1. Yeo W, Pang E, Liem GS, Suen JJS, Ng RYW, Yip CCH, et al. Menopausal symptoms in relationship to breast cancer-specific quality of life after adjuvant cytotoxic treatment in young breast cancer survivors. Health Qual Life Outcomes. 2020;18(1):1-9. [
doi:10.1186/s12955-020-1283-x]
2. Bellver J, Donnez J. Introduction: Infertility etiology and offspring health. FertilSteril. 2019; 111(6):1033-35. [
doi:10.1016/j.fertnstert.2019.04.043]
3. Mobaraki M, Faraji A, Dolati P, Ataei M, Manshadi HRD. Molecular Mechanisms of Cardiotoxicity: A Review on the Major Side-effect of Doxorubicin. Indian J Pharm Sci. 2017; 79(3):335-44. [
doi:10.4172/pharmaceutical-sciences.1000235]
4. Wang Y, Liu M, Johnson SB, Yuan G, Arriba AK, Zubizarreta ME, et al. Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation. Toxicol Appl Pharmacol. 2019;15(381):1-11. [
doi:10.1016/j.taap.2019.114714] [
pmid: 31437492]
5. Niringiyumukiza JD, Cai H, Chen L, Li Y, Wang L, Zhang M, et al. Protective properties of glycogen synthase kinase-3 inhibition against doxorubicin-induced oxidative damage to mouse ovarian reserve. Biomed Pharmacother. 2019; 116:108963. [
doi:10.1016/j.biopha.2019.108963]
6. Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY). 2011;3(8):782-93. [
doi:10.18632/aging.100363] [
pmid: 21869459]
7. Siddiqui AJ, Jahan S, Singh R, Saxena J, Ashraf SA, Khan A, et al. Plants in Anticancer Drug Discovery: From Molecular Mechanism to Chemoprevention. Biomed Res Int. 2022; 2022:1-18. [
DOI:10.1155/2022/5425485]
8. Rad P, Safari F, Mohammadi J, Delaviz H. Preserved Ovarian Function After Toxicity WithDoxorrubicin in Rats: Protective Effect of Nasturtium Officinale Extract. Iran JToxicol. 2021;15(1):57-64. [
doi:10.32598/IJT.15.1.747.1]
9. Campos LB, Gilglioni EH, Garcia RF, Brito Mdo N, Natali MR, Ishii-Iwamoto EL, et al. Cimicifuga racemosa impairs fatty acid β-oxidation and induces oxidative stress in livers of ovariectomized rats with renovascular hypertension. Free Radic Biol Med. 2012;53(4):680-89. [
doi:10.1016/j.freeradbiomed.2012.05.043]
10. Morelli V, Naquin C. Alternative therapies for traditional disease states: menopause. Am Fam Physician. 2002;66(1):129-34. [
Link]
11. Kim CD, Lee WK, Lee MH, Cho HS, Lee YK, Roh SS. Inhibition of mast cell-dependent allergy reaction by extract of black cohosh (Cimicifuga racemosa). ImmunopharmacolImmunotoxicol. 2004; 26(2):299-308. [
