Ethics code: IR.LUMS.REC.1400.153


1- Assistant Professor of Pharmacology and Toxicology, Occupational Environment Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran.
2- Assistant Professor of Nutritional Sciences, Department of Nutrition, School of Health and Nutrition, Lorestan University of Medical Sciences, Khorramabad, Iran
3- Pharmacy Student; Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran
4- Pharmacy Student; Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran.
5- Assistant Professor; Dept. of Toxicology, Faculty of Pharmacy, Lorestan University of Medical Sciences, Khorramabad, Iran. , hamidrezamohammadi65@yahoo.com
Full-Text [PDF 712 kb]
(161 Downloads)
|
Abstract (HTML) (525 Views)
Full-Text: (105 Views)
Introduction
Tuberculosis (TB) is a life-threatening bacterial disorder and a major public health problem across the globe. According to the World Health Organization (WHO) report in 2021, more than 4000 people lose their lives and 29000 cases of TB are diagnosed worldwide each day [1]. Rifampin (RIF), isoniazid (INH), ethambutol, and pyrazinamide are the first-line medications prescribed in TB [2]. The standard treatment for TB consists of a combination therapy using RIF, INH, ethambutol, and pyrazinamide for the first two months. Subsequently, pyrazinamide and ethambutol are discontinued, and the medication therapy is followed by a combination of INH and RIF for the next four months [3].
RIF, a brownish-red crystalline powder, was first introduced as an effective agent for the treatment of TB in 1968 [4]. This medication can suppress RNA synthesis in bacteria by binding to the β-subunit of DNA-dependent RNA polymerase. Resistance against RIF is usually caused by a mutation in this subunit [5]. Ample evidence has demonstrated that RIF is significantly associated with unfavorable treatment outcomes when it is used at the recommended dosage based on international guidelines [6, 7]. The most frequent adverse effects of RIF treatment are reported to be hepatotoxicity, immune-allergic disorders, renal failure, and skin reaction [7, 8]. Nonetheless, the main deterrent to extensive utilization of RIF is its potential for hepatotoxicity, which can be severe and even fatal if used concurrently with other anti-TB drugs [9, 10].
The liver is the most important organ that regulates the biochemical activities of the human body, playing a major role in the metabolism of carbohydrates, proteins, amino acids, and lipids. Therefore, liver damage is associated with various metabolic dysfunctions [11]. The RIF-induced hepatotoxicity is mainly related to the accumulation of fat, cholestasis, and stress on the cells’ endoplasmic reticulum [12]. It has been observed that RIF-induced hepatotoxicity mainly manifests as increased levels of liver transaminases, total bilirubin, bile acid, fat accumulation, and LP, as well as decreased levels of liver GSH [12, 13, 14]. Furthermore, oxidative stress plays a pivotal role in the development of RIF-induced hepatic damage [15, 16].
So far, multiple studies have been carried out to assess the beneficial effects of various compounds, such as natural medicinal ingredients, synthetic compounds, and food supplements, against anti-TB drugs-induced hepatotoxicity [12, 17, 18]. Moreover, the use of antioxidant compounds is a successful tool to mitigate hepatotoxicity induced by anti-TB drugs [16, 19]. Antioxidant compounds donate electrons to reactive radicals and mitigate the adverse effects in cells [16]. As a sulfur-containing molecule, Tua is among the most plentiful free amino acids in the human body. It has been involved in different physiological interactions in a wide array of cell types and organs [20]. In addition, Tua has been demonstrated to exert a direct impact on oxidative stress mediated by pharmaceuticals and Environmental pollutants [21]. Recently, there has been a boost in interest among researchers to assess the protective effect of Tau against anti-TB drug-induced oxidative stress [21, 22]. Nonetheless, the effects of Tua against RIF-induced hepatotoxicity have not been investigated to date. In light of the aforementioned issues, the present study sought to evaluate the likely protective effects of Tua against RIF-induced liver damage. Our focus was on the assessment of alterations in the indices related to oxidative stress and biochemical parameters.
Materials and Methods
Rifampin (RIF) and taurine (Tua) were obtained from Sigma-Aldrich (St. Louis, MO, USA). 2, 4, 6-tripyridyl-s-triazine (TPTZ), Thiobarbituric acid, Tris-Acetate EDTA, Trichloroacetic acid (TCA), 2′,7′‑Dichlorofluorescein diacetate (DCFH-DA), Potassium Chloride (KCl), Dinitrophenylhydrazine (DNPH), sodium citrate, ferric chloride, were purchased from Merck (Darmstadt, Germany). All other reagents and solvents were prepared from reliable local suppliers.
