Ethics code: IR.ARAKMU.AEC.1401.012.


Hosseinzadeh M, Amini M, Seif F, Baazm M, Ganji A, Siasi A et al . Study of the protective effects of ginger extract on oxidative stress induced by X-ray radiation in the peripheral blood and liver tissue of male rats. IJT 2024; 18 (4) :217-223
URL:
http://ijt.arakmu.ac.ir/article-1-1363-en.html
1- Medical Student Research Committee, School of Medicine, Arak University of Medical Sciences, Arak, Iran , hosseinzadeh.1376@yahoo.com
2- Medical Student Research Committee, School of Medicine, Arak University of Medical Sciences, Arak, Iran
3- Medical Physics, Department of Radiotherapy and Medical Physics, School of Para-Medicine, Arak University of Medical Sciences and Khansari Hospital. Arak, Iran
4- Anatomical Sciences, Department of Anatomy, School of Medicine, Arak University of Medical Sciences. Arak, Iran
5- Department of Immunology, School of Medicine Molecular and Medicine Research Center, Arak University of Medical Sciences. Arak, Iran
6- Clinical Biochemistry, Department of Biochemistry and Genetics, School of Medicine, Arak University of Medical Sciences. Arak, Iran
Full-Text [PDF 750 kb]
(501 Downloads)
|
Abstract (HTML) (1459 Views)
Full-Text: (643 Views)
Introduction
Radiation exposure from natural or artificial sources may be frequently occurring in our daily lives [1]. Radiation therapy is a vital therapeutic approach for human malignancies. However, excessive radiation exposure may negatively affect individuals, such as those involved in nuclear accidents, medical professionals, and patients with cancer [2]. In the latter group, radiation exposure exerts harmful effects on normal cells and tissues surrounding the cancer tumor [3].
Radiation-induced damages to body organs and tissues occur through either direct or indirect mechanisms of action. It can be directly mediated by DNA single- or double-strand breaks and chromosomal damage or indirectly by the generation of reactive oxygen species (ROS), which can also lead to cellular and tissue damage [4, 5]. Reactive oxygen species are highly reactive molecules that interact with lipids, proteins, and nucleic acids in order to induce damage to these biomolecules, leading to oxidative stress and cellular and/or tissue dysfunctions [6]. The production of ROS can occur through the radiolysis of water molecules, forming hydroxyl radicals as potent oxidizing agents with the ability to damage cellular components [7]. The accumulation of ROS can lead to a variety of pathophysiological conditions, including cancer, neurodegenerative disorders, and cardiovascular diseases [8].
To date, several preventive strategies have been described to reduce the side effects of radiation exposure and prevent the development of oxidative stress [9]. In this context, natural products have traditionally been used for nutritional and therapeutic purposes [10]. Several in-vivo and in-vitro studies have shown that polyphenol-rich phytochemicals, such as resveratrol, quercetin, and ginger, may be used as preventive and therapeutic drugs against several diseases [11]. These compounds have a variety of pharmacological activities, such as antioxidant, anti-inflammatory, anti-microbial, and anti-cancer effects [11].
Zingiber officinale Rosc., commonly known as ginger, belongs to the Zingiberaceae family and is a medicinal plant characterized by its fragrant and spicy rhizomes [12]. Ginger contains a large amount of active compounds, such as phenolic compounds and terpenes [13]. Multiple studies have suggested that ginger can protect the body from oxidative stress-induced injuries [14]. The antioxidant properties of this medicinal plant have been reported numerous times in the literature. Akullo, et al. have reported that ginger extracts show a significant amount of total phenols, flavonoids, and vitamin C contents compared to garlic [16]. Another study conducted by Yang, et al. revealed that both cultivated and wild ginger have considerable antioxidant activity and high nutritional value, which may be used as a nutritional supplement or therapeutic agent [17]. Danwilai, et al. studied the antioxidant effect of ginger extract supplements on patients with cancer who were under chemotherapy treatment and compared it with a placebo group. The study data revealed that the patients in the ginger group had significantly higher serum levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), together with increased levels of total glutathione (GSH/GSSG) on day 64 than those in the placebo group. However, malondialdehyde (MDA) and NO2−/NO3− levels in patients receiving ginger were significantly lower than those of the placebo [18].
