Ethics code: E03/23 (date of approval: 11 March 2023)


1- Senior Researcher, Biomodeling and Translational Medicine Laboratory, FSBSI East-Siberian Institute of Medical and Ecological Research, Angarsk, Russia
2- Senior Researcher, Biomodeling and Translational Medicine Laboratory, FSBSI East-Siberian Institute of Medical and Ecological Research, Angarsk, Russia. , novik-imt@mail.ru
3- Senior Researcher, Biomodeling and Translational Medicine Laboratory, FSBSI East-Siberian Institute of Medical and Ecological Research, Angarsk, Russia.
4- Officer, Biomodeling and Translational Medicine Laboratory, FSBSI East-Siberian Institute of Medical and Ecological Research, Angarsk, Russia
5- Junior Researcher, Biomodeling and Translational Medicine Laboratory, FSBSI East-Siberian Institute of Medical and Ecological Research, Angarsk, Russia.
6- Professor & Head; Biomodeling and Translational Medicine Laboratory, FSBSI East-Siberian Institute of Medical and Ecological Research, Angarsk, Russia.
Full-Text [PDF 515 kb]
(215 Downloads)
|
Abstract (HTML) (1137 Views)
Full-Text: (371 Views)
Introduction
Copper nanocomposites are promising materials for the development of antiseptic agents on their basis and the creation of highly effective antibacterial drugs without the use of antibiotics [1, 2]. It has been proven that copper nanocomposites are able to successfully suppress the growth of bacteria. In addition, an important aspect of the use of copper nanocomposites is the creation of anticancer drugs, including those combined with chemotherapy agents [3].
The widespread use of copper nanoparticles (NPs) in medical applications has prompted researchers to expand their studies on the toxicity of copper nanomaterials to microorganisms, animals, and humans.
Most scientific studies indicate that the toxicity of copper-based NPs is associated with their accumulation around the cells, their dissolution, and subsequent adhesion to the cell membrane caused by electrostatic interactions. The adhesion of NPs and the subsequent release of copper ions disrupt the cell membrane, facilitating the penetration of copper NPs and ions into cells. The release of copper ions can cause increased levels of reactive oxygen species (ROS), protein oxidation, decreased production of adenosine triphosphate, and DNA damage [4]. Based on the literature review, copper NPs modulate cells, cytokines, and growth factors involved in the mechanism or repair of damaged tissues. This compound is more efficient than the plain copper ions [3, 5]. The histological assessment of copper NPs has stimulated the reparative functions of the tissues, demonstrating the active proliferation of fibroblasts, collagen deposits, and re-epithelialization of damaged organs [5].
A number of researchers have demonstrated that copper NPs can act as therapeutic agents or as drug carriers to provide a controlled release of anticancer drugs and inhibit cancer cell proliferation [6].
Additionally, fucoidan-modified CuO NPs have demonstrated the ability to modulate cancer cell apoptosis through the activation of apoptosis-related proteins. These include B-cell lymphoma 2 (BCL2), Bcl-2-associated X protein (BAX), caspase-3, and poly (ADP-ribose) polymerase [7]. Further, copper NPs functionalized with chrysin as a radiosensitizer have improved the effect of irradiation in Ehrlich ascites carcinoma in vivo [6, 8]. Given the importance of copper as an essential element for cellular metabolism, it is essential to evaluate the biological activities of this nanocomposite in terms of biosafety in animals and humans.
Arabinogalactan is a natural water-soluble heterosaccharide and was obtained from the wood of the Siberian larch (Larix sibirica). The arabinogalactan macromolecule has a highly branched structure, unlike other polysaccharides used in pharmaceuticals, which makes it possible to use it as a “host” molecule to create inclusion complexes on it. One aspect of the bioavailability of arabinogalactan is its adhesion to enterocytes [9]. It has been experimentally shown that the use of arabinogalactan as a matrix doubled the bioavailability of drugs [10]. The structural features of arabinogalactan, such as its high content of hydroxyl groups,
determine its significant potential in the formation of nanostructures. Moreover, the advantages of arabinogalactan include low molecular weight, water solubility, and the ability to facilitate transmembrane transport [11].
Aim of the Study: Our main aim in conducting this study was to analyze the morphological, biochemical, and genotoxic effects of exposure in rats to a copper oxide nanocomposite
encapsulated in an arabinogalactan polymer matrix.
Materials and Methods
Copper Nanocomposite Characterization: Cu2OAg nanocomposites were purchased from Favorsky Institute of Chemistry SB RAS (Irkutsk, Russia). The methodology for the synthesis of this nanocomposite is presented in an earlier publication [11]. The nanocomposite is an easily dissolving, odorless, and green material in a fine powder form. The nanocomposite is
encapsulated in purified arabinogalactan without having other impurities. The NPs were in spherical shape (7%) with a distribution of 5-10 nm with an average size of 7.7 nm. The typical microstructures are granules that are fairly uniform in size. The NPs were coated with a layer of arabinogalactan macromolecules, i.e., representing residues of galactose and arabinose.