A total of 36 male Wistar albino rats were purchased from the animal house of the Pharmacy Faculty of Lorestan University of Medical Sciences. The mean weight of the rats before the intervention was 220±30 grams. The animals were held in standard laboratory conditions (an environmental temperature of 21±3°C and a 12:12 h light-dark cycle, along with 40±5% of relative humidity). Rats were allowed to eat and drink (laboratory chow) ad libitum. All experiments were performed following the internationally accepted principles for the care of laboratory animals and achieved the approval of the Ethics Committee of Lorestan University of Medical Sciences, Khorramabad, Iran (approval code: IR.LUMS.REC.1400.153).
After the rats became acclimated to the laboratory environment, they were assigned to six groups of six rats each (n=6 × 6). Group I (controls) received only saline solution (0.5 ml/rat). Hepatic damage was induced by the oral administration of 100 mg/kg/day RIF for two consecutive weeks, using saline as the solvent (Group II). Groups III, IV, and V were treated respectively with Tua at 100, 500, and 1000 mg/kg/day for the next 14 days, in addition to the 100 mg/kg/day of RIF. Tua was also dissolved in saline and injected peritoneally into animals approximately four hours before RIF administration. Group V continued to receive single Tua treatment at 1000 mg/kg/day for 21 days to investigate the possible liver damage.
Biochemical Studies: Liver function enzymes (AST and ALT) are hepatocyte products found in large amounts in the cells. When liver cells are damaged or destroyed, AST and ALT are released into the bloodstream [23]. At the end of the treatment period, the animals were anesthetized with diethyl ether, and the whole blood from each animal was taken via the inferior vena cava using a sterile syringe. The blood sample tubes were placed at normal room temperature for 30 minutes until they clotted completely, and then centrifuged for 15 minutes (5000 rpm) to remove the supernatants. After the serum separation, the samples were kept at -20 degrees Celsius until further analysis. An auto-analyzer device (RA1000 model, manufactured by Technicon, America) was used for the measurement of serum ALT, LDH, and AST Spectrophotometrically. Appropriate commercial kits were also obtained throughout the biochemical examinations (Pars-Azmoon®; Tehran, Iran).
Reactive Oxygen Species (ROS) Assay: The ROS formed in the rat’s liver was assessed via the measurement of the fluorescent probe of 2, 7-dichlorofluorescein diacetate (DCF-DA). In brief, 5 mL of the ice-cooled buffer of Tris–HCl (250 mM, pH = 7.4, and temperature = 4ºC) was used to homogenize the liver tissue samples. Following that, 1 mL of Tris–HCl buffer (40 mM) and 10 μM of DCF-D were used to dissolve 100μL of the tissue homogenates. We incubated the obtained mixture for 15 minutes at 37ºC in the dark. The fluorescence intensity of the samples was finally measured by a fluorimeter at excitation and emission wavelengths of 490 nm and 590 nm, respectively [24].
Lipid Peroxidation (LPO) Assay: The levels of thiobarbituric acid reactive substances were measured for the assessment of LPO in damaged liver tissue [23]. In a nutshell, a total volume of 5 mL of cold potassium chloride (1.15% w: v) was used to homogenize 500 mg of liver tissue samples. Thereafter, 500 μL of the obtained homogenate was dissolved in a mixture of 1 mL thiobarbituric acid (0.6%, w: v) and 3 mL phosphoric acid (1% w: v). The samples were kept in water at 100ºC for 45 minutes and were then cooled down at room temperature. Finally, we added 2 mL of n-butanol to each sample and centrifuged the contents at 10,000g for 10 minutes. The absorbances of supernatants were then recorded at 532 nm (BioTek®, USA, EPOCH® plate reader) [25].
Ferric Reducing Antioxidant Power (FRAP) Assay: The total antioxidant capacity (TAC) of each liver tissue sample was assessed by the FRAP assay. This method is based on the ability of serum to regenerate Fe+3 to Fe+2 in the presence of the substance, tripyridyltriazine (TPTZ). The regeneration rate of each sample was measured by increasing the concentration of Fe+2-TPTZ complex at 593 nm on a spectrophotometer. In brief, 25 mL of acetate buffer (300 mM) was mixed with 2.5 mL of 2, 4, 6-tripyridyl-s-triazine (TPTZ, 10 mM in hypo-chloric acid), and ferric chloride (2.5 mL, 20 mM) to prepare the fresh FRAP reagent. Following that, we added liver homogenates (100 μL, 10% w:v) to the FRAP reagent (900 μL) and incubated the mixture at 37ºC in the dark for 5 minutes. On a final note, samples were centrifuged at 10,000g (4°C) for 15 minutes, and the supernatant absorbance was read at 595 nm for each sample.