Aim of the Study: This study aimed to investigate whether ginger can mitigate the oxidative effects of X-ray. To the best of our knowledge, no study has yet reported the protective effect of ginger extract against X-rays. Therefore, we evaluated the impact of ginger extract on markers of oxidative stress in the serum and liver tissue samples of adult male rats after exposure to X-ray radiation.
Materials and Methods
Animals: A total of 36 male Sprague–Dawley rats weighing 220 ± 20 g were purchased from the Pasteur Institute of Iran (Tehran, Iran). Animals were acclimated in cages with free access to water and food. All experiments were performed according to the guidelines of the Animal Ethics Committee of Arak University (Ethics approval: IR.ARAKMU.AEC.1401.012).
Preparation of Ginger Extract: To prepare the ginger extract, 30 g of ginger was ground into powder and then soaked in 300 ml of pure ethanol inside a Soxhlet extractor. To ensure saturation, the extraction process took 4 hours at 78°C. Subsequently, the ethanol part of the extract was evaporated on a rotary evaporator at 89°C to produce the ginger crude extract. The obtained extract samples were weighed, dissolved in 10% dimethyl sulfoxide (DMSO), and stored at a temperature of 4°C in a refrigerator until further experimental steps.
Experimental Design: The experimental design is summarized in Figure 1. All rats were randomly allocated into six groups of six rats each. Control and treatment groups received 0.5 ml of normal saline and 10% DMSO, respectively. The rats in the sham group were treated with 0.5 ml of normal saline and placed in the X-ray device that was turned off during the same treatment period. The fourth group of animals (GE group) received ginger extract at 100 mg/kg, while those in the fifth group (X-ray treatment) were administered 0.5 ml normal saline and exposed to whole body X-radiation at an energy level of 6 MV and a dose of 4 Gy (Elekta precise Linac) once daily. The sixth rat group was treated with the combination of Ginger (100 mg/kg) and X-ray with an energy of 6 MV and a dose of 4 Gy (Elekta precise Linac). All treatments were orally administered once a day over four weeks. Animals were anesthetized 24 hours after X-ray exposure, and the blood samples were taken by cardiac puncture. Subsequently, the collected serum samples were stored at -70°C for further tests. Finally, the animals were sacrificed, and their livers were removed. All dissected tissues were stored at -70°C until further processing and experiments.
Preparation of Liver Homogenates: The frozen tissue samples (~100 mg) were cut into small pieces and then homogenized on ice in 1 mL cold PBS (pH=7.4, 0.1 M). Next, the homogenized tissue samples were centrifuged at 10,000 rpm for 15 min at 4°C. The supernatant was collected for each sample and used to measure the oxidative stress parameters. All parameters were expressed per mg of wet tissue samples for the evaluation of oxidative stress markers in the liver and serum samples.
Measurement of Malondialdehyde: The MDA levels were determined to estimate the rate of the production of lipid peroxidation in the liver tissue and serum samples of the X-ray-exposed rats. Measurement of MDA levels was carried out using an established procedure as described previously [19]. Briefly, MDA in the samples (liver homogenate or plasma) reacted with thiobarbituric acid (TBA) to generate MDA-TBA adducts. Subsequently, the thiobarbituric acid reactive substance was extracted by n-butanol, and the absorbance of the final product was read at 532 nm on a plate reader (BioTek Epoch Microplate, USA).
Measurement of Total Antioxidant Capacity: The ferric-reducing ability of the plasma method was used to assess the total antioxidant capacity (TAC) in the samples [20]. In this method, the antioxidant capacity of a sample is determined by reducing Fe+3 to Fe+2 under acidic conditions. The complex between Fe2+ and 2,4,6-tri(2-pyridyl)-s-triazine produces a blue color solution with the absorbance read at 593 nm. The absorbance of this complex should correlate with the antioxidant capacity of the samples.
Measurement of Total Oxidative Status: Total oxidant status (TOS) was measured based on the method described earlier by Erel [20]. This method is based on the oxidation of Fe2+ to Fe3+ by the oxidants in the serum and liver tissue homogenate samples. The ferric ion reacts with xylenol orange in an acidic medium to form a colored complex. The absorbance of the Fe3+-xylenol orange complex is directly related to the concentration of oxidants in the samples.
Statistical Analyses: Kolmogorov-Smirnov test was used to assess the normal distribution of data within each group. Additionally, the one-way analysis of variance (ANOVA) followed by Tukey’s post hoc tests was performed to examine the statistical variations among the groups. The results from each test are reported as the mean ± standard error of the mean (SEM). The statistical significance level among pairs of data sets was set at P<0.05.

