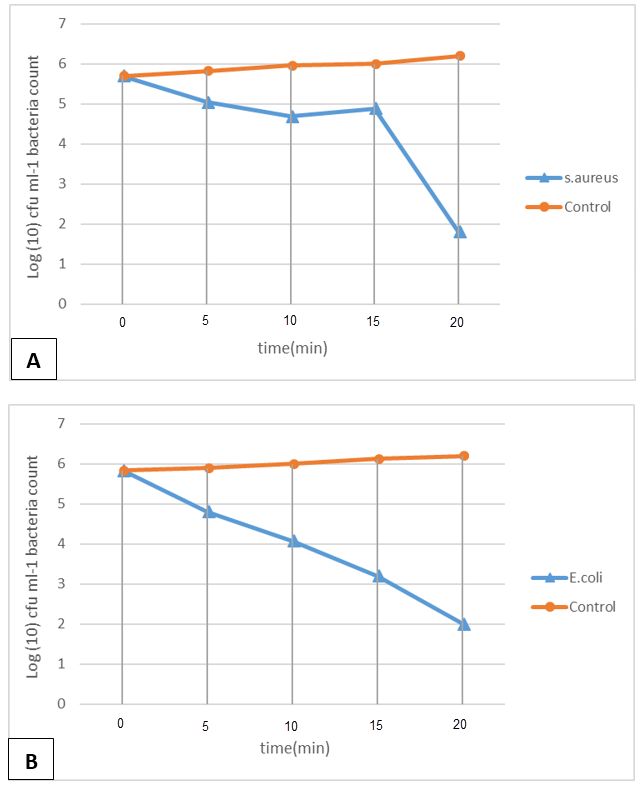
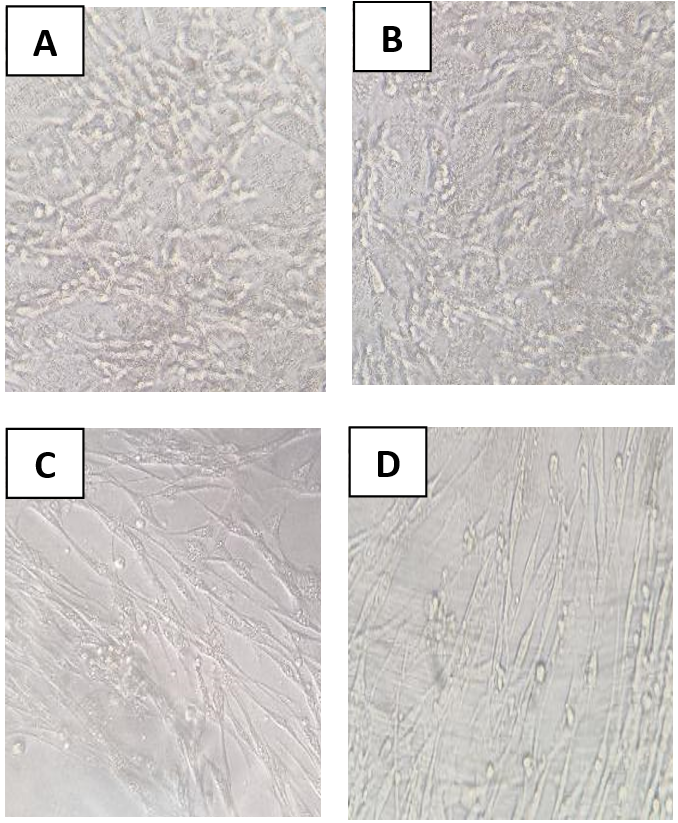
1. Wilson RM, Walker JM, Yin K. Different Concentrations of Lactobacillus acidophilus Cell Free Filtrate Have Differing Anti-Biofilm and Immunomodulatory Effects. Front Cell Infect Microbiol. 2021;11:737392. [
DOI:10.3389/fcimb.2021.737392] [
PMID] [
]
2. Dodds DR. Antibiotic resistance: A current epilogue. Biochem Pharmacol. 2017;134:139-46. [
DOI:10.1016/j.bcp.2016.12.005] [
PMID]
3. Santajit S, Indrawattana N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed Res Int. 2016;2016:2475067. [
DOI:10.1155/2016/2475067] [
PMID] [
]
4. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1-12. [
DOI:10.1086/595011] [
PMID]
5. Soleymanzadeh Moghadam S, Khodaii Z, Fathi Zadeh S, Ghooshchian M, Fagheei Aghmiyuni Z, Mousavi Shabestari T. Synergistic or Antagonistic Effects of Probiotics and Antibiotics- Alone or in Combination- on Antimicrobial-Resistant Pseudomonas aeruginosa Isolated from Burn Wounds. Archives of Clinical Infectious Diseases. 2018;13(3). [
DOI:10.5812/archcid.63121]
6. Kadri SS. Key Takeaways From the U.S. CDC's 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit Care Med. 2020;48(7):939-45. [
DOI:10.1097/CCM.0000000000004371] [
PMID] [
]
7. Spizek J, Sigler K, Rezanka T, Demain A. Biogenesis of antibiotics-viewing its history and glimpses of the future. Folia Microbiol (Praha). 2016;61(4):347-58. [
DOI:10.1007/s12223-016-0462-y] [
PMID]
8. Peral MC, Martinez MA, Valdez JC. Bacteriotherapy with Lactobacillus plantarum in burns. Int Wound J. 2009;6(1):73-81. [
DOI:10.1111/j.1742-481X.2008.00577.x] [
PMID] [
]
9. Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics on wound healing: A review of animal and human studies. Int Wound J. 2020;17(6):1687-94. [
DOI:10.1111/iwj.13451] [
PMID] [
]
10. Yan F, Polk DB. Probiotics and immune health. Curr Opin Gastroenterol. 2011;27(6):496-501. [
DOI:10.1097/MOG.0b013e32834baa4d] [
PMID] [
]
11. Campana R, van Hemert S, Baffone W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017;9:12. [
DOI:10.1186/s13099-017-0162-4] [
PMID] [
]
12. Nagoba BS, Selkar SP, Wadher BJ, Gandhi RC. Acetic acid treatment of pseudomonal wound infections--a review. J Infect Public Health. 2013;6(6):410-5. [
DOI:10.1016/j.jiph.2013.05.005] [
PMID]
13. Loo AE, Wong YT, Ho R, Wasser M, Du T, Ng WT, et al. Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLoS One. 2012;7(11):e49215. [
DOI:10.1371/journal.pone.0049215] [
PMID] [
]
14. Fijan S, Frauwallner A, Langerholc T, Krebs B, Ter Haar Nee Younes JA, Heschl A, et al. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. Biomed Res Int. 2019;2019:7585486. [
DOI:10.1155/2019/7585486] [
PMID] [
]
15. Sadeghi-Bazargani H, Maghsoudi H, Soudmand-Niri M, Ranjbar F, Mashadi-Abdollahi H. Stress disorder and PTSD after burn injuries: a prospective study of predictors of PTSD at Sina Burn Center, Iran. Neuropsychiatr Dis Treat. 2011;7:425-9. [
