Ethics code: IR.IAU.Z.REC.1401.017


Abbasi H, Asaadi Tehrani G, Asle-Rousta M. Effects of Thymol and Menthol on Diethylnitrosamine-induced Changes in SOX2 Expression and TGF-β/SMAD3 Signaling in Mice’s Liver and Kidneys. IJT 2024; 18 (4) :196-202
URL:
http://ijt.arakmu.ac.ir/article-1-1374-en.html
1- Department of Genetics, Zanjan Branch, Islamic Azad University, Zanjan, Iran.
2- Department of Genetics, Zanjan Branch, Islamic Azad University, Zanjan, Iran & Aerospace and Mechanical Engineering Department, Notre Dame University, Indiana, USA
3- Department of Physiology, Zanjan Branch, Islamic Azad University, Zanjan, Iran. , mrousta58@gmail.com
Full-Text [PDF 839 kb]
(382 Downloads)
|
Abstract (HTML) (1078 Views)
Full-Text: (370 Views)
Introduction
Nitrosamines, one of the causes of cancer, are primarily formed when nitrogen oxide reacts with secondary amines. The most commonly found nitrosamine in agricultural foods is diethylnitrosamine (DEN) [1]. This compound has the potential to cause the formation of DNA adducts, which is mediated by cytochrome P450 enzymes, particularly cytochrome P450 family-2, subfamily-E, member 1. As a result, DEN is considered a potent hepatocarcinogenic agent and is often used to induce hepatocellular carcinoma in rodents for medical research purposes. The age and gender of the animals are factors that affect the tumorigenesis of DEN. For example, the liver is most susceptible to DEN during the 7-15 days of infancy. Further, since estrogen has an anti-hepato-carcinogenic effect while androgens have carcinogenic potential, male animals are more likely than females to develop DEN-induced tumorigenesis [2]. Estrogens are known to inhibit tumorigenesis by preventing the production of interleukin-6 in the liver’s Kupffer cells [3]. In addition to affecting the liver, DEN also has harmful effects on the kidneys, as demonstrated by two earlier studies [4, 5].
Based on the available literature, the DEN damaging effect begins with oxidative stress and inflammation, progresses to fibrosis, and ultimately culminates in hepatic tumor development [6]. Toll-like receptor-4 (TLR4) signaling is the fundamental determinant in DEN-induced inflammation [7]. The activation of the TLR4/myeloid differentiation primary response 88 (MyD88) signaling pathway triggers the transforming growth factor (TGF)-β pathway and results in fibrosis [8]. The stimulation of TGF-β/SMAD3 signaling can cause fibrosis in various organs, such as the liver, kidneys, heart, and lungs. Consequently, inhibiting this pathway can prevent fibrosis, suppress tumorogenesis, and hinder tumor progression [9]. Transcription factor SRY-related, high-mobility box (SOX)-2 is crucial in mammalian embryogenesis. Nevertheless, it has been observed that SOX-2 expression changes in many tumors, contributing to increased tumor growth, metastasis, drug resistance, and reduced survival rates [10]. Studies indicate that there is a significant correlation between TGF-β and SOX-2 expression [11], along with DEN’s ability to induce SOX-2 expression [12].
Thymol (2-isopropyl-5-methylphenol) and menthol (2-isopropyl-5-methyl-cyclohexanol) are two forms of monoterpenes that possess pharmacological properties. Thymol is commonly extracted from Thymus vulgaris but can also be found in other plant species, such as Ocimumgratissimum L., Origanum L., Trachyspermumammi (L.), Satureja L., Monarda L., Carum copticum L., Oliveriadecumbens, and Anemopsis californica. Menthol is also abundant in Mentha canadensis L. and Mentha x piperita L. [13, 14]. Studies have demonstrated that these compounds exhibit antioxidant, anti-inflammatory, antitumor, and hepato- and nephro-protective effects [13-16]. Conversely, there are several research reports about the protective effects of these compounds on the liver and kidneys [17-20].
Aim of the Study: Based on the available evidence, we hypothesized that thymol and menthol could prevent the molecular changes associated with fibrosis and tumor induction caused by DEN in mice’s liver and kidneys. Therefore, this study was designed to investigate the effect of DEN on TGF-β/SMAD3 signaling and SOX-2 expression in the liver and kidneys of mice that had received DEN during infancy across two periods.