Liver Glutathione (GSH) Levels: Each liver tissue sample (200 mg) was homogenized in EDTA buffer (0.02M) at a ratio of 1:10 w/v. Thereafter, the homogenate (5 mL) was mixed with deionized water (4 mL) and CA 50% (1 mL) and centrifuged at 3000 g for 15 minutes. Finally, each supernatant (2 mL) was mixed well with DTNB 0.01 M (0.1 mL) and Tris buffer 0.4 M (4 mL). After agitation at 25°C for 10 minutes, the absorbance was read at 734 nm, and total GSH content was calculated based on the slope of the standard curve.
Statistical Analyses: In the current study, the findings were expressed as means±standard deviations (SD). We also used the statistical test of one-way analysis of variance (ANOVA) followed by Dunnett’s t-test to normalize the derived values. All statistical analyses were performed using GraphPad InStat-3 software. Between-group differences with a probability level was considered significant at P<0.05.
Results
Effect of Tua on Biochemical Parameters: 24 hours after receiving the last drug dose, the rats were anaesthetized with diethyl ether, and whole blood was collected from them. Administering 100 mg/kg RIF significantly elevated the serum levels of ALT, AST, LDH, and bilirubin compared to those in the controls (Figure 1). However, there were no significant differences between the 1000 mg/kg Tua group and the controls in terms of the mentioned serum parameters. As displayed in Figure 1, receiving Tua at all doses (100, 500, and 1000 mg/kg) four hours before RIF administration could significantly suppress the elevation of ALT, AST, and LDH levels in serum. Nonetheless, the serum levels of bilirubin were significantly decreased only in the rats receiving Tau at 1000 mg/kg (Figure 1D).
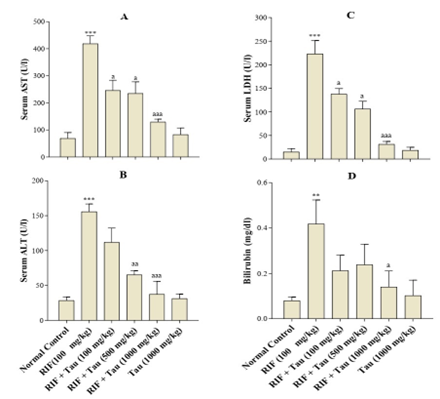
Figure 1. Various effects of exposure to taurine (Tau) for two weeks on hepatotoxicity induced by rifampin (RIF) in adult male rats. Values are expressed as mean ± SD (n=6). ∗∗P < 0.01, ∗∗∗P < 0.001 compared to the control group. # P<0.05; ## P<0.01, ### P<0.001 compared to the RIF group
Effect of Tua on Oxidative Stress Markers: As illustrated in Figure 2A, liver GSH levels in animals exposed to RIF were significantly decreased in comparison to the control group (P<0.001). Furthermore, the treatment of RIF-exposed rats with Tua at 500mg/kg or 1000 mg/kg significantly increased the GSH level compared to those rats that received RIF alone. Nonetheless, the GSH level in the rats that were given RIF plus Tua (100 mg/kg) was not significantly different from those that were treated with RIF alone. The levels of ROS and LPO were significantly higher (P<0.001) in the rats that were treated with RIF compared to those in the control group (Figure 2, panels B and C). The treatment of RIF-exposed rats with Tua at a dose of 100, 500, or 1000 milligrams per kilogram of body weight significantly decreased the ROS and LPO levels compared to those that received RIF alone. However, there were no significant between-group differences in terms of ROS and LPO levels comparing the Tua group (1000 mg/kg) and the normal control group. In addition, this study pointed out that TAC significantly decreased as a result of RIF administration. On the contrary, significant improvements were observed in the antioxidant capacity of the rats that were treated with RIF after they were given Tua at 500 or 1000 mg/kg (Figure 2D).