The electronic spectra of Cu/Ag NPs represent a wide, low-resolution band with a maximal absorption range of 237-245 nm. Then, the plot smoothly descended into the long-wave region with a second maximum but less intense visible region at 670 nm. Based on the position of λ max 237 nm and the intensity, the absorption band in the UV region of the spectrum was attributed to the complex with charge transfer from Ag ligand to Cu (II) ions. The less intense band was in the visible region with
λ max 670 nm [11].
Animals and Experimental Design: Three-month-old white female rats (n=40; weight 200-220 g.) were divided into four groups of 10 animals each as follows: two experimental (EX1 and EX2) and two control groups (C1 and C2). Animals in groups EX1 and EX2 were orally given the mixture with a probe (1 ml) of an aqueous solution of Cu2OAg at 500 μg/kg for 10 days. Animals in groups C1 and C2 received distilled water in a similar manner. The investigation was carried out in 2 phases: Groups EX1 and C1 were withdrawn from the
experiment immediately after exposure (early period), while groups EX2 and C2 were given the treatment 4 months after the study completion (long-term exposure). The treatment dosage (500 μg/kg) was based on the concentration observed in similar studies conducted earlier [12, 13]. Those studies reported the most pronounced alterations in the brain, liver, and kidney morphology at the same dosage (500 µg/kg).
All animals were kept under 12 hours of alternating light-dark cycles, on a ventilated shelf, and under controlled temperature and humidity (22-25°C and 55-60% humidity). The animals were obtained from the vivarium of the Federal State Budgetary Scientific Institution “East Siberian Institute of Medical and Ecological Research” (FSBSI ESIMER) and were kept on a standard diet and drinking water ad libitum (BioPro Russia). All animal experiments were approved by the Ethics Committee of FSBSI ESIMER (approval code: E03/23; date of approval: 11 March 2023, amended/approved every 6 months). The experiments were also carried out in compliance with the humane rules of animal treatment, consistent with the requirements of the International Recommendations for Biomedical Research Using Animals (World Health Organization, Geneva, 1985), UK Animals (Scientific Procedures) Act (UK, 1986), and National Institutes of Health guide for the care and use of laboratory animals (NIH Publications #: 8023, revised 1978).
Hematological Investigations: To study the number of erythrocytes and hemoglobin
concentration, blood samples were taken from the tail vein of the rats after 14-15 hours of fasting. The hemoglobin level was determined using a set of reagents using the hemoglobin-cyanide method (Vital Diagnostics LLC, St. Petersburg, Russia). The number of erythrocytes was counted in five large squares of the Goryaev chamber using the standard method [14]. The following parameters were analyzed in the blood sera: total protein, albumin, urea, glucose, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and triglycerides. A commercial reagent kit was used based on the manufacturer's instructions to determine the levels of these parameters (Vital Diagnostics LLC, St. Petersburg, Russia) to determine the levels of these parameters. To determine the leukocyte formula, blood samples were taken from the tail vein of the rats by the standard method [15]. Various leukocyte populations were counted (per 100 cells) immersed in oil under an Olympus BX51 microscope at ×1000 magnification.
Histological Examinations: For morphological studies of the brain tissue, the animals
underwent euthanasia followed by decapitation. The brain from each animal was removed and fixed in 10% neutral buffered formalin solution, dehydrated with ascending concentrations of ethanol (70, 80, 90, 95, and 100%), and embedded in paraffin wax for histological studies (HistoMix; BioVitrum, Russia). Next, using an HM 400 microtome (Microm, Germany), serial horizontal sections were made at 4-5µm thickness (Bregma: 6.10 mm level and Interanural: 3.90 mm). These were stained with hematoxylin and eosin on histological slides for examination by light microscopy [16]. On the stained sections, the total number of neurons per unit area, glial cells, degenerated neurons (i.e., wrinkled dark-colored neurons without clearly
distinguishable nuclei and cytoplasms), and the number of neuronophagies were counted. In the liver sections, the number of stellate Kupffer cells and polynuclear hepatocytes was counted. The areas of the Shumlyansky-Bowman capsule were determined in each of the kidney sections.
Genotoxic Investigations: To determine the genotoxic effects of the nanocomposite
compound on the brain and blood, we used a DNA-comet method based on an established protocol [17]. The preparations were stained with SYBR Green I and examined under an OLYMPUS BX-51 microscope, with an OLYMPUS RX-420 digital camera attached
(magnification ×100). Images of DNA comets (100 cells from each animal) were analyzed using the CASP 1.2.2 program. The percentage of DNA fragments in the comets (% of DNA in the tail) was used as an indicator of DNA damage in each sample.