doi:10.1081/IPH-120037728]
12. Guo Y, Yin T, Wang X, Zhang F, Pan G, Lv H, et al. Traditional uses, phytochemistry, pharmacology and toxicology of the genus Cimicifuga: A review. J Ethnopharmacol. 2017; 209:264-82. [
doi:10.1016/j.jep.2017.07.040]
13. Ruhlen RL, Sun GY, Sauter ER. Black Cohosh: Insights into its Mechanism(s) of Action. Integr Med Insights. 2008. [
doi:10.4137/117863370800300002]
14. Liske E, Hänggi W, Henneicke-von Zepelin HH, Boblitz N, Wüstenberg P, Rahlfs VW. Physiological investigation of a unique extract of black cohosh (Cimicifugaera cemosae rhizoma): A 6-month clinical study demonstrates no systemic estrogenic effect. J Womens Health Gend Based Med. 2002; 11(2):163-74. [
doi:10.1089/152460902753645308]
15. Fernandes ES, Celani MFS, Fistarol M, Geber S. Effectiveness of the short-term use of Cimicifuga racemosa in the endothelial function of postmenopausal women: A double-blind, randomized, controlled trial. Climacteric.2020;23(3):245-51. [
doi:10.1080/13697137.2019.1682542]
16. Rabenau M, Unger M, Drewe J, Culmsee C. Metabolic switch induced by Cimicifuga racemosa extract prevents mitochondrial damage and oxidative cell death. Phytomedicine. 2019;52:107-6. [doi: 10.1016/j.phymed.2018.09.177] [
doi:10.1016/j.phymed.2018.09.177]
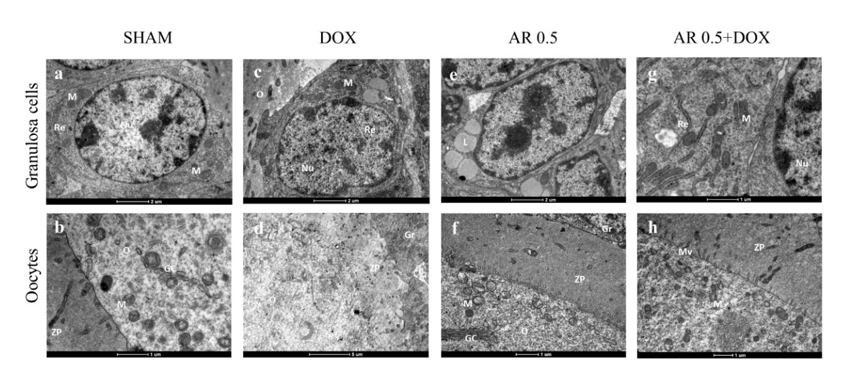
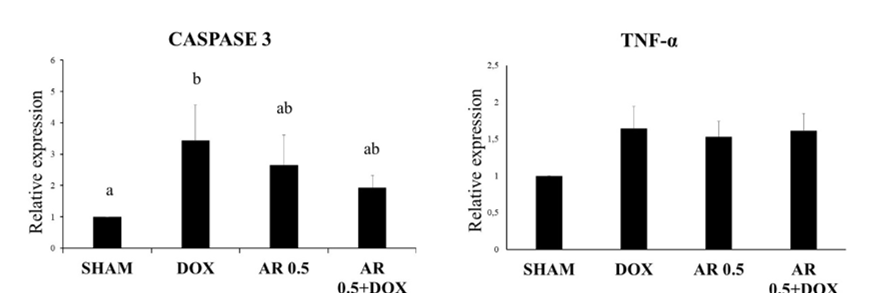
17. de Assis EIT, Azevedo VAN, De Lima Neto MF, Costa FC, Paulino LRFM, Barroso PAA, et al. Protective Effect of Cimicifuga racemosa (L.) Nutt Extract on Oocyte and Follicle Toxicity Induced by Doxorubicin during In vitro Culture of Mice Ovaries. Animals (Basel). 2022;13(1):1-18. [
doi:10.3390/ani13010018]
18. Azouz AA, Ali SE, Abd-Elsalam RM, Emam SR, Galal MK, Elmosalamy SH, et al. Modulation of steroidogenesis by Actaea racemosa and vitamin C combination, in letrozole induced polycystic ovarian syndrome rat model: promising activity without the risk of hepatic adverse effect. Chin Med. 2021;16(1):1-17. [
doi:10.1186/s13020-021-00444-z]
19. Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4A):609-614. [
doi:10.1590/S1519-69842002000400008]
20. Gouveia BB, Barberino RS, Dos Santos Silva RL, Lins TLBG, da Silva Guimarães V, do Monte APO, et al. Involvement of PTEN and FOXO3a Proteins in the Protective Activity of Protocatechuic Acid Against Cisplatin-Induced Ovarian Toxicity in Mice. Reprod Sci. 2021;28(3):865-76. [