Figure 1: Schematic representation of the steps of the study.
Results
Effect of Ginger Pre-treatment on X-ray-induced Lipid Peroxidation: As shown in Figures 1A and 1B, ionizing radiation-induced lipid peroxidation in samples from the experimental rats as indicated by the significantly elevated levels of MDA in the serum and liver tissue samples compared to those of the control group (P<0.05). Ginger pre-treatment prior to X-ray irradiation significantly decreased MDA levels in the serum and liver samples compared to that of the X-ray irradiated group (P<0.05). However, the ginger pre-treatment failed to return the MDA levels to those of the control group.
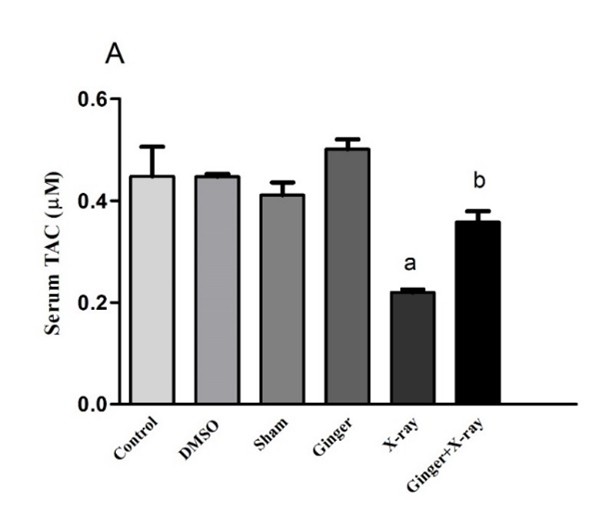
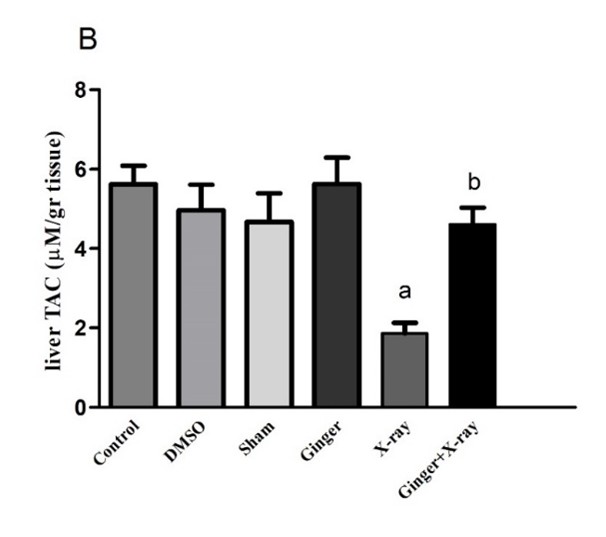
Effect of Ginger Pre-treatment on the TAC Level in X-ray-irradiated Rats: As shown in Figures 2A and 2B, the TAC levels in the serum and liver tissue samples were significantly decreased in the X-ray-exposed group as compared to that of the controls. Ginger pre-treatment, prior to X-ray irradiation, significantly elevated the TAC levels in both serum and liver tissue compared to the group that was exposed to X-ray alone (P<0.05). An insignificant difference was found between the TAC levels of the controls and those treated with ginger alone.


|
|
Figure 2: Comparison of the level of malondialdehyde (MDA) in the serum (A) and liver tissue (B) of the rats in different groups. Ginger pre-treatment significantly decreased MDA levels compared to the X-ray-irradiated group. Data are presented as mean ± SEM (n=6).
a: shows a significant difference between the control group and X-ray irradiation.
b: shows a significant difference between ginger pre-treatment and X-ray irradiation (P<0.05). |
Effect of Ginger Pre-treatment on TOS Levels in the X-ray-irradiated Rats: As shown in Figures 3A and 3B, the serum and liver levels of TOS were significantly higher in the X-ray-exposed group than those of the controls (P<0.05). Ginger pre-treatment before exposure to X-ray significantly diminished the TOS level in the serum and liver tissue samples compared to those of the X-ray-irradiated group. The ginger pre-treatment returned the TOS levels of the liver tissue samples to those of the controls. As reflected by all figures, the levels of MDA, TAC, and TOS in the serum and liver tissue samples of the rats treated with DMSO were not significantly different from those of the saline-treated animals. Therefore, 10% DMSO had no major interfering effect on our results.