Statistical Analyses: We used the Statistica software package, version 6.0 (license no: AXXR004E642326FA), for the statistical analyses of the study data. The morphological and genotoxic data were identified by the median and interquartile range, Me (Q25-Q75). The data reflecting the biochemical and hematological parameters were tabulated as the means and standard error of the means. The normal distribution of the data was checked based on
Shapiro-Wilk test. To compare the significance of differences in the results obtained among the groups, the parametric Student's t-test and the nonparametric Mann-Whitney U test were used. The null hypotheses about the absence of differences between the groups were rejected when the significance level of the corresponding statistical test reached P≤0.05.
Results
Hematological Findings: The results of the biochemical parameters from the peripheral blood samples of the rats treated with the nanocomposite compound showed that early in the treatment period, the blood glucose and triglyceride levels increased. However, in the long-term phase, these parameters returned to normal levels except for the level of hemoglobin and urea, which remained high (Table 1). The study of hematological parameters in the peripheral blood of the experimental rats showed a major increase in the number of basophils. In the long-term period of the study, the number of basophils remained significantly higher than that of the controls, although the number was lower compared to that in the early phase of the treatment period (Table 2).
Table 1: Biochemical parameters of the peripheral blood of white rats exposed to Cu2OAg at early and long-term periods of examination, M±m.
| Indicators |
C1 |
EX1 |
| Early period |
| ALT, U/l |
229.38±16.56 |
248.61±22.26 |
| AST, U/l |
104.50±11.68 |
82.11±5.57 |
| Glucose, mmol/l |
6.06±0.45 |
7.81±0.32* |
| Total protein, g/l |
67.01±1.55 |
65.15±0.93 |
| Albumin, g/l |
34.37±0.89 |
37.65±0.81 |
| Urea, mmol/l |
16.67±1.69 |
17.46±0.79 |
| Triglycerides, mmol/l |
0.42±0.05 |
1.13±0.24* |
| Creatinine, µmol/l |
55.63±6.55 |
41.40±3.96 |
| Long-term period |
| Indicators |
C2 |
EX2 |
| ALT, U/l |
253.14±27.52 |
319.13±19.12 |
| AST, U/l |
73.44±9.88 |
87.79±2.99 |
| Glucose, mmol/l |
6.63±0.30 |
6.71±0.29 |
| Total protein, g/l |
70.37±0.34 |
70.82±0.53 |
| Table1 Continue |
| Albumin, g/l |
44.50±3.06 |
39.28±0.75 |
| Urea, mmol/l |
16.29±0.52 |
17.76±0.36# |
| Triglycerides, mmol/l |
1.11±0.34 |
0.68±0.12 |
| Creatinine, µmol/l |
54.23±1.49 |
59.42±1.58 |
|
|
|
|
|
Keys - *: Differences are statistically significant in comparison with C1 at P<0.05;
#: Differences are statistically significant in comparison with C2 at P<0.05.
Table 2: Hematological parameters of the leukocyte formula of the peripheral blood of white rats under the influence of Cu2OAg at early and long-term period of examination, M±m.
| Early period |
| Indicators |
C1 |
EX1 |
| Plasma cells, % |
0.75±0.75 |
1.00±0.50 |
| Erythrocytes, 1012/l |
2.00±2.005,56±0.08 |
1.13±0.08 |
| Young cells, %Hemoglobin, g/l |
0.00±0.00145,01±2.43 |
0.75±2.27 |
| Stab neutrophils, % |
4.00±1.47 |
6.50±1.13 |
| Segmented neutrophils, % |
30.50±5.17 |
31.63±3.78 |
| Eosinophils, % |
1.75±0.62 |
2.50±1.06 |
| Basophils, % |
0.50±0.28 |
1.88±0.47* |
| Monocytes, % |
7.25±1.88 |
8.00±1.84 |
| Lymphocytes, % |
53.25±7.08 |
46.63±4.28 |
| Long-term period |
| Erythrocytes, 1012/l |
5.30±0.14 |
5.19±0,11 |
| Hemoglobin, g/l |
137.66±3.89 |
149.15±1.62# |
| Stab neutrophils, % |
1.50±0.86 |
3.60±0.92 |
| Segmented neutrophils, % |
26.25±3.68 |
27.80±1.88 |
| Eosinophils, % |
4.50±1.89 |
2.60±0.92 |
| Basophils, % |
0.00±0.00 |
0.40±0.40# |
| Monocytes, % |
11.75±2.32 |
9.80±1.88 |
| Lymphocytes, % |
55.50±4.17 |
56.20±2.39 |
Keys - *: Differences are statistically significant in comparison with C1 at P<0.05;
#: Differences are statistically significant in comparison with C2 at P<0.05.
Histological and Morphometric Findings: The blood volume in the brain vessels was normal, and the vascular wall structures were unaltered. There was a pronounced decrease in the total number of normal neurons per unit area of the brain tissue samples compared to that of the controls. The number of glial cells in the experimental group was also significantly lower than that of the control group. There was a sharp increase in the number of degenerated neurons and neuronophagy compared to those found in the control group (Figures 1, 2).