Materials and Methods
Animal Grouping: A total of 48 Balb/C male mice, aged 11-14 days, were used for this experimental research. The mice were obtained from the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences (Tehran, Iran) and were separated into six groups, each consisting of eight mice, as outlined below:
1. The control group (C) did not receive any treatment.
2. The Menthol group (M) was given 50mg/kg of menthol (dissolved in dimethyl sulfoxide) three times a week for 26 weeks [21].
3. The Thymol group (T) was given 10mg/kg thymol (dissolved in dimethyl sulfoxide) for 26 weeks [22].
4. The Diethyl-nitrosamine group (N) was given an intraperitoneal (IP) injection of 25mg/kg DEN (dissolved in normal saline) at the age of 14 days [23].
5. The Diethyl-nitrosamine-Menthol group (NM) received menthol in addition to the DEN injections.
6. The Diethyl-nitrosamine-Thymol group (NT) received thymol in addition to the DEN injections.
All animal procedures were conducted consistent with the ethical principles of the university and were reviewed and approved by the Ethics Committee of the Islamic Azad University, Zanjan Branch, Zanjan, Iran (Approval Code: IR.IAU.Z.REC.1401.017).
Study Scheme: We acquired DEN, thymol, and menthol from Sigma Aldrich Co. (St. Louis, MO, USA). After one day of DEN injection, we started administering thymol and menthol treatment to the mice by gavage for a continuous period of 26 weeks. Following four weeks of DEN injection, we anesthetized four animals from each group with Ketamine (100 mg/kg, IP) and Xylazine (10 mg/kg, IP) [24] to investigate their liver and kidney tissue for the expression of TGF-β, SMAD3, and SOX-2, using real-time polymerase chain reaction (PCR). The remaining animals continued receiving treatment until the end of the 26th week, after which they were sacrificed.
Real-time Polymerase Chain Reaction: Initially, RNA was extracted using an RNA extraction kit (Pars-Tous Co., Mashhad, Iran). Subsequently, the RNA concentration and quality were determined through spectrophotometry at 260 nm and by calculating the A260/A280 ratio. Next, cDNA was generated following the guidelines provided in the Easy cDNA Synthesis Kit (Pars-Tous Co., Mashhad, Iran). For the real-time PCR, we utilized the qPCRBIOSy Green Mix Lo-ROX from PCR BIOSYSTEMS in the Rotor-Gene Q device (Qiagen Co., Hilden, Germany). The thermal cycling conditions involved primary denaturation for one cycle at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 30 sec, annealing at 52°C for 30 sec, and extension at 72°C for 20 sec. The melting curve analysis was carried out to verify the single PCR product for each primer. The primers were synthesized by Gen Fan Avaran (Tehran, Iran), the sequences of which are presented in Table 1. Finally, the 2-ΔΔCT method was employed to normalize the amplification of each target to its corresponding mRNA levels of glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH), as per the recommendations of Livak and Schmittgen [25].
Statistical Analyses: The study data are presented as the means ± the standard error of the means (SEM) for each of the study groups. One-way analysis of variance was used to identify any discrepancies between the
Table 1. Primer sequences used in real-time PCR*
| Sequences |
|
Gene |
| 5'-GTTGTCTCCTGCGACTTCA-3’ |
F |
GAPDH |
| 5'-GGTGGTCCAGGGTTTCTTA-3' |
R |
| 5'-AACTTCTGTCTGGGACCCTG-3’ |
F |
TGF-β |
| 5'- CCGGGTTGTGTTGGTTGTAG-3' |
R |
| 5’- GTCAAAGAACACCGATTCCA-3’ |
F |
SMAD3 |
| 5'- TCAAGCCACCAGAACAGAAG-3' |
R |
| 5'-GCGGAGTGGAAACTTTTGTCC-3' |
F |
SOX-2 |
| 5'-GGGAAGCGTGTACTTATCCTTCT-3' |
R |
*Polymerase chain reaction
experimental groups. The statistical variations among the groups were determined using the Least Significant Difference and Tukey post-hoc tests. The statistical significance between the pairs of study groups was set at P<0.05.Results
The Effects of Thymol and Menthol on TGF-β Expression in the Liver and Kidneys: The expression of TGF-β in the liver of mice receiving DEN showed a significant increase at the end of the fourth and twenty-sixth weeks compared to the control group (P<0.001). The treatment of both thymol and menthol prevented the increase in the expression of TGF-β in the liver of mice receiving DEN; as a result, its expression in the NT and NM groups was significantly reduced compared to the N group (P<0.001) (Figure 1).