|
|
Figure 3: Comparison of the level of total antioxidant capacity in the serum (A) and liver tissue (B) between the control and treatment groups. MDA levels were significantly increased due to ginger pre-treatment in comparison to X-ray-exposed rats. Data are presented as mean ± SEM (n=6).
a: shows a significant difference between the control and X-ray-irradiated groups.
b: shows a significant difference between the rats receiving ginger pre-treatment and those exposed to
X-ray alone (P<0.05). |
Discussion
The findings of this study demonstrated that whole-body (4 Gy) X-ray irradiation in the rats induced oxidative stress in the serum and liver tissue samples by increasing the levels of MDA and TOS while decreasing the TAC levels. Ginger treatment before the exposure of the animals to X-ray alleviated oxidative stress by augmenting the TAC while declining the MDA and TOS levels in the serum and liver tissue samples. It has previously been shown that X-ray exposure exerts detrimental effects on the body [22]. Specifically, ionizing radiation generates ROS in biological systems either by forming exogenous ROS or inducing the production of endogenous ROS. Excessive ROS formation destroys the body’s antioxidant system and disrupts the redox balance [21]. Oxidative stress is the outcome of overwhelming levels of ROS, which leads to adverse and irreversible modifications in cellular compartments [22].
The cellular and organelle membranes are highly susceptible to ROS damage because of their high contents of polyunsaturated fatty acids (PUFAs) [23]. The ROS molecules attack PUFAs in a process that is known as lipid peroxidation, damaging phospholipids directly and starting a chain reaction to induce membrane damage [23]. Peroxidation of some well-known PUFAs, such as arachidonic acid, results in the formation of peroxides, which in turn produces MDA via a retro-Diels-Alder reaction [24]. The main aldehyde product of lipid peroxidation is MDA, which has the ability to react with DNA bases and cause mutagenic lesions [23].
As reported in the reviewed literature, during oxidative stress, MDA increases while TAC decreases in the body tissues and organs [25]. Therefore, it seems logical that X-ray irradiation, as a source of generating ROS in this study, has led to a rise in MDA and a decline in the TAC levels in both serum and liver samples. In our previous research on a rat model of whole-body irradiation, X-ray exposure led to a significant elevation in MDA levels and a significant decrease in TAC levels compared to those found in the controls [26]. In another study conducted by Motallebzadeh, et al., X-ray radiation of the whole brain at a single dose of 25 Gy resulted in a significant increase in the MDA levels with a significant decrease in the TAC levels found in the brainstem tissue samples [27]. Ionizing radiation also increases the levels of TOS, oxidative stress index, and lipid hydroperoxide in rats receiving 5 Gy gamma-radiations [28].
Many pre-clinical and clinical studies have suggested that natural products contain potential antioxidants with the capacity to alleviate oxidative stress-induced damage and combat oxidative stress-related diseases [29]. Thus, the present study aimed to use a natural, plant-derived material, such as ginger extract, to ameliorate the oxidative stress induced by whole-body X-ray exposure to rats. The results provided evidence that ginger has the ability to reestablish redox balance in the body tissues by reducing oxidative markers and raising the anti-oxidative indices. Numerous studies have previously shown the antioxidant activity of ginger in different body organs. Nwachukwu, et al. investigated the protective effects of garlic, ginger, and onion extracts on the oxidative stress markers in the liver, kidney, and heart of X-ray-exposed rats. The results revealed that the extracts exerted antioxidant effects on the three organs by increasing the activity of SOD and CAT and a significant decrease in lipid peroxidation [30].
Ginger extract pre-treatment also mitigated the adverse effects of varying doses of γ-ray (2, 4, and 8 Gy) in the kidney tissue samples of male Wistar rats. The irradiated rats that received ginger extract had ameliorated histological images and stronger antioxidant status in the kidneys compared to the animals that received γ-ray alone, as reported by another study [31]. Inducing oxidative stress in rat eyes results in a significant decrease in the activities of SOD, GPx, and CAT, as well as a significant increase in the levels of MDA. However, ginger pre-treatment ameliorated the oxidative status by raising anti-oxidative enzymes while reducing the lipid peroxidation marker MDA, as compared to that of the controls [32].