doi:10.1007/s43032-020-00305-4]
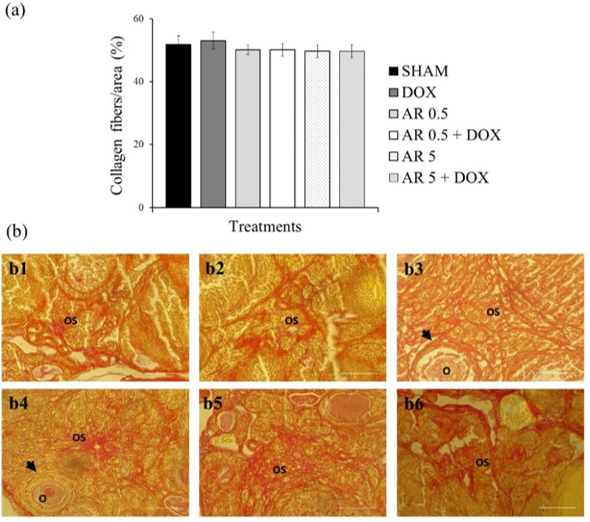
21. Rittié L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. Methods Mol Biol. 2017;1627:395-407. [
doi:10.1007/978-1-4939-7113-8_26]
22. Barberino RS, Menezes VG, Ribeiro AEAS, Palheta RCJr, Jiang X, Smitz JEJ, et al. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol Reprod. 2017;96:1244-55. [
doi:10.1093/biolre/iox053]
23. Kayedpoor P, Mohamadi S, Karimzadeh-Bardei L, Nabiuni M. Anti-inflammatory effect of silymarin on ovarian immunohistochemical localization of TNF-α associated with systemic inflammation in polycystic ovarian syndrome.Int J Morphol. 2017;35:723-32. [
doi:10.4067/S0717-95022017000200054]
24. Suh KS, Choi EM, Jung WW, Kim YJ, Hong SM, Park SY, et al. Deoxyactein protects pancreatic β-cells against methylglyoxal-induced oxidative cell damage by the upregulation of mitochondrial biogenesis. Int J Mol Med;40(2):539-48. [
doi:10.3892/ijmm.2017.3018]
25. Choi EM. Deoxyactein Isolated from Cimicifuga racemosa protects osteoblastic MC3T3-E1 cells against antimycin A-induced cytotoxicity. J Appl Toxicol. 2013;33(6):488-94. [
doi:10.1002/jat.1784]
26. Schmid D, Woehs F, Svoboda M, Thalhammer T, Chiba P, Moeslinger T. Aqueous extracts of Cimicifuga racemosa and phenolcarboxylic constituents inhibit production of proinflammatory cytokines in LPS-stimulated human whole blood. Can J PhysiolPharmacol. 2009;87(11):963-72. [
doi:10.1139/Y09-091]
27. Roti Roti EC, Leisman SK, Abbott DH, Salih SM. Acute doxorubicin insult in the mouse ovary is cell- and follicle-type dependent. PLoS ONE. 2012;7:42293. [
doi:10.1371/journal.pone.0042293]
28. Ben-Aharon I, Meizner I, Granot T, Uri S, Hasky N, Rizel S, et al. Chemotherapy-induced ovarian failure as a prototype for acute vascular toxicity. Oncologist. 2012;17(11):1386-93. [
doi:10.1634/theoncologist.2012-0172] [
pmid:22956534]
29. Lliberos C, Liew SH, Zareie P, La Gruta NL, Mansell A, Hutt K. Evaluation of inflammation and follicle depletion during ovarian ageing in mice. Sci Rep.2021;11(1):1-15. [
doi:10.1038/s41598-020-79488-4] [
pmid:33432051]
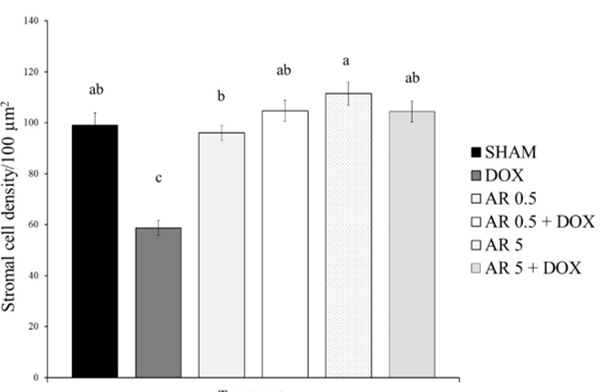
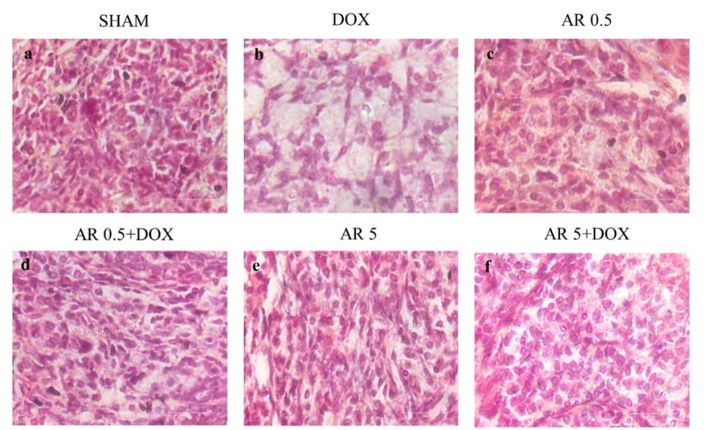
30. Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction. 2016;152(3):245-60. [
doi:10.1530/REP-16-0129] [
pmid: 27491879]
