327. [doi: 10.1186/s12906-022-03802-y] [pmid: 36482339]
2. Mostafa RE, El-Marasy SA, Abdel Jaleel GA, Bakeer RM. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon. 2020; 6(2):e03330. [doi: 10.1016/j.heliyon.2020.e03330] [pmid: 32025584]
3. Khan RA. Natural products chemistry: The emerging trends and prospective goals. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society. 2018; 26(5):739-53. [doi: 10.1016/j.jsps.2018.02.015] [pmid: 29991919]
4. Aycan IO, Elpek O, Akkaya B, Kirac E, Tuzcu H, Kaya S, et al. Diclofenac induced gastrointestinal and renal toxicity is alleviated by thymoquinone treatment. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2018; 118:795-804. [doi: 10.1016/j.fct.2018.06.038] [pmid: 29935248]
5. Alabi QK, Akomolafe RO. Kolaviron Diminishes Diclofenac-Induced Liver and Kidney Toxicity in Wistar Rats Via Suppressing Inflammatory Events, Upregulating Antioxidant Defenses, and Improving Hematological Indices. Dose-response : a publication of International Hormesis Society. 2020; 18(1):1559325819899256. [doi: 10.1177/1559325819899256] [pmid: 32165871]
6. Hassan RA, Hozayen WG, Abo Sree HT, Al-Muzafar HM, Amin KA, Ahmed OM. Naringin and Hesperidin Counteract Diclofenac-Induced Hepatotoxicity in Male Wistar Rats via Their Antioxidant, Anti-Inflammatory, and Antiapoptotic Activities. Oxidative medicine and cellular longevity. 2021; 2021:9990091. [doi: 10.1155/2021/9990091] [pmid: 34422219]
7. Olayaki LA, Adeyemi WJ, Yinusa JS, Adedayo GA. Omega-3 fatty acids moderate oxidative and proinflammatory events in experimental hepatotoxicity in Wistar rats: comparison with livolin. Synergy. 2018; 7:17-24. [doi: 10.1016/j.synres.2018.08.001]
8. Nematollahi S, Pishdad GR, Zakerkish M, Namjoyan F, Ahmadi Angali K, Borazjani F. The effect of berberine and fenugreek seed co-supplementation on inflammatory factor, lipid and glycemic profile in patients with type 2 diabetes mellitus: a double-blind controlled randomized clinical trial. Diabetology & metabolic syndrome. 2022; 14(1):120. [doi: 10.1186/s13098-022-00888-9] [pmid: 35999562]
9. Sriuttha P, Sirichanchuen B, Permsuwan U. Hepatotoxicity of Nonsteroidal Anti-Inflammatory Drugs: A Systematic Review of Randomized Controlled Trials. International journal of hepatology. 2018; 2018:5253623. [doi: 10.1155/2018/5253623] [pmid: 29568654]
10. Subramanian SP, Prasath GS. Trigonelline improves insulin sensitivity and modulates glucose homeostasis in high fat fed-streptozotocin induced type 2 deiabetic rats. Journal of Pharmaceutical Research. 2014; 8(4):563-9.
11. Srinivasan K. Fenugreek (Trigonella foenum graecum): A review of health beneficial physiological effects. Food Reviews International. 2006; (22):203-24. [doi: 10.1080/87559120600586315]
12. Abduljawad SH. The ameliorative effect of Fenugreek (Trigonella foenum graecum L.) seeds on obese albino rats. Advances in Environmental Biology. 2015; 9(11):26-32.
13. Bahmani M, Shirzad H, Mirhosseini M, Mesripour A, Rafieian-Kopaei M. A Review on Ethnobotanical and Therapeutic Uses of Fenugreek (Trigonella foenum-graceum L). Journal of evidence-based complementary & alternative medicine. 2016; 21(1):53-62. [doi: 10.1177/2156587215583405] [pmid: 25922446]
14. Esposito S, Noviello S, Leone S, Ianniello F, Ascione T, Gaeta GB. Clinical efficacy and tolerability of levofloxacin in patients with liver disease: a prospective, non comparative, observational study. Journal of chemotherapy. 2006; 18(1):33-7. [doi: 10.1179/joc.2006.18.1.33] [pmid: 16572891]
15. Olayinka ET, Ore A, Ola OS. Influence of Different Doses of Levofloxacin on Antioxidant Defense Systems and Markers of Renal and Hepatic Dysfunctions in Rats. Advances in Toxicology. 2015; (3):1-7. [doi: 10.1155/2015/385023]
16. Uslu GA, Uslu H, Adali Y. Hepatoprotective and nephroprotective effects of Trigonella foenum-graecum L. (Fenugreek) seed extract against sodium nitrite toxicity in rats. Biomedical Research. 2019; 6(5):3142-50.
17. Fatima NS, Masood J. Fenugreek seeds attenuate thioacetamide induced liver damage. Pakestan Journal of Pharmatic Sciences. 2021; 34(3):933-42.
18. Badr MI. Effect of fenugreek seeds on kidney structure and some physiological parameters in rats. Middle East Journal of Applied Sciences. 2017; 7(4):967-73.
19. Sayed AA, Khalifa M, Abd el-Latif FF. Fenugreek attenuation of diabetic nephropathy in alloxan-diabetic rats: attenuation of diabetic nephropathy in rats. Journal of physiology and biochemistry. 2012; 68(2):263-9. [doi: 10.1007/s13105-011-0139-6] [pmid: 22237966]
20. Nasri H, Nematbakhsh M, Ghobadi S, Ansari R, Shahinfard N, Rafieian-Kopaei M. Preventive and curative effects of ginger extract against histopathologic changes of gentamicin induced tubular toxicity in rats. International Journal of Preventive Medicine. 2013; 4:316-21.
Type of Study:
Research |
Subject:
General
















