Hydroalcoholic extract of ginger has also increased the activity of three antioxidant enzymes (i.e., GPx, SOD, and CAT) in the testes of ethanol-treated male Wistar rats [33]. In addition, ginger has significantly diminished the levels of lipid peroxidation marker MDA in testes [33]. By detecting the same parameters in the kidney tissue of ginger- and ethanol-administered male Sprague-Dawley rats, Fathi, et al. have suggested that antioxidant and anti-renotoxicity effects of ginger are mediated through a pathway that activates nuclear factor erythroid 2-related factor 2 and tumor necrosis factor-α [34]. An in-vivo study in animal models has shown that low single-dose radiation (ranging from 0.02 to 1.0 Gy) may induce liver damage [35]. Therefore, ginger can be used as a hepatoprotective natural product against ionizing radiation-induced tissue damage.
Conclusions
According to the results of the current research, X-ray irradiation can induce oxidative stress in the liver. The main finding of this investigation is that ginger pre-treatment prior to X-ray irradiation may have a protective effect on the oxidative damage induced by X-ray radiation. The present data suggested that ginger ameliorated oxidative stress by mitigating lipid peroxidation, reducing TOS levels, and enhancing the TAC of serum and liver tissue. Taken together, the present study provided evidence to suggest ginger as a natural plant-derived candidate for the alleviation of radiation-induced oxidative stress and organ damage.
Conflict of Interests
Authors declare that they have no conflict of interest.
Funding
This paper was supported by Grant Number 6891 from the Vice-Chancellor for Research Affairs of Arak University of Medical Sciences, Arak, Iran.
Acknowledgement
This work was extracted from a thesis and funded by Grant #: 6891 and partially by Grant #: 3743, that were provided by the Vice-Chancellor for Research Affairs of Arak University of Medical Sciences.
Compliance with Ethical Guidelines
The study protocol was reviewed and approved by the Ethics Committee of Arak University of Medical Sciences (Ethics approval code: IR.ARAKMU.AEC.1401.012.)
Authors' Contributions
MH, MA, and FS: Designed the study protocol. All authors assisted in conducting the experiments and interpreting the data. AT and SR: Assisted in writing the initial drafts of the manuscript. All authors reviewed and approved the final version of the manuscript.
References
1. Lowe D, Roy L, Tabocchini MA, Rühm W, Wakeford R, Woloschak GE, et al. Radiation dose rate effects: what is new and what is needed? Radiation and environmental biophysics. 2022;61(4):507-43.[ doi: 10.1007/s00411-022-00996-0][pmid: 36241855]
2. Jain S. Radiation in medical practice & health effects of radiation: Rationale, risks, and rewards. Journal of family medicine and primary care. 2021;10(4):1520-4. [ doi: 10.4103/jfmpc.jfmpc_2292_20] [pmid: 34123885]
3. Wang JS, Wang HJ, Qian HL. Biological effects of radiation on cancer cells. Military Medical Research. 2018;5(1):20.[ doi: 10.
1186/s40779-018-0167-4][pmid: 29958545]
4. Nickoloff JA, Sharma N, Taylor L. Clustered DNA Double-Strand Breaks: Biological Effects and Relevance to Cancer Radiotherapy. Genes. 2020;11(1).[ doi: 10.3390/genes11010099][pmid: 31952359]
5. Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. International journal of molecular sciences. 2021;22(9).[ doi: 10.3390/ijms22094642][pmid: 33924958]
6. Babak F, Rajabi S, Sakhaie MH, Jalali-Mashayekhi* F. Carvacrol ameliorating effects on trimethyltin chloride-induced neurotoxicity by modulating the interplay between Nrf2/Keap1/ARE pathway and sirt1. Research Journal of Pharmacognosy. 2022;9(2):53-61.[doi:10.