31. Oktem O, Oktay K. A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res. 2007;67(21):10159-62. [
doi:10.1158/0008-5472.CAN-07-2042]
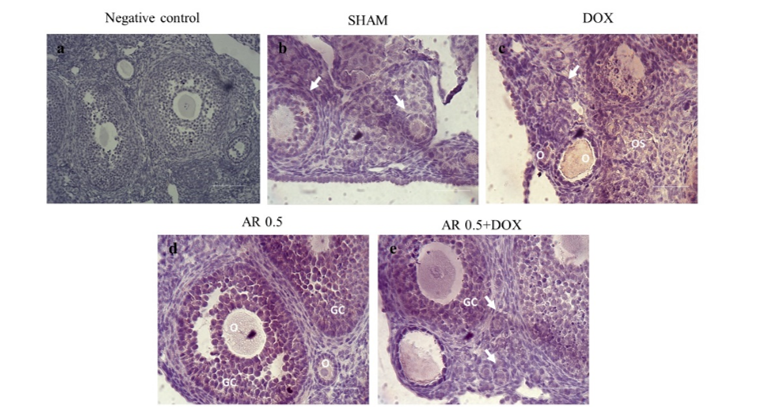
32. Silva JRV, Lima FEO, Souza ALP, Silva AWB. Interleukin-1β and TNF-α systems in ovarian follicles and their roles during follicular development, oocyte maturation and ovulation. Zygote. 2020; 28(4):270-77. [
doi:10.1017/S0967199420000222]
33. Marcinkiewicz JL, Krishna A, Cheung CM, Terranova PF. Oocytic tumor necrosis factor alpha: localization in the neonatal rat ovary and throughout follicular development in the adult rat. Biol Reprod. 1994;1251-60. [
doi:10.1095/biolreprod50.6.1251]
34. Roby KF, Terranova PF. Localization of tumor necrosis factor (TNF) in the rat and bovine ovary using immune-cytochemistry and cell blot: evidence for granulosal production. In: Hirshfield, A.N. (eds) Growth Factors and the Ovary. Springer, Boston, MA. 1989:273-78. [
doi:10.1007/978-1-4684-5688-2_31]
35. Sancho-Tello M, Tash J, Roby K, Terranova PF. Effects of lipopolysaccharide on ovarian function in the pregnant mare serum gonadotropin-treated immature rat. Endocrine Journal. 1993;503-11. [
doi:10.2527/jas1987.6561768x]
36. Strauss JF, Fitz Gerald GA. Steroid Hormones and Other Lipid Molecules Involved in Human Reproduction. Yen and Jaffe's Reproductive Endocrinology. 2019;75-114. [
doi:10.1016/b978-0-323-47912-7.00004-4]
37. Nagata S. Apoptosis and Clearance of Apoptotic Cells. Annu Rev Immunol. 2018; 36:489-517. [
doi:10.1146/annurev-immunol-042617-053010]










































 , Ernando Igo Teixeira de Assis1
, Ernando Igo Teixeira de Assis1 

 , Antônia Venância Nunes Azevedo1
, Antônia Venância Nunes Azevedo1 

 , Laís Raiane Feitosa Melo Paulino1
, Laís Raiane Feitosa Melo Paulino1 

 , Mariana Aragão Matos Donato2
, Mariana Aragão Matos Donato2 

 , Christina Alves Peixoto2
, Christina Alves Peixoto2 

 , Alane Pains Oliveira do Monte3
, Alane Pains Oliveira do Monte3 

 , Maria Helena Tavares de Matos3
, Maria Helena Tavares de Matos3 

 , Alana Nogueira Godinho4
, Alana Nogueira Godinho4 

 , Jordânia Marques de Oliveira Freire4
, Jordânia Marques de Oliveira Freire4 

 , Ricássio De Sousa Barberino3
, Ricássio De Sousa Barberino3 

 , Anderson Weiny Barbalho Silva1
, Anderson Weiny Barbalho Silva1 

 , José Roberto Viana Silva *5
, José Roberto Viana Silva *5