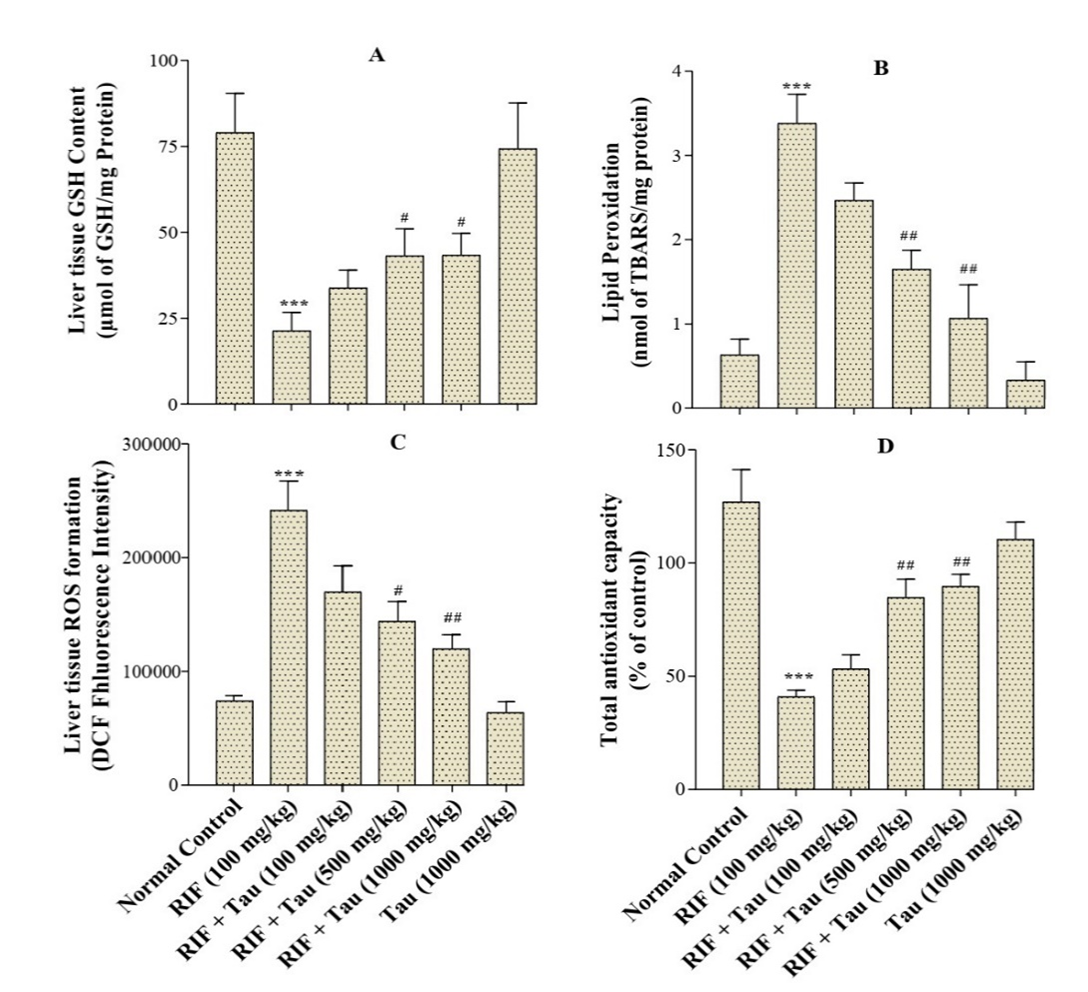
Figure 2. Various effects of exposure to taurine (Tau) for two weeks on hepatotoxicity induced by rifampin (RIF) in adult male rats. Values are expressed as mean±SD (n=6). ∗∗∗P<0.001 compared to the control group; # P<0.05, ## P<0.01 compared to the RIF group
Discussion
Drug-induced liver injury is a general concept that refers to all classes of medications that can cause liver dysfunction [26]. Liver injury is considered a serious concern in the treatment of TB with a variety of drugs. Isoniazid, pyrazinamide, and rifampin are among the most widely used first-line anti-TB drugs, whose hepatotoxic effects are well documented [16, 27, 28]. Based on the findings of this study, the administration of RIF to rats for two consecutive weeks led to significant liver damage. The liver damage was manifested by considerable elevation in blood levels of AST, ALT, LDH, bilirubin, and oxidative stress markers. In agreement with the current research, previous studies have reported that RIF causes hyperbilirubinemia, increases serum aminotransferases, and leads to pathological changes in liver tissue, including hepatocyte necrosis and cholestasis [12, 14, 29, 30].