22127/rjp.2022.320430.1820]
7. Aranda-Rivera AK, Cruz-Gregorio A, Arancibia-Hernández YL, Hernández-Cruz EY, Pedraza-Chaverri J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen. 2022; 2(4):437-78. [doi: 10.3390/oxygen2040030]
8. Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Archives of toxicology. 2023;97(10):2499-574.[ doi: 10.1007/s00204-023-03562-9][pmid: 37597078]
9. Nuszkiewicz J, Woźniak A, Szewczyk-Golec K. Ionizing Radiation as a Source of Oxidative Stress-The Protective Role of Melatonin and Vitamin D. International journal of molecular sciences. 2020;21(16).[ doi: 10.3390/ijms21165804][pmid: 32823530]
10. Fischer N, Seo EJ, Efferth T. Prevention from radiation damage by natural products. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2018;47:192-200.[ doi: 10.1016/j.phymed.2017.11.005][pmid: 30166104]
11. Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, et al. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Frontiers in pharmacology. 2022;13:806470.[ doi: 10.3389/fphar.2022.806470][pmid: 35237163]
12. Zhang M, Zhao R, Wang D, Wang L, Zhang Q, Wei S, et al. Ginger (Zingiber officinale Rosc.) and its bioactive components are potential resources for health beneficial agents. Phytotherapy research : PTR. 2021;35(2):711-42.[ doi: 10.1002/ptr.6858][pmid:32954562]
13. Mao QQ, Xu XY, Cao SY, Gan RY, Corke H, Beta T, et al. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods (Basel, Switzerland). 2019;8(6).[ doi: 10.3390/foods80
60185][pmid:31151279]
14. Anwar S, Almatroudi A, Allemailem KS, Jacob Joseph R, Khan AA, Rahmani AH. Protective Effects of Ginger Extract against Glycation and Oxidative Stress-Induced Health Complications: An In Vitro Study. Processes. 2020; 8(4).[doi: 10.3390/pr8040468]
15. Akinyemi AJ, Ademiluyi AO, Oboh G. Aqueous extracts of two varieties of ginger (Zingiber officinale) inhibit angiotensin I-converting enzyme, iron(II), and sodium nitroprusside-induced lipid peroxidation in the rat heart in vitro. J Med Food. 2013;16(7):641-6.[ doi: 10.1089/jmf.2012.0022][pmid: 23875904]
16. Akullo JO, Kiage-Mokua BN, Nakimbugwe D, Ng’ang’a J, Kinyuru J. Phytochemical profile and antioxidant activity of various solvent extracts of two varieties of ginger and garlic. Heliyon. 2023;9(8):
e18806.[ doi: 10.1016/j.heliyon.2023.e18806][pmid: 37576272]
17. Yang J, Li Y, He Y, He H, Chen X, Liu T, et al. Wild vs. Cultivated Zingiber striolatum Diels: Nutritional and Biological Activity Differences. Plants (Basel, Switzerland). 2023;12(11).[ doi: 10.3390/
plants12112180][pmid: 37299159]
18. Danwilai K, Konmun J, Sripanidkulchai B, Subongkot S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer management and research. 2017;9:11-8.[ doi: 10.2147/CMAR.S124016][pmid: 28203106]
19. Samimi F, Baazm M, Eftekhar E, Rajabi S, Goodarzi MT, Jalali Mashayekhi F. Possible antioxidant mechanism of coenzyme Q10 in diabetes: impact on Sirt1/Nrf2 signaling pathways. Research in pharmaceutical sciences. 2019;14(6):524-33.[doi: 10.4103/1735-5362.272561][pmid: 32038732]
20. Erel O. A new automated colorimetric method for measuring total oxidant status. Clinical biochemistry. 2005;38(12):1103-11.[ doi: 10.1016/j.clinbiochem.2005.08.008][pmid:16214125]
21. Dong S, Lyu X, Yuan S, Wang S, Li W, Chen Z, et al. Oxidative stress: A critical hint in ionizing radiation induced pyroptosis. Radiation Medicine and Protection. 2020;1(4):179-85.[ doi:10.