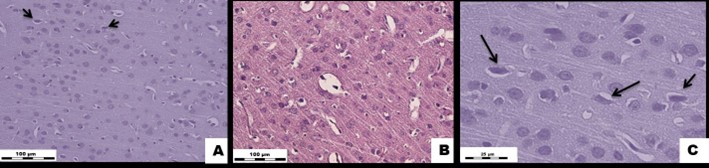
Figure 1: White rat brain tissue exposed to Cu2OAg at a dose of 500 µg/kg during the early period.
(A) EX1 group, (B) C1 group.↑ - degeneratively altered neurons. H&E stained. Magnification × 400.
(C) EX1 group. ↑ - degeneratively altered neurons. H&E stained. Magnification × 1000.
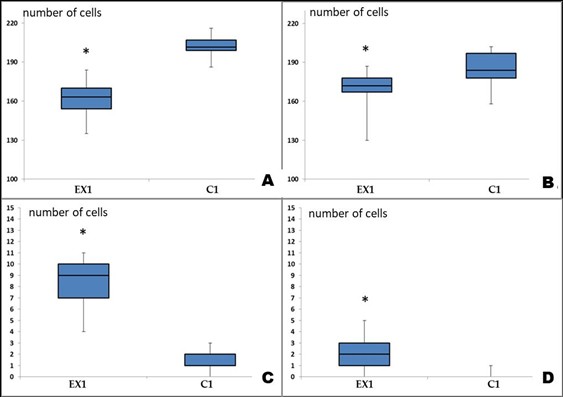
Figure 2: Changes in the composition of cell populations of the sensorimotor cerebral cortex of albino rats exposed to Cu2OAg at a dose of 500 μg/kg during the early period.
(A) total number of neurons per unit area (0.2 mm2); (B) total number of gliocytes per unit area; (C) number of degeneratively changed neurons; (D) number of neuronophagy events. Note: * - differences are statistically significant compared with the control at P<0.05.
Histological and Morphometric Assessments of the Brain: In the long-term phase, the blood volume in the brain vessels remained normal, and the vascular wall structures were
unaltered. A significant decrease in the total number of normal neurons per unit area persisted compared to the controls; however, there were no significant differences compared to that observed in the early observation period. The number of glial cells in the experimental group was also significantly lower than that of the control group. Such changes, even after four months of restoration of the cell population of glial cells, did not occur. There was a sharp increase in the number of degenerate neurons compared to that of the control group. The number of neuronophagy in the long-term period of the experiment did not differ from that of the control group (Figures 3 & 4).
Histological Examination of the Liver: In the early experimental period, the blood volume in the sinusoidal capillaries, central veins, and portal tract veins remained normal. The portal tracts were not dilated and had no signs of sclerosis and inflammation; nonetheless, the central veins were dilated. The beam-radial structure of the hepatic lobules was preserved. The number of stellate Kupffer macrophages in the sinusoidal capillaries did not differ from that of the controls. The number of polynuclear hepatocytes also did not significantly change compared to those found in the control group. Moreover, in the long-term experimental period, none of the indicators changed (Figure 5).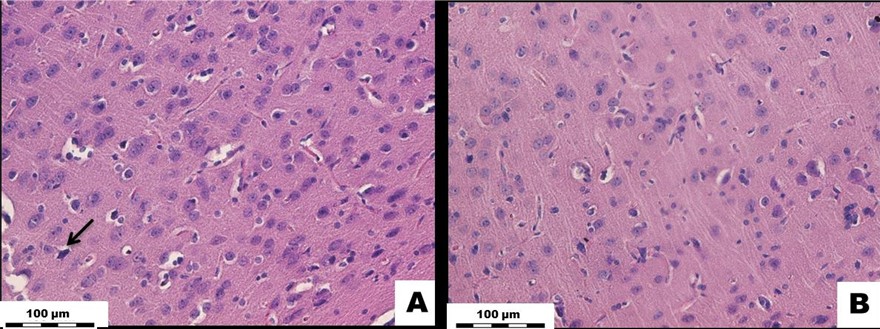
Figure 3: White rat brain tissue exposed to Cu2OAg at a dose of 500 µg/kg during the long-term period. (A) EX2 group, (B) C2 group.↑ - degeneratively altered neurons. H&E stained. Magnification × 400.