The results of real-time PCR showed that there was no significant difference in the renal expression of TGF-β at the end of the fourth week in all different groups. DEN injection after twenty-six weeks caused a significant increase in its expression in the kidney compared to the control group (P<0.001). The expression of TGF-β in the NM and NT groups was significantly lower compared to the N group (P<0.001) (Figure 1).
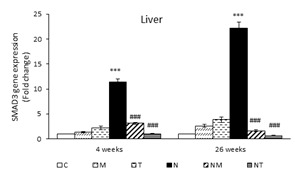
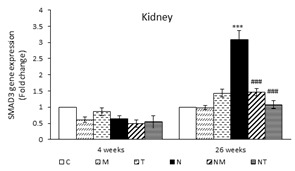
The Effects of Thymol and Menthol on SMAD3 Expression in the Liver and Kidneys: At the end of the fourth and twenty-sixth weeks, the expression of SMAD3 in the liver of the N group was significantly higher compared to that of the control group (P<0.001). In both samples, the SMAD3 expression in the liver of NT and NM groups was significantly lower compared to the N group (P<0.001) (Figure 2).
At the end of the fourth week, no significant difference was observed in the expression of SMAD3 in the kidneys of all study groups. However, at the end of the twenty-sixth week, the SMAD3 expression was higher in the N group compared to the control (P<0.001). The expression of SMAD3 in the kidneys of the NM and NT groups showed a significant decline compared to that of the N group (P<0.001) (Figure 2).
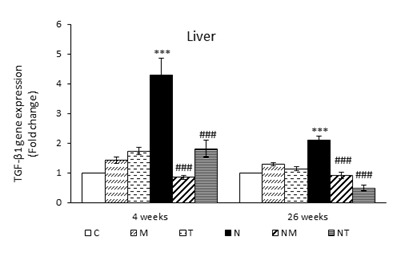
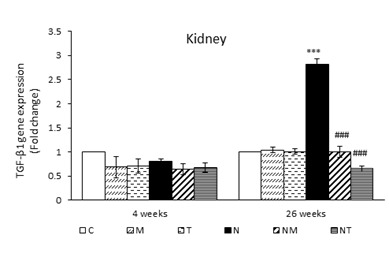
Figure 1. Expression of TGF-β in liver and kidney. Results are presented as means ± SEM. Each group contained four mice. ***P<0.001 compared to group C and ### P<0.001 compared to group N.
Figure 2. Expression of SMAD3 in liver and kidney. Results are presented as means ± SEM. Each group contained four mice. ***P<0.001 compared to group C and ###P<0.001 compared to group N.
The Effects of Thymol and Menthol on SOX-2 Expression in the Liver and Kidneys: The expression of SOX-2 was also significantly increased in the liver of animals receiving DEN in both samples compared to group C (P<0.001). Nevertheless, its expression was significantly lower in the NT and NM groups compared to the N group (P<0.001) (Figure 3).
After four weeks, there was no significant difference in the renal expression of SOX-2 among the various groups. However, after 26 weeks, the N group had significantly higher SOX-2 expression compared to that of the control group (P<0.001). The NM and NT groups had significantly lower levels of SOX-2 expression compared to that of the N group (P<0.001) (Figure 3). In both samples, the expression of TGF-β, SMAD3, and SOX-2 in the liver and kidneys of the T and M groups were not significantly different from those of the control (Figures 1, 2, and 3).
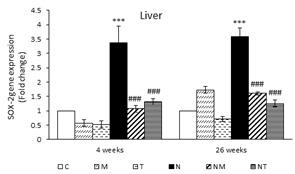
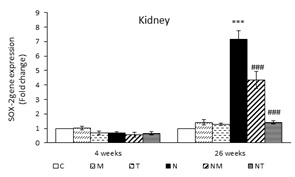
Figure 3. Expression of SOX-2 in liver and kidney. Results are presented as means ± SEM. Each group contained four mice. ***P<0.001 compared to group C and ###P<0.001 compared to group N.