1016/j.radmp.2020.10.001]
22. Lundgren CAK, Sjöstrand D, Biner O, Bennett M, Rudling A, Johansson AL, et al. Scavenging of superoxide by a membrane-bound superoxide oxidase. Nature chemical biology. 2018;14(8):788-93.[ doi: 10.1038/s41589-018-0072-x][pmid: 29915379]
23. Su LJ, Zhang JH, Gomez H, Murugan R, Hong X, Xu D, et al. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative medicine and cellular longevity. 2019;2019:5080843.[ doi: 10.1155/2019/50
80843][pmid: 31737171]
24. Yang B, Fritsche KL, Beversdorf DQ, Gu Z, Lee JC, Folk WR, et al. Yin-Yang Mechanisms Regulating Lipid Peroxidation of Docosahexaenoic Acid and Arachidonic Acid in the Central Nervous System. Frontiers in neurology. 2019;10:642.[ doi: 10.3389/fneur.2019.00642][ pmid: 31275232]
25. Khajehnasiri F, Mortazavi SB, Allameh A, Akhondzadeh S, Hashemi H. Total antioxidant capacity and malondialdehyde in depressive rotational shift workers. Journal of environmental and public health. 2013;2013:150693.[ doi: 10.1155/2013/150693]
[pmid: 23690799]
26. Salehi S, Bayatiani M, Yaghmaei P, Rajabi S, Goodarzi M, Jalali Mashayekhi F. Protective effects of resveratrol against X-ray irradiation by regulating antioxidant defense system. Radioprotection. 2018;53(4):293-8.[ doi:10.1051/radiopro/
2018034]
27. Motallebzadeh E, Aghighi F, Vakili Z, Talaei SA, Mohseni M. Neuroprotective effects of alpha-lipoic acid on radiation-induced brainstem injury in rats. Research in pharmaceutical sciences. 2023;18(2):202-9.[ doi: 10.4103/1735-5362.367798]
[pmid: 36873276]
28. Alkis H, Demir E, Taysi MR, Sagir S, Taysi S. Effects of Nigella sativa oil and thymoquinone on radiation-induced oxidative stress in kidney tissue of rats. Biomedicine & pharmacotherapy= Biomedecine & pharmacotherapie. 2021;139:111540.[ doi: 10.10
16/j.biopha.2021.111540][pmid:33831837]
29. Chen W, Jia Z, Pan MH, Anandh Babu PV. Natural Products for the Prevention of Oxidative Stress-Related Diseases: Mechanisms and Strategies. Oxidative medicine and cellular longevity. 2016;2016:4628502.[ doi: 10.1155/2016/4628502]
[pmid: 26925192]
30. Nwachukwu KC, Asagba SO, Nwose C, Okoh MP. Radiation protection and anti-oxidative effects of garlic, onion and ginger extracts, x-ray exposed albino rats as model for biochemical studies. African Journal of Biochemistry Research. 2014;8(9):
166-73. [doi:10.5897/AJBR2014.0794]
31. Saberi H, Keshavarzi B, Shirpoor A, Gharalari FH, Rasmi Y. Rescue effects of ginger extract on dose dependent radiation-induced histological and biochemical changes in the kidneys of male Wistar rats. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;94:569-76.[ doi: 10.1016/j.biopha.
2017.07.128][pmid:28780473]
32. Akbari A, Nasiri K, Heydari M. Ginger (Zingiber officinale Roscoe) extract can improve the levels of some trace elements and total homocysteine and prevent oxidative damage induced by ethanol in rat eye. Avicenna journal of phytomedicine. 2020;10(4):365-71.[pmid: 32850293]
33. Akbari A, Nasiri K, Heydari M, Mosavat SH, Iraji A. The Protective Effect of Hydroalcoholic Extract of Zingiber officinale Roscoe (Ginger) on Ethanol-Induced Reproductive Toxicity in Male Rats. Journal of evidence-based complementary & alternative medicine. 2017;22(4):609-17.[ doi: 10.1177/21565872
16687696][pmid: 29228791]
34. Fathi R, Akbari A, Nasiri K, Chardahcherik M. Ginger (Zingiber officinale roscoe) extract could upregulate the renal expression of NRF2 and TNFα and prevents ethanol-induced toxicity in rat kidney. Avicenna journal of phytomedicine. 2021;11(2):134-45.[pmid: 33907672]
35. Sun Q, Mao W, Jiang H, Zhang X, Xiao J, Lian Y. The Effect of Protracted Exposure to Radiation on Liver Injury: A Cohort Study of Industrial Radiographers in Xinjiang, China. International journal of environmental research and public health. 2018;15(1).[ doi: 10.3390/ijerph15010071] [pmid:29300360]
Type of Study:
Research |
Subject:
Special










































 , Mohammad Amini2
, Mohammad Amini2 
 , Fatemeh Seif3
, Fatemeh Seif3 

 , Maryam Baazm4
, Maryam Baazm4 

 , Ali Ganji5
, Ali Ganji5 

 , Amirshayan Siasi2
, Amirshayan Siasi2 
 , Farideh Jalali-Mashayekhi6
, Farideh Jalali-Mashayekhi6 