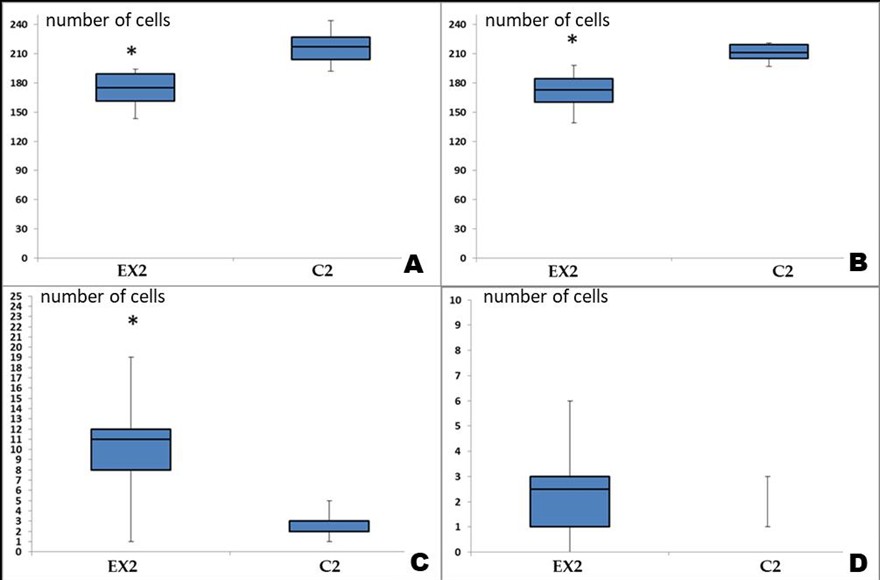
Figure 4: Changes in the composition of cell populations of the sensorimotor cerebral cortex of albino rats exposed to Cu2OAg at a dose of 500 μg/kg during the long-term period.
(A) total number of neurons per unit area (0.2 mm2); (B) total number of gliocytes per unit area; (C) number of degeneratively changed neurons; (D) number of neuronophagy events. Note: * - differences are statistically significant compared with the control at P<0.05.
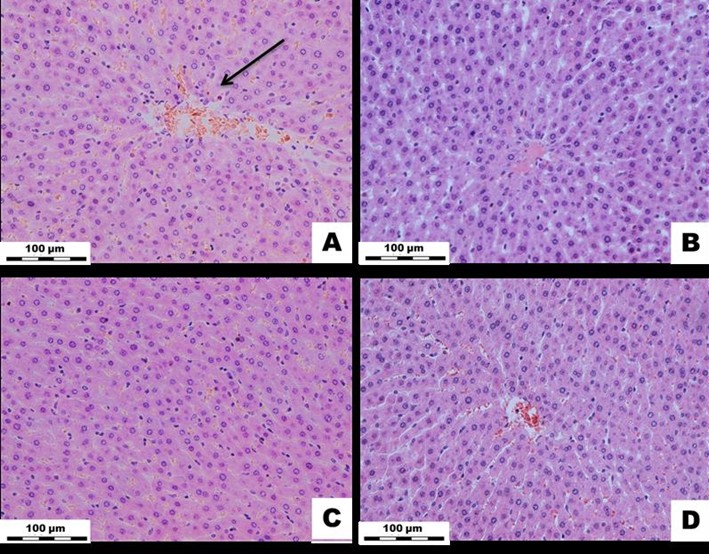
Figure 5: White rat liver tissue exposed to Cu2OAg at a dose of 500 µg/kg during the early and long-term period. (A) EX1 group, (B) C1 group, (C) EX2 group, (D) C2 group.↓ - dilatation of the central vein. H&E stained. Magnification × 400.
Histological Examinations of the Kidneys: In the early stage of the experiments, the blood supply to the cortical and medulla of the kidneys remained normal, and there were no disturbances in the blood circulation of these organs. The wall structures of the renal arteries and arterioles remained normal in the interstitial spaces, and the renal glomeruli structures were preserved. No inflammatory or necrotic foci were found in the renal tissue samples. The epithelia of the proximal and distal renal tubules also appeared normal. In the cortical substance of the kidneys, the areas of the Shumlyansky-Bowman capsules were not significantly different from those found in the control group. However, in the long-term period, none of the indicators changed (Figure 6).
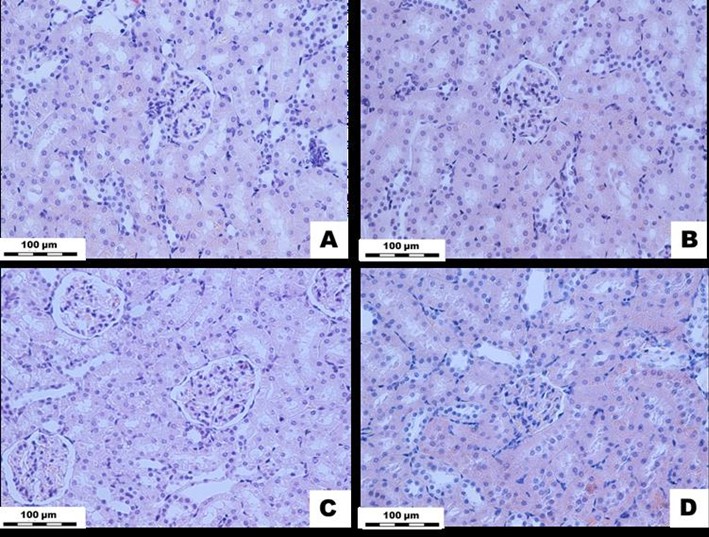
Figure 6: White rat kidney tissue exposed to Cu2OAg at a dose of 500 µg/kg during the short-
and long-term periods. (A) EX1 group, (B) C1 group, (C) EX2 group, (D) C2 group. H&E stained.