In general, liver injury happens in approximately 1%-2% of patients receiving RIF as monotherapy against TB [27]. Nevertheless, not all mechanisms involved in RIF-induced liver injury are elucidated. Based on previous studies, ROS production and oxidative stress are among the important mechanisms in liver injury induced by RIF [14, 16]. The current study revealed that RIF could increase the ROS generation in the liver. In particular, ROS leads to LPO in cell membranes, cellular antioxidant depletion, inhibition of the antioxidant defense system, and cross-linking reactions among cellular components [16, 18]. The elevation of ROS production in this study was evident by the increased level of malondialdehyde, which was an indicator of LPO in liver tissue. It is suggested that RIF can induce ROS production through the cytochrome P450 (CYP450) system in the liver. Furthermore, RIF has been shown to induce numerous CYPs, including CYP1A, CYP2A, CYP2B, CYP2C, and CYP3A [31, 32]. It has also been found that CYPs contribute to liver oxidative damage via ROS generation [33, 34].
Moreover, diminished liver GSH has been proposed as another mechanism involved in hepatic injury caused by exposure to RIF and INH [17, 35, 36]. In this study, the RIF treatment led to a marked decrease in GSH level in the liver tissue of the rats. GSH serves as a potent intracellular antioxidant that protects important cellular components from damage caused by free radicals[23, 37]. In response to ROS accumulation, a cellular defensive mechanism begins to exhibit through enzymatic antioxidant defense systems, such as glutathione peroxidase (GPxs), catalase, and superoxide dismutase [38]. The enzyme GPxs uses GSH as a source of reducing equivalents to convert H2O2 and lipid hydroperoxides to water and their corresponding alcohols, respectively [39]. As suggested by the obtained results, the reduction of GSH levels could diminish the activity of GPxs, and consequently reinforce RIF to increase oxidative stress levels in liver tissue.
In addition, it was revealed that the co-administration of Tua and RIF led to a considerable protection against the RIF-induced liver damage. The administration of Tua inhibited LPO and prevented GSH depletion in RIF-exposed rats. Consistent with the current research, previous studies have demonstrated that Tua could decrease the levels of ALT, AST, ROS, and LPO, and induce GSH restoration in animals exposed to pharmaceutical and environmental chemicals [21]. For instance, previous animal experiments have reported a hepatoprotective effect for Tua against hepatic damage induced by acetaminophen [40], methimazole [41], cyclosporine A [42], and Phenytoin [43]. Furthermore, a review of earlier literature revealed that Tua acts as a protective agent against liver injury, induced by heavy metals [44, 45], ethanol [46], and carbon tetrachloride [47]. It is demonstrated that Tua acts as an antioxidant and inhibits LPO in many tissues via scavenging oxygen-free radicals. Moreover, previous investigations have pinpointed that Tua can serve as a membrane stabilizer and subsequently prevents the aberrant membrane permeability due to oxidative damage [48]. Finally, Tua has other physiological functions in the body, including osmoregulation and bile flow control in the liver tissue [49].
Conclusions
As evidenced by the findings of this study, Tua has a protective effect against RIF-induced hepatic damage in rats. This protective effect is mediated by improving antioxidant defenses against oxidative stress. Nonetheless, more investigations are warranted for a comprehensive understanding of other mechanisms through which Tua plays a role against RIF-induced liver injury.
Ethical Considerations
The study protocol was reviewed and approved by the Research Ethics Committee, Lorestan University of Medical Sciences, Khorramabad, Iran (Approval #: IR.LUMS.REC.1400.153).
Authors' Contributions
HM and AGB conceived and designed the experiment. HM and RS collected the experimental data. SM performed statistical analyses. AGB and HM collaborated on the interpretation of the findings. AGB and SH wrote the manuscript’s first draft. SM participated in finalizing and editing the manuscript drafts. All authors cooperated fairly equally in writing the final version of the manuscript.
Conflict of Interests
The authors declare that they have no conflict of interest.
Funding
The current study was funded by Lorestan University of Medical Sciences (Grant number: 37429).
Acknowledgement
The authors of this manuscript wish to express their gratitude to Lorestan University of Medical Sciences, Khorramabad, Iran. The data of this study were extracted from the thesis submitted by the third author, Reza Shahmohammadloo.