Discussion
There exists strong evidence that DEN is harmful to both the liver and kidneys in animal models [4, 5]. The compound DEN has been known as a potent carcinogen [2, 26]. In the current study, 14-day-old male mice were intraperitoneally injected with DEN (25 mg/kg). The dosage and injection time were determined based on a previous study conducted by Connor et al. [23]. In that study, the injection of this dose in 14-16-day-old mouse pups led to hepatocarcinoma in 26-40-week-old mice. Consequently, the study also measured the expression of the desired genes at the end of the twenty-sixth week. The results of both samples, taken 4 weeks and 26 weeks after DEN injection, showed elevated expression of TGF-β, SMAD3, and SOX-2 in the liver. The expression of these factors increased in the kidney after 26 weeks; however, no significant changes were found at the end of the fourth week. The ability of DEN to increase the expression of SOX-2, TGF-β, and SMAD3 is consistent with the findings reported in the studies conducted by Shen et al. [12] and Perumal et al. [27].
Previous research has shown that a decline in SOX-2 expression in the heart, spleen, and kidneys indicates aging [28], while a high expression can mean tumorigenesis and resistance to treatment [29]. In our N group, the increased expression of SOX-2 in the liver and kidneys suggests changes toward tumorigenesis. DEN tumorigenesis in the liver began earlier than that in the kidneys, as the expression of SOX-2 in the kidneys remained unchanged at the end of the fourth week. However, treatment with thymol and menthol effectively prevented the increase in SOX-2 expression in both mice organs. Although there is no report on the inhibitory effects of menthol and thymol on SOX-2, research has shown that they can boost the levels of signal transducers and activators of transcription 3 (STAT3) [30, 31]. These alterations can likely lead to squamous cell carcinoma [32]. Since STAT3 activation plays an important role in the induction of hepato-carcinogenesis by DEN [33], the ability of menthol and thymol to prevent the increase in SOX-2 expression in mice injected with DEN may prevent the induction of carcinoma. Therefore, we suggest evaluating the expression and activity of STAT3 in the mice liver and kidneys of mice across different animal groups of the current study.
Earlier studies have reported that TGF-β/SMAD3 signaling is crucial in liver and kidney diseases. In this context, SMAD3 plays a significant role in inflammation and fibrosis, whereas SMAD2 activation has shown protective effects [34, 35]. Inflammation is a main factor in developing fibrogenesis as it activates the TGF-β/SMAD3 signaling pathway [36]. The over-expression of TGF-β can lead to epithelial-mesenchymal transition, extracellular matrix deposition, organ fibrosis, and cancer. As a result, targeting TGF-β has become an accepted strategy in cancer treatment [37, 38].
The pathogenesis of DEN begins with inflammation and can lead to fibrosis and carcinoma [6]. Therefore, suppressing TGF-β/SMAD3 signaling is critical in reducing the harmful effects of DEN. For this reason, we determined the expression of inflammatory factors four weeks after DEN injection in both organs. While TGF-β and SMAD3 expression did not change in the kidneys of DEN-injected animals at the end of the fourth week, it seems that the activation of the fibrogenesis pathway in the kidneys of mice injected with DEN during infancy has a delay of several months compared to that of the liver. Menthol and thymol treatments prevented the increase of TGF-β and SMAD3 expression in both organs.
There is also evidence available in support of the anti-fibrogenesis effect of menthol and thymol. Ogaly et al. [39] found that the essential oil of Mentha piperita L. (which contains high amounts of menthol) reduced the expression of TGF-β and SMAD3 in the liver of rats receiving carbon tetrachloride, as well as α-smooth muscle actin and desmin expression. Additionally, the inhibitory effect of thymol on the expression of TGF-β in bleomycin-induced pulmonary fibrosis has been established [40].
Lastly, studies have revealed a significant correlation between SOX-2 and TGF-β/SMAD3 signaling. In non-small-cell lung cancer, SOX-2 enhances the activity of the TGF-β/SMAD3 pathway [41]. Further, Weina et al. [11] have demonstrated that TGF-β elevates the expression of SOX-2. Based on this finding, the co-expression of these genes in the liver (at the end of the 4th and 26th weeks) and kidney (at the end of the 26th week) of animals exposed to DEN validates the findings of previous research. The ability of menthol and thymol to alter the expression of these genes in the liver and kidneys of NT and NM animals indicates their anticancer properties. To support this assertion, further histopathological investigations are warranted.