Magnification × 400.
DNA Fragmentation: The percentage of DNA fragmentations of the "tails of the comets" in the brain tissue samples did not differ between the experimental and control groups in either early or late periods of the experimental phases. At the same time, significantly higher levels of DNA fragmentations were detected in the blood cells of the experimental groups during the early period of the study compared to those observed in the controls (Table 3).
Table 3: Results of the study of DNA fragmentation in the blood during subacute exposure to Cu2OAg nanocomposite at 500 µg/kg at various points. Me (Q25-Q75).
| Early period |
| Indicators |
C1 |
EX1 |
P |
| % DNA in "comet tails" |
2.39 (1.20-4.46) |
3.47 (1.85- 6.03)* |
0.00001 |
| Long-term period |
| Indicators |
C2 |
EX2 |
P |
| % DNA in "comet tails" |
1.38 (0.45-2.75) |
1.21 (0.51- 2.51) |
0.26 |
Note: * - differences are statistically significant in comparison with C2 at P<0.05.
Discussion
In this study, the effect of copper nanocomposite compound on the white rats was assessed
by the assessment of morpho-functional changes in their various bodily organs. Immediately after exposure to the nanocomposite compounds, the glucose and triglyceride levels in the rats’ blood increased. It is likely that this event is associated with the mobilization of liver glycogen or an increase in the process of gluconeogenesis in response to the effect of the copper oxide nanocomposite. An increase in the glucose level in the blood has also been reported when copper NPs were used as bait in farm animals [18]. A change in the triglyceride contents also indicates dysfunction of metabolic processes in the rats’ body.
Examination of the leukocyte number in the peripheral blood in the presence of Cu2OAg has shown an increase in the number of neutrophils. In the late post-experimental period, the number of neutrophils decreased compared to that in the early period in the experimental group. However, the number remained significantly high compared to that of the control. This observation indicates the occurrence of an inflammatory process that persists through the late experimental period. In addition, there was an increase in the number of basophils in the experimental groups, which might indicate the development of inflammatory processes in the GI tract and liver of the experimental animals or the possibility of allergic reactions. At the same time, the absence of an increase in the number of Kupffer cells in the experimental groups compared to the controls indicates that the effect of nanocomposite on the liver occurs without an inflammatory reaction in the organ.
The effect of the Cu2OAg nanocomposite compound did not result in any morphological alteration in the kidneys. At the same time, an increase in the blood urea level in the experimental animals during the late experimental period might indicate a dysfunction of the kidneys. The effect of the nanocomposite compound on the brain tissue resulted in the most significant alterations. We observed a sharp decrease in the total number of neurons and glial cells in the sensorimotor cortex. It is likely that the decrease in the number of neurons is caused both by the direct toxic effect of the copper nanocomposite compound and by disruptions in the tissue trophic processes mediated by a decrease in the number of gliocytes. It has been reported that copper NPs can affect the sodium and potassium channels in neurons [19, 20], thereby disrupting their normal function. Thus, the rats exposed to copper NPs showed a decline in their learning and memory [21].
Reportedly, copper NPs can accumulate in neurons in significant amounts [22]. Presumably, NPs are able to penetrate from the bloodstream into gliocytes via endocytosis and then move to neurons [22]. In the late post-treatment period, that is, four months after the end of the experiments, the sensorimotor cortex of the brain did not restore the population of gliocytes and retained a large number of degenerate neurons per unit area (0.2 mm2), as compared to the controls. At the same time, this variable was not significantly different from that observed in the early experimental period. These pathological processes in the brain persisted in the late post-contact period; nonetheless, their intensity did not rise under the influence of the copper oxide nanocomposite compound.
The study of the genotoxic effects of the nanocomposite compound showed that there was an increase in DNA fragmentation in the rats’ blood cells. At the same time, the genotoxic properties of the nanocomposite were noted only in the early period of the experiments. At present, it has been demonstrated that the genotoxicity of NPs is the result of two main mechanisms: primary or secondary, both of which can be realized together or separately. Primary genotoxicity is caused by the direct interaction of NPs with the genome and requires physical contact of substances with DNA in the nucleus, which leads to its pathological alterations. The main damaging factors as the secondary mechanism are ROS or the release of toxic ions after the dissolution of the NPs [23].