References
- Organization WH. Global tuberculosis report. WHO; 2021. [Link]
- Zumla A, Nahid P, Cole ST. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov. 2013;12(5):388-404. [DOI: 10.1038/nrd4001] [PMID: 23629506]
- Horsburgh Jr CR, Barry III CE, Lange C. Treatment of tuberculosis. N Engl J Med. 2015;373(22):2149-60. [DOI: 10.1056/NEJMra1413919] [PMID: 26605929]
- Oliveira M, Chellini P, Amorim T. Simultaneous determination of rifampicin, isoniazid, pyrazinamide and ethambutol in fixed-dose combination antituberculosis pharmaceutical formulations: a review. Analytical Methods. 2018;10(10):1103-16. [DOI:10.1039/C7AY02686B]
- Ashna H, Kaffash A, Khaledi A, Ghazvini K. Mutations of rpob gene associated with rifampin resistance among mycobacterium tuberculosis isolated in tuberculosis regional reference laboratory in northeast of iran during 2015-2016. Ethiop J Health Sci. 2018;28(3):299-304. [DOI: 10.4314/ejhs.v28i3.7] [PMID: 29983529]
- Ramappa V, Aithal GP. Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J Clin Exp Hepatol. 2013;3(1):37-49. [DOI: 10.1016/j.jceh.2012.12.001] [PMID: 25755470]
- Abulfathi AA, Decloedt EH, Svensson EM, Diacon AH, Donald P, Reuter H. Clinical pharmacokinetics and pharmacodynamics of rifampicin in human tuberculosis. Clin Pharmacokinet. 2019;58(9):1103-29. [DOI: 10.1007/s40262-019-00764-2] [PMID: 31049868]
- Grosset J, Leventis S. Adverse effects of rifampin. Rev Infect Dis.1983;5(Supplement_3):S440-S6. [DOI: 10.1093/clinids/5.supplement_3.s440] [PMID: 6356277]
- Ijaz K, Jereb JA, Lambert LA, Bower WA, Spradling PR, McElroy PD, et al. Severe or fatal liver injury in 50 patients in the United States taking rifampin and pyrazinamide for latent tuberculosis infection. Clin Infect Dis. 2006;42(3):346-55. [DOI:10.1086/499244]
- Sridhar A, Sandeep Y, Krishnakishore C, Sriramnaveen P, Manjusha Y, Sivakumar V. Fatal poisoning by isoniazid and rifampicin. Indian Jo Nephrol. 2012;22(5):385-7. [DOI: 10.4103/0971-4065.103930] [PMID: 23326053]
- Singh A, Bhat T, Sharma O. Clinical biochemistry of hepatotoxicity. J Clinic Toxicol S. 2011;4:1-19. [DOI:10.4172/2161-0495.S4-001]
- Zhuang X, Li L, Liu T, Zhang R, Yang P, Wang X, et al. Mechanisms of isoniazid and rifampicin-induced liver injury and the effects of natural medicinal ingredients: A review. Front Pharmacol. 2022:13:1037814. [DOI: 10.3389/fphar.2022.1037814] [PMID: 36299895 ]
- Xiang XX. Studies on characteristics of liver injury model induced by rifampicin and isoniazid in mice. Chinese Pharmacol Bull. 2019;12:586-90. [Link]
- Kim J-H, Nam WS, Kim SJ, Kwon OK, Seung EJ, Jo JJ, et al. Mechanism investigation of rifampicin-induced liver injury using comparative toxicoproteomics in mice. Int J Mol Sci. 2017;18(7):1417. [DOI: 10.3390/ijms18071417] [PMID: 28671602]
- Naseer A, Hussain A, Aslam B, Muhammad F, Mohsin M, Bari MU, et al. Vitamin E and selenium attenuate hepatotoxicity, nephrotoxicity and oxidative stress induced by rifampicin in rabbits. Pakistan Vet J. 2020;40(3):227-82. [DOI:10.29261/pakvetj/2020.026]
- Yew WW, Chang KC, Chan DP. Oxidative stress and first-line antituberculosis drug-induced hepatotoxicity. Antimicrob Agents Chemother. 2018;62(8):e02637-17. [DOI: 10.1128/AAC.02637-17] [PMID: 29784840]
- Attri S, Rana S, Vaiphei K, Sodhi C, Katyal R, Goel R, et al. Isoniazid–and rifampicin–induced oxidative hepatic injury–protection by N–acetylcysteine. Hum Expe Toxicol. 2000;19(9):517-22. [DOI: 10.1191/096032700674230830] [PMID: 11204554]