Conclusions
Based on the current study findings, it can be concluded that monoterpenes menthol and thymol suppress the expression of SOX2 and down-regulate TGF-β/SMAD3 signaling in the liver and kidneys of mice injected with DEN. Menthol and thymol are highly likely to prevent the occurrence of molecular changes that initiate fibrosis and cancer in these organs in animal models and potentially in humans.
Conflict of Interests
The authors declare no conflict of interest with any internal or external entities in conducting this research project.
Funding
This study was funded by the authors and did not receive any funding from any other external sources.
Acknowledgement
The authors are thankful to Yasaman Peirovy, Zahra Mellaei, Samaneh Alijanian, and Mohana Karimi for their valuable collaboration with the animal experiments conducted during this study.
Compliance with Ethical Guidelines
All animal procedures were conducted based on ethical principles and were reviewed and approved by the Ethics Committee of the Islamic Azad University, Zanjan Branch, Zanjan, Iran (Approval Code: IR.IAU.Z.REC.1401.017).
Authors' Contributions
HA conducted the experiments and collected the data. GAT conceived and crafted the experiments. MAR conceptualized and designed the experiments, analyzed the data, and wrote and revised the various drafts of the manuscript.
References
- Park JE, Seo JE, Lee JY, Kwon H. Distribution of seven N-nitrosamines in food. Toxicological Research. 2015; 31:279-88. [doi:10.5487/TR.
2015.31.3.279], [pmid: 26483887]
- Tolba R, Kraus T, Liedtke C, Schwarz M, Weiskirchen R. Diethylnitrosamine (DEN)-induced carcinogenic liver injury in mice. Laboratory Animals. 2015;49(1):59-69. [doi:10.1177/0023677215570086]
- Sander LE, Trautwein C, Liedtke C, Kowdley K, McCaughan G. Is interleukin-6 a gender-specific risk factor for liver cancer?. Hepatology 2007; 46(4):1304-5. [doi:10.1002/hep.21982]
- Rezaie A, Fazlara A, Karamolah MH, Shahriari A, Zadeh HN, Pashmforosh M. Effects of Echinacea purpurea on hepatic and renal toxicity induced by diethylnitrosamine in rats. Jundishapur Journal of Natural Pharmaceutical Products. 2013;8(2):60-4. [pmid: 24624189]
- Owumi SE, Aliyu‐Banjo NO, Danso OF. Fluoride and diethyl-nitrosaminecoexposure enhances oxido‐inflammatory responses and caspase‐3 activation in liver and kidney of adult rats. Journal of Biochemical and Molecular Toxicology. 2019;33(7):e22327. [doi: 10.1002/jbt.22327], [pmid: 30920066]
- Ding YF, Wu ZH, Wei YJ, Shu L, Peng YR. Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular carcinoma induced by diethylnitrosamine. Journal of Cancer Research and Clinical Oncology. 2017; 143:821-34. [doi:10.1007/s00432-017-2364-z], [pmid: 28238064]
- Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21(4):504-16. [doi: 10.1016/
j.ccr.2012.02.007], [pmid: 22516259]
- Seki E, De Minicis S, Österreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nature Medicine. 2007;13(11):1324-32. [doi:10.1038/nm1663], [pmid: 17952090]
- Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chemico-biological interactions.2018;292:76-83. [doi:10.1016/j.cbi.2018.07.008], [pmid: 30017632]
- Wuebben EL, Rizzino A. The dark side of SOX2: cancer-a comprehensive overview. Oncotarget. 2017;8(27):44917-43. [doi:10.18632/oncotarget.16570], [pmid: 28388544]
- Weina K, Wu H, Knappe N, Orouji E, Novak D, Bernhardt M, et al. TGF‐β induces SOX 2 expression in a time‐dependent manner in human melanoma cells. Pigment Cell & Melanoma Research. 2016;29(4):453-458. [doi:10.1111/pcmr.12483], [pmid: 27105574]
- Shen J, Yokota S, Yokoi H, Suzuki T. Diethylnitrosamine-induced expression of germline-specific genes and pluripotency factors, including vasa and oct4, in medaka somatic cells. Biochemical and Biophysical Research Communications. 2016;478(2):858-863. [doi:10.1016/j.bbrc.2016.08.039] [pmid: 27514449]
- Kamatou GP, Vermaak I, Viljoen AM, Lawrence BM. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013; 96:15-25. [doi: 10.1016/j.phytochem.2013.08.005], [pmid: 24054028]
- Escobar A, Perez M, Romanelli G, Blustein G. Thymol bioactivity: A review focusing on practical applications. Arabian Journal of Chemistry. 2020;13(12):9243-9269. [doi: 10.1016/j.arabjc.2020.11.009]
- Bastaki SM, Adeghate E, Amir N, Ojha S, Oz M. Menthol inhibits oxidative stress and inflammation in acetic acid-induced colitis in rat colonic mucosa. American Journal of Translational Research. 2018;10(12):4210. [pmid: 30662664]
- Sampaio LA, Pina LT, Serafini MR, Tavares DD, Guimarães AG. Antitumor effects of carvacrol and thymol: a systematic review. Frontiers in Pharmacology. 2021; 12:702487. [doi: 10.3389/fphar.