DOI:10.2147/NDT.S23041] [
PMID] [
]
16. Soleymanzadeh Moghadam S, Mohammad N, Ghooshchian M, FathiZadeh S, Khodaii Z, Faramarzi M, et al. Comparison of the effects of Lactobacillus plantarum versus imipenem on infected burn wound healing. Med J Islam Repub Iran. 2020;34:94. [
DOI:10.47176/mjiri.34.94] [
PMID] [
]
17. Valdez JC, Peral MC, Rachid M, Santana M, Perdigon G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect. 2005;11(6):472-9. [
DOI:10.1111/j.1469-0691.2005.01142.x] [
PMID]
18. Divyashree S, Anjali PG, Somashekaraiah R, Sreenivasa MY. Probiotic properties of Lactobacillus casei - MYSRD 108 and Lactobacillus plantarum-MYSRD 71 with potential antimicrobial activity against Salmonella paratyphi. Biotechnol Rep (Amst). 2021;32:e00672. [
DOI:10.1016/j.btre.2021.e00672] [
PMID] [
]
19. Appiah T, Boakye YD, Agyare C. Antimicrobial Activities and Time-Kill Kinetics of Extracts of Selected Ghanaian Mushrooms. Evid Based Complement Alternat Med. 2017;2017:4534350. [
DOI:10.1155/2017/4534350] [
PMID] [
]
20. Mogana R, Adhikari A, Tzar MN, Ramliza R, Wiart C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Med Ther. 2020;20(1):55. [
DOI:10.1186/s12906-020-2837-5] [
PMID] [
]
21. Messick CR, Rodvold KA, Pendland SL. Modified time-kill assay against multidrug-resistant Enterococcus faecium with novel antimicrobial combinations. J Antimicrob Chemother. 1999;44(6):831-4. [
DOI:10.1093/jac/44.6.831] [
PMID]
22. Primavilla S, Pagano C, Roila R, Branciari R, Ranucci D, Valiani A, et al. Antibacterial Activity of Crocus sativus L. Petals Extracts against Foodborne Pathogenic and Spoilage Microorganisms, with a Special Focus on Clostridia. Life (Basel). 2022;13(1). [
DOI:10.3390/life13010060] [
PMID] [
]
23. Elikaei A, Vazini H, Javani Jouni F, Zafari J. Investigating Cytotoxic Effects of Juniperus Excelsa Extract on Esophageal Cancer Cell Line KYSE-30 and Normal Fibroblast Cell Line HU02. Medical Laboratory Journal. 2019;13(5):13-8. [
DOI:10.29252/mlj.13.5.13]
24. Remzova M, Zouzelka R, Brzicova T, Vrbova K, Pinkas D, Rossner P, et al. Toxicity of TiO(2), ZnO, and SiO(2) Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials. Nanomaterials (Basel). 2019;9(7). [
DOI:10.3390/nano9070968] [
PMID] [
]
25. Piatek J, Krauss H, Ciechelska-Rybarczyk A, Bernatek M, Wojtyla-Buciora P, Sommermeyer H. In-Vitro Growth Inhibition of Bacterial Pathogens by Probiotics and a Synbiotic: Product Composition Matters. Int J Environ Res Public Health. 2020;17(9). [
DOI:10.3390/ijerph17093332] [
PMID] [
]
26. Saadatzadeh A, Ameri A, Moghimipour E, Bahmani B, Gholipour S. Comparison of Antibacterial Efficacy of Probiotic Mouthwash with Chlorhexidine Against Common Oral Pathogens: An In-Vitro Study. Jundishapur Journal of Natural Pharmaceutical Products. 2018;13(1). [
DOI:10.5812/jjnpp.65029]