Under oxidative stress, free radicals interact with DNA, causing purine or pyrimidine oxidation and molecular breaks. Damages can be repaired or lead to gene mutations or damage to chromosomes. Nanoparticles can also damage genetic materials through other molecules
interacting with DNA, such as protein kinases that regulate DNA replication [24]. The toxicity of copper NPs is most likely due to the increased generation of ROS in cells, leading to their oxidative damage [23].
Conclusions
The effect of copper oxide nanocomposite compound at 500 μg/kg on the body of experimental rats for 10 days w:as char:acterized by a dysfunction of normal metabolism, along with the resultant cytotoxicity and/or genotoxic effects. To date, the dosage of copper NPs for use as an antibacterial agent or reparative drug is not well known. Therefore, it is not possible to evaluate the toxicity of copper NPs as a medicinal agent. However, the revealed toxicity of copper NPs encapsulated in an arabinogalactan matrix under the experimental conditions of the current study should be alarming. Lastly, the study findings should be taken into account when planning for the application of this agent for its medicinal properties.
Conflict of Interests
The authors declare no conflict of interest.
Funding
This research project was funded solely by the authors and received no funding from any internal or external sources.
Acknowledgement
The authors acknowledge the support of the management and staff of the Biomodeling and Translational Medicine Laboratory, East-Siberian Institute of Medical and Ecological Research, Angarsk, Russia.
Compliance with Ethical Guidelines
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the FSBSI ESIMER (identification code: E03/23; date of approval: 11 March 2023).
Authors' Contributions
Conceptualization: E.A.T. and M.A.N.; methodology: L.M.S., V.A.T., V.A.V., and A.A.P.; software: M.A.N. and V.A.T.; validation: E.A.T., M.A.N. and L.M.S.; formal analysis: L.M.S.; investigation: E.A.T., M.A.N., V.A.T., A.A.P., and E.V.B.; resources: L.M.S.; data curation: M.A.N. and E.V.B; writing—original draft preparation: E.A.T.; writing—review and editing: L.M.S. and M.A.N.; visualization: E.A.T. and M.A.N.; supervision: L.M.S.; project administration: L.M.S. and E.A.T.; funding acquisition: L.M.S. All authors read and approved the final manuscript.
References
- Nevezhina AV, Fadeeva TV. Prospects for the creation of antimicrobial drugs based on copper nanoparticles and copper oxides. Acta Biomedica Scientifica (East Siberian Biomedical Journal). 2021;6(6-2):37-50. [doi: 10.29413/ABS.2021-6.6-2.5]
- Mitra D, Kang ET, Neoh KG. Antimicrobial copper –based materials and coatings: Potential multifaceted biomedical applications. ACS Appl Mater Interfaces. 2020;12(19):21159 -21182. [doi: 10.1021/
acsami.9b17815] [pmid:31880421]
- Shurygina IA, Shurygin MG. Prospects for the use of metal nanoparticles for the purposes of regenerative medicine. Siberian Medical Review. 2018;4:31-37. [doi: 10.20333/2500136-2018-4-31-37]
- Ramos-Zúñiga J, Bruna N, Pérez-Donoso JM. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023;24:10503.[ doi: 10.3390/ijms241310503] [pmid: 37445681]
- Gopal A, Kant V, Gopalakrishnan A, Tandan SK, Kumar D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. European Journal of Pharmacology. 2014;731:8-19. [doi: 10.1016/j.ejphar.2014.02.033] [pmid: 24632085]
- Woźniak-Budych MJ, Staszak K, Staszak M. Copper and Copper-Based Nanoparticles in Medicine-Perspectives and Challenges. Molecules. 2023;28(18):6687. [doi: 10.3390/molecules28186687] [ pmid: 37764463]
- Phull A-R, Ali A, Dhong KR, Zia M, Mahajan PG, Park H-J. Synthesis, characterization, anticancer activity assessment and apoptosis signaling of fucoidan mediated copper oxide nanoparticles. Arab J Chem. 2021;14:103250. [doi:10.1016/j.
arabjc.2021.103250]
- Abdelhakm LO, Kandil EI, Mansour SZ, El-Sonbaty SM. Chrysin Encapsulated Copper Nanoparticles with Low Dose of Gamma Radiation Elicit Tumor Cell Death Through p38 MAPK/NF-κB Pathways. Biol Trace Element Res. 2023:1-20. [doi: 10.1007/s12011-023-03596-1] [pmid: 36905557]
- Khvostov MV, Tolstikova TG, Borisov SA, Dushkin AV. Application of natural polysaccharides in pharmaceuticals. Bioorganic Chemistry. 2019;45(6):563-575. [doi: 10.1134/S1068162019060219]
- Date AA, Nagarsenker MS. Novel delivery systems of atorvastatin should be evaluated for pharmacodynamics instead of pharmacokinetics. JPP. 2007;59:1583-1584. [doi: 10.1211/jpp.59.