2021.702487], [pmid: 34305611]
- Saravanan S, Pari L. Protective effect of thymol on high fat diet induced diabetic nephropathy in C57BL/6J mice. Chemico-biological interactions. 2016; 245:1-11. [doi: 10.1016/j.cbi.2015.11.033]
- Anter A, Ahmed AS, Hammad AS, Almalki WH, Abdel Hafez SM, Kasem AW, et al. The severity of acute kidney and lung injuries induced by cecal ligation and puncture is attenuated by menthol: Role of proliferating cell nuclear antigen and apoptotic markers. Frontiers in Medicine. 2022; 9:904286. [doi:10.3389/fmed.2022.
904286], [pmid: 35814769]
- Dou X, Yan D, Liu S, Gao L, Shan A. Thymol alleviates LPS-induced liver inflammation and apoptosis by inhibiting NLRP3 inflammasome activation and the AMPK-mTOR-autophagy pathway. Nutrients. 2022;14(14):2809. [doi: 10.3390/nu14142809], [pmid: 35889766]
- Matouk AI, El-Daly M, Habib HA, Senousy S, Naguib Abdel Hafez SM, Kasem AW, et al. Protective effects of menthol against sepsis-induced hepatic injury: Role of mediators of hepatic inflammation, apoptosis, and regeneration. Frontiers Pharmacology. 2022; 13:952337. [doi:10.3389/fphar.2022.952337], [pmid: 36120368]
- Santo SG, Romualdo GR, Santos LA, Grassi TF, Barbisan LF. Modifying effects of menthol against benzo (a) pyrene‐induced forestomach carcinogenesis in female Swiss mice. Environmental Toxicology. 2021;36(11):2245-55. [doi:10.
1002/tox.23338], [pmid: 34331502]
- Jafari A, Rasmi Y, Hajaghazadeh M, Karimipour M. Hepatoprotective effect of thymol against subchronic toxicity of titanium dioxide nanoparticles: biochemical and histological evidences. Environmental toxicology and pharmacology. 2018; 58:29-36. [doi:10.1016/j.etap.2017.12.010], [pmid: 29289817]
- Connor F, Rayner TF, Aitken SJ, Feig C, Lukk M, Santoyo-Lopez J, et al. Mutational landscape of a chemically-induced mouse model of liver cancer. Journal of Hepatology. 2018; 69(4):840-850. [doi:10.1016/j.jhep.2018.06.009], [pmid: 29958939]
- Gergye CH, Zhao Y, Moore RH, Lee VK. A comparison of ketamine or etomidate combined with xylazine for intraperitoneal anesthesia in four mouse strains. American Association for Laboratory Animal Science. 2020;59(5):519-30. [doi:10.30802/
AALAS-JAALAS-19-000129], [pmid: 32723425]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001,25(4), 402-408. [doi:10.1006/meth.2001.1262], [pmid: 1
1846609]
- Schulien I, Hasselblatt P. Diethylnitrosamine-induced liver tumorigenesis in mice. Methods in Cell Biology. 2021; 163:137-52. [doi:10.1016/bs.mcb.2020.08.006], [pmid: 33785162]
- Perumal N, Perumal M, Halagowder D, Sivasithamparam N. Morin attenuates diethylnitrosamine-induced rat liver fibrosis and hepatic stellate cell activation by co-ordinated regulation of Hippo/Yap and TGF-β1/Smad signaling. Biochimie. 2017; 140:10-19. [doi:10.