27. Saadatzadeh A, Fazeli MR, Jamalifar H, Dinarvand R. Probiotic Properties of Lyophilized Cell Free Extract of Lactobacillus casei. Jundishapur J Nat Pharm Prod. 2013;8(3):131-7. [
DOI:10.17795/jjnpp-8564] [
PMID] [
]
28. Zahradnik RT, Magnusson I, Walker C, McDonell E, Hillman CH, Hillman JD. Preliminary assessment of safety and effectiveness in humans of ProBiora3, a probiotic mouthwash. J Appl Microbiol. 2009;107(2):682-90. [
DOI:10.1111/j.1365-2672.2009.04243.x] [
PMID]
29. Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651-9. [
DOI:10.1016/S0140-6736(08)60207-X] [
PMID]
30. Quinto EJ, Jiménez P, Caro I, Tejero J, Mateo J, Girbés T. Probiotic Lactic Acid Bacteria: A Review. Food and Nutrition Sciences. 2014;05(18):1765-75. [
DOI:10.4236/fns.2014.518190]
31. Goudarzi L, Kermanshahi RK, Moosavi-Nejad Z, Dalla MM. Evaluation of antimicrobial activity of probiotic lactobacillus strains against growth and urease activity of proteus spp. Journal of Medical Bacteriology. 2017;6(3-4):31-43.
32. Ershadian M, Branch D, Azad I. The Antimicrobial and Co-aggregation effects of probiotic lactobacilli against some pathogenic bacteria. Iranian Journal of Medical Microbiology. 2015;9(3):14-22.
33. Anas M, Eddine HJ, Mebrouk K. Antimicrobial activity of Lactobacillus species isolated from Algerian raw goat's milk against Staphylococcus aureus. World Journal of Dairy & Food Sciences. 2008;3(2):39-49.
34. Collado MC, Meriluoto J, Salminen S. Adhesion and aggregation properties of probiotic and pathogen strains. European Food Research and Technology. 2007;226(5):1065-73. [
DOI:10.1007/s00217-007-0632-x]
35. Prashant, Tomar SK, Singh R, Gupta SC, Arora DK, Joshi BK, et al. Phenotypic and genotypic characterization of lactobacilli from Churpi cheese. Dairy Science and Technology. 2009;89(6):531-40. [
DOI:10.1051/dst/2009029]
36. Dolati M, Tafvizi F, Salehipour M, Movahed TK, Jafari P. Inhibitory effects of probiotic Bacillus coagulans against MCF7 breast cancer cells. Iran J Microbiol. 2021;13(6):839-47. [
DOI:10.18502/ijm.v13i6.8089] [
PMID] [
]
37. Jafari-Nasab T, Khaleghi M, Farsinejad A, Khorrami S. Probiotic potential and anticancer properties of Pediococcus sp. isolated from traditional dairy products. Biotechnol Rep (Amst). 2021;29:e00593. [
DOI:10.1016/j.btre.2021.e00593] [
PMID] [
]
38. Dehghani N, Tafvizi F, Jafari P. Cell cycle arrest and anti-cancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells. Bioimpacts. 2021;11(4):245-52. [
DOI:10.34172/bi.2021.32] [
PMID] [
]
39. Nami Y, Haghshenas B, Haghshenas M, Abdullah N, Yari Khosroushahi A. The Prophylactic Effect of Probiotic Enterococcus lactis IW5 against Different Human Cancer Cells. Front Microbiol. 2015;6:1317. [
DOI:10.3389/fmicb.2015.01317] [
PMID] [
]










































 , Sara Minaeian2
, Sara Minaeian2 

 , Ali Majidpour
, Ali Majidpour 

 , Mahdi Adabi3
, Mahdi Adabi3 

 , Reza Hosseini Doust *4
, Reza Hosseini Doust *4