11.0017] [pmid: 17976271]
- Aleksandrova, G.P., Sukhov, B.G., Trofimov, B.A., Sapozhnikov, A.N., Boymirzaev, A.S.Nanobiocomposites of pharmacophoric iron and bismuth oxides with arabinogalactan matrix. Russian Journal of General Chemistry. 2020; 90 (4): 672-679. [doi:10.
1134/S1070363220040180]
- Titov EA, Sosedova LM, Novikov MA. Alteration of the brain tissue of white rats induced by the action of a silver nanocomposite encapsulated on a polymer matrix. Pathological Physiology and Experimental Therapy. 2015;59(4):41-44. [pmid: 27116877]
- Titov EA, Sosedova LM, Novikov MA, Zvereva MV, Rukavishnikov VS, Lakhman OL. The analysis of acute and subacute toxicity of silver selenide nanoparticles encapsulated in arabinogalactan polymer matrix. Polymers. 2022; 14(15):3200. [doi:10.3390/polym14153200]
- Dolgov VV, Menshikov VV. Clinical laboratory diagnostics. National leadership; Moscow: GEOTAR-Media. 2016; p.688.
- Zapadnyuk I P, Zapadnyuk V I, Zakharia E A, Zapadnyuk BV. Laboratory animals: breeding, maintenance, use in the experiment, 3rd edition. Kiev:Vishcha school;1983; 134-154.
- Durnev AD, et al. Application of the method of alkaline gel electrophoresis of isolated cells to assess the genotoxic properties of natural and synthetic compounds; Moscow: Official. 2006;1-27.
- Magdolenova Z, Drlickova M, Henjum K, Rundén-Pran E, Tulinska J, Bilanicova, D, et al. Coating-dependent induction of cytotoxicity and genotoxicity of iron oxide nanoparticles. Nanotoxicology. 2015;9:44-56. [doi: 10.3109/17435390.2013.
847505] [pmid: 24228750]
- Robinson RR, Feirtag J, Slavin JL. Effects of dietary arabinogalactan on gastrointestinal and blood parameters in healthy human subjects. J Am CollNutr. 2001;20(4):279-285. [doi: 10.1080/07315724.2001.
10719048] [pmid: 11506055]
- Lee IC, Ko JW, Park SH, Lim JO, Shin IS, Moon C, et al. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int J Nanomedicine. 2016;16(11):2883-2900. [doi: 10.2147/IJN.
S106346] [pmid: 27366066]
- Sizova EA, Korolev VL, Makaev ShA, Miroshnikova EP, Shakhov VA. Morpho-biochemical parameters of blood in broilers with correction of the diet with salts and Cu nanoparticles. Agricultural Biology. 2016;51(6):903-911.[doi:10.15389/agrobiology.2016.
6.903eng]
- Xu LJ, Zhao JX, Zhang T, Ren GG, Yang Z. In vitro study on influence of nano particles of CuO on CA1 pyramidal neurons of rat hippocampus potassium currents. Environ Toxicol. 2009; 24:211-217. [doi: 10.1002/tox.20418] [pmid: 18623077]
- Liu Z, Liu S, Ren G, Zhang T, Yang Z. Nano-CuO inhibited voltage-gated sodium current of hippocampal CA1 neurons via reactive oxygen species but independent from G-proteins pathway. J Appl Toxicol. 2011;31:439-445. [doi: 10.1002/jat.1611] [pmid: 21218498]
- Sajjad H, Sajjad A, Haya RT, Khan MM, Zia M. Copper oxide nanoparticles: In vitro and in vivo toxicity, mechanisms of action and factors influencing their toxicology. Comp Biochem Physiol C Toxicol Pharmacol. 2023;271:109682. [doi: 10.1016/j.cbpc.2023.109682][ pmid: 37328134.]
- Morsy EA, Hussien AM, Ibrahim MA, Farroh KY, Hassanen EI. Cytotoxicity and Genotoxicity of Copper oxide Nanoparticles in chickens. Biol Trace Elem Res.2021;199(12):4731-4745.[doi: 10.1007/s12011-021-02595-4][pmid: 33484442]
Type of Study:
Research |
Subject:
General










































 , Мikhail Aleksandrovich Novikov *2
, Мikhail Aleksandrovich Novikov *2 

 , Vera Aleksandrovna Vokina3
, Vera Aleksandrovna Vokina3 

 , Vera Aleksandrovna Tyutrina4
, Vera Aleksandrovna Tyutrina4 

 , Anna Aleksandrovna Pankova5
, Anna Aleksandrovna Pankova5 

 , Ekaterina Vladimirovna Buynova5
, Ekaterina Vladimirovna Buynova5 

 , Larisa Mikhailovna Sosedova6
, Larisa Mikhailovna Sosedova6 