1016/j.biochi.2017.05.017], [pmid:28552397]
- Carrasco-Garcia E, Moreno-Cugnon L, Garcia I, Borras C, Revuelta M, Izeta A, et al. SOX2 expression diminishes with ageing in several tissues in mice and humans. Mechanisms of Ageing and Development. 2019; 177:30-6. [doi:10.1016/j.mad.2018.03.008], [pmid: 29574045]
- Tam WL, Ng HH. Sox2: masterminding the root of cancer. Cancer cell. 2014;26(1):3-5. [doi:10.1016/j.ccr.2014.06.024], [pmid: 25026204]
- Gholijani N, Gharagozloo M, Farjadian S, Amirghofran Z. Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. Journal of Immunotoxicology. 2016;13(2):157-164. [doi:10.3109/
1547691X.2015.1029145], [pmid: 25812626]
- Kim MH, Park SJ, Yang WM. Inhalation of essential oil from Mentha piperita ameliorates PM10-exposed asthma by targeting IL-6/JAK2/STAT3 pathway based on a network pharmacological analysis. Pharmaceuticals 2020;14(1):2. [doi:10.3390/ph14010002], [pmid: 33374928].
- Bass AJ, Wang TC. An inflammatory situation: SOX2 and STAT3 cooperate in squamous cell carcinoma initiation. Cell Stem Cell 2013;12(3):266-268. [doi: 10.1016/j.stem.2013.02.004].
- Miyakoshi M, Yamamoto M, Tanaka H, Ogawa K. Serine 727 phosphorylation of STAT3: An early change in mouse hepatocarcinogenesis induced by neonatal treatment with diethylnitrosamine. Molecular Carcinogenesis. 2014;53(1):67-76. [doi:10.1002/mc.21949], [pmid: 22911886]
- Lan HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. International Journal of Biological Sciences. 2011,7(7):1056-1067. [doi:10.7150/ijbs.7.1056], [pmid: 2192
7575].
- Fabregat I, Moreno‐Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, et al. IT‐LIVER Consortium. TGF‐β signalling and liver disease. FEBS journal. 2016; 283(12):2219-2232. [doi:10.1111/
febs.13665], [pmid: 26807763]
- Bonniaud P, Margetts PJ, Ask K, Flanders K, Gauldie J, Kolb M. TGF-β and Smad3 signaling link inflammation to chronic fibrogenesis. The Journal of Immunology. 2005;175(8):5390-5395. [doi:10.4049/
jimmunol.175.8.5390], [pmid: 16210645]
- Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Molecular cancer. 2022;21(1):104. [doi:10.1186/s12943-022-01569-x], [pmid: 35461253]
- Xin X, Cheng X, Zeng F, Xu Q, Hou L. The Role of TGF-β/SMAD Signaling in Hepatocellular Carcinoma: from Mechanism to Therapy and Prognosis. International Journal of Biological Sciences. 2024;20(4):1436-1451.[doi:10.7150/ijbs.89568], [pmid: 38385079].
- Ogaly HA, Eltablawy NA, Abd-Elsalam RM. Antifibrogenic influence of Mentha piperita L. essential oil against CCl4‐induced liver fibrosis in rats. Oxidative Medicine and Cellular Longevity. 2018; (1): 4039753. [doi:10.1155/2018/4039753], [pmid: 29849890].
- Hussein RM, Arafa ES, Raheem SA, Mohamed WR. Thymol protects against bleomycin-induced pulmonary fibrosis via abrogation of oxidative stress, inflammation, and modulation of miR-29a/TGF-β and PI3K/Akt signaling in mice. Life Sciences. 2023; 314:121256. [doi:10.1016/j.lfs.2022.121256], [pmid: 36
549352].
41. Wang L, Yang H, Lei Z, Zhao J, Chen Y, Chen P, et al. Repression of Tif1γ by SOX2 promotes TGF-β-induced epithelial–mesenchymal transition in non-small-cell lung cancer. Oncogene 2016;35(7):867-877. [doi:10.1038/onc.2015.
141], [pmid: 25961934].
Type of Study:
Research |
Subject:
Special