- Baskaran UL, Sabina EP. Clinical and experimental research in antituberculosis drug-induced hepatotoxicity: a review. J Integr Med. 2017;15(1):27-36. [DOI: 10.1016/S2095-4964(17)60319-4] [PMID: 28088257]
- Singh D, Cho WC, Upadhyay G. Drug-induced liver toxicity and prevention by herbal antioxidants: an overview. Front Physiol. 2016;6:363. [DOI: 10.3389/fphys.2015.00363] [PMID: 26858648]
- Schaffer S, Kim HW. Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther. 2018;26(3):225-41. [DOI: 10.4062/biomolther.2017.251] [PMID: 29631391]
- Nikkhah E, Shirani K, Rezaee R, Karimi G. Protective effects of taurine against hepatotoxicity induced by pharmaceuticals and Environmental chemicals. Toxicol Environ Chem. 2021;103(1):56-84. [DOI:10.1080/02772248.2021.1892113]
- Heidari R, Babaei H, Eghbal MA. Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arch Industrial Hyg Toxicol. 2013;64(2):201-10. [DOI: 10.2478/10004-1254-64-2013-2297] [PMID: 23819928]
- Ghaffarian-Bahraman A, Shahroozian I, Jafari A, Ghazi-Khansari M. Protective effect of magnesium and selenium on cadmium toxicity in the isolated perfused rat liver system. Acta Med Iran. 2014;52(12):872-8. [PMID: 25530047]
- Ghaffarian-Bahraman A, Arabnezhad M-R, Keshavarzi M, Davani-Davari D, Jamshidzadeh A, Mohammadi-Bardbori A. Influence of cellular redox environment on aryl hydrocarbon receptor ligands induced melanogenesis. Toxicol In Vitro. 2022;79:105282. [DOI: 10.1016/j.tiv.2021.105282] [PMID: 34856342]
- Abdoli N, Sadeghian I, Mousavi K, Azarpira N, Ommati MM, Heidari R. Suppression of cirrhosis-related renal injury by N-acetyl cysteine. Curr Res Pharmacol Drug Discov. 2020;1:30-8. [DOI: 10.1016/j.crphar.2020.100006] [PMID: 34909640]
- David S, Hamilton JP. Drug-induced liver injury. US Gastroenterol Hepatol Rev. 2010;6:73-80. [PMID: 21874146]
- Tostmann A, Boeree MJ, Aarnoutse RE, De Lange WC, Van Der Ven AJ, Dekhuijzen R. Antituberculosis drug‐induced hepatotoxicity: concise up‐to‐date review. J Gastroenterol Hepatol. 2008;23(2):192-202. [DOI: 10.1111/j.1440-1746.2007.05207.x] [PMID: 17995946]
- Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5(1):58. [DOI: 10.1038/s41572-019-0105-0] [PMID: 31439850]
- Fountain FF, Tolley EA, Jacobs AR, Self TH. Rifampin hepatotoxicity associated with treatment of latent tuberculosis infection. Am J Med Sci. 2009;337(5):317-20. [DOI: 10.1097/MAJ.0b013e31818c0134] [PMID: 19295414]
- Tan W, Zhao K, Xiang J, Zhou X, Cao F, Song W, et al. Pyrazinamide alleviates rifampin-induced steatohepatitis in mice by regulating the activities of cholesterol-activated 7α-hydroxylase and lipoprotein lipase. Eur J Pharm Sci. 2020;151:105402. [DOI: 10.1016/j.ejps.2020.105402] [PMID: 32492461]
- Burk O, Koch I, Raucy J, Hustert E, Eichelbaum M, Brockmöller Jr, et al. The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (PXR) and constitutively activated receptor (CAR). J Biol Chem. 2004;279(37):38379-85. [DOI: 10.1074/jbc.M404949200] [PMID: 15252010]
- Sousa M, Pozniak A, Boffito M. Pharmacokinetics and pharmacodynamics of drug interactions involving rifampicin, rifabutin and antimalarial drugs. J Antimicrob Chemother. 2008;62(5):872-8. [DOI: 10.1093/jac/dkn330] [PMID: 18713760]
- Hrycay EG, Bandiera SM. Involvement of cytochrome P450 in reactive oxygen species formation and cancer. Adv Pharmacol. 2015;74:35-84. [DOI: 10.1016/bs.apha.2015.03.003] [PMID: 26233903]
- Bondy SC, Naderi S. Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species. Biochem Pharmacol. 1994;48(1):155-9. [DOI: 10.1016/0006-2952(94)90235-6] [PMID: 8043018]
- Enriquez-Cortina C, Almonte-Becerril M, Clavijo-Cornejo D, Palestino-Domínguez M, Bello-Monroy O, Nuño N, et al. Hepatocyte growth factor protects against isoniazid/rifampicin-induced oxidative liver damage. Toxicol Sci. 2013;135(1):26-36. [DOI: 10.1093/toxsci/kft134] [PMID: 23764483]
- Mujahid M, Siddiqui HH, Hussain A, Hussain MS. Hepatoprotective effects of Adenanthera pavonina (Linn.) against anti-tubercular drugs-induced hepatotoxicity in rats. Pharmacognosy J. 2013;5(6):286-90. [DOI:10.1016/j.phcgj.2013.08.003]
- Vairetti M, Di Pasqua LG, Cagna M, Richelmi P, Ferrigno A, Berardo C. Changes in glutathione content in liver diseases: an update. Antioxidants. 2021;10(3):364. [DOI: 10.3390/antiox10030364] [PMID: 33670839]
- Ighodaro O, Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018;54(4):287-93. [DOI:10.1016/j.ajme.2017.09.001]
- Handy DE, Loscalzo J. The role of glutathione peroxidase-1 in health and disease. Free Radic Biol Med. 2022;188:146–61. [DOI: 10.1016/j.freeradbiomed.2022.06.004]
- Das J, Ghosh J, Manna P, Sil PC. Acetaminophen induced acute liver failure via oxidative stress and JNK activation: protective role of taurine by the suppression of cytochrome P450 2E1. Free Radic Res. 2010;44(3):340-55. [DOI: 10.3109/10715760903513017 ] [PMID: 20166895]
- Heidari R, Babaei H, Eghbal MA. Ameliorative effects of taurine against methimazole-induced cytotoxicity in isolated rat hepatocytes. Sci Pharm. 2012;80(4):987-1000. [DOI: 10.3797/scipharm.1205-16] [PMID: 23264945]
- Hagar HH. The protective effect of taurine against cyclosporine A-induced oxidative stress and hepatotoxicity in rats. Toxicol Lett. 2004;151(2):335-43. [DOI: 10.1016/j.toxlet.2004.03.002] [PMID: 15183458]
- Eghbal MA, Taziki S, Sattari MR. Mechanisms of phenytoin‐induced toxicity in freshly isolated rat hepatocytes and the protective effects of taurine and/or melatonin. J Biochem Mol Toxicol. 2014;28(3):111-8. [DOI: 10.1002/jbt.21542]
- Hwang D-F, Wang L. Effect of taurine on toxicity of cadmium in rats. Toxicol. 2001;167(3):173-80. [DOI: 10.1016/s0300-483x(01)00472-3] [PMID: 11578796]
- Sinha M, Manna P, Sil PC. Taurine, a conditionally essential amino acid, ameliorates arsenic-induced cytotoxicity in murine hepatocytes. Toxicol In Vitro. 2007;21(8):1419-28. [DOI: 10.1016/j.tiv.2007.05.010] [PMID: 17624716]
- Balkan J, KANBAGli Ö, Aykaç-Toker G, Uysal M. Taurine treatment reduces hepatic lipids and oxidative stress in chronically ethanol-treated rats. Biol Pharm Bull. 2002;25(9):1231-3. [DOI: 10.1248/bpb.25.1231] [PMID: 12230126]
- Erman F, Balkan J, Cevikbaş U, Kocak-Toker N, Uysal M. Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids. 2004;27(2):199-205. [DOI: 10.1007/s00726-004-0105-5] [PMID: 15338317]
- Piao F, Aadil RM, Suleman R, Li K, Zhang M, Wu P, et al. Ameliorative effects of taurine against diabetes: a review. Amino Acids. 2018;50(5):487-502. [DOI: 10.1007/s00726-018-2544-4] [PMID: 29492671]
- Miyazaki T, Matsuzaki Y. Taurine and liver diseases: a focus on the heterogeneous protective properties of taurine. Amino Acids. 2014;46(1):101-10. [DOI: 10.1007/s00726-012-1381-0] [PMID: 22918604]
Type of Study:
Research |
Subject:
General










































 , Salman Mohammadi2
, Salman Mohammadi2 

 , Reza Shahmohammadloo3
, Reza Shahmohammadloo3 

 , Salma Heidari4
, Salma Heidari4 

 , Hamidreza Mohammadi *5
, Hamidreza Mohammadi *5 




