Ethics code: 922/KEP-UNISM/VIII/2023


Hadi S, Setiawan D, Viogenta P, Nastiti K, Andiarsa D. Protective Effects of the Extract of Combretum indicum Flowers and Leaves Against Methanol-induced Liver Damage and Formaldehyde Formation. IJT 2024; 18 (4) :189-195
URL:
http://ijt.arakmu.ac.ir/article-1-1377-en.html
1- Department of Pharmacy, Faculty of Mathematics and Natural Sciences, Lambung Mangkurat University. South Kalimantan, Indonesia
2- Department of Pharmacy, Faculty of Mathematics and Natural Sciences, Lambung Mangkurat University. South Kalimantan, Indonesia , deni.setiawan@ulm.ac.id
3- Department of Pharmacy, Faculty of Health, Sari Mulia University, Banjarmasin, South Kalimantan, Indonesia
4- Research Organization for Health, National Research and Innovation Agency, Republic of Indonesia. Bogor, Indonesia
Full-Text [PDF 1052 kb]
(736 Downloads)
|
Abstract (HTML) (1472 Views)
Full-Text: (544 Views)
Introduction
Methanol is easily breathed in or absorbed through the skin due to its volatile properties [1]. Alcohol Dehydrogenase (ADH) is an enzyme found in the gastric mucosa and is used by the liver to metabolize methanol into formaldehyde [2]. Subsequently, formaldehyde is converted into formic acid or formate by the enzyme aldehyde dehydrogenase (ALDH), leading to its accumulation in tissues [3]. This accumulation is detrimental to the body since formic acid interferes with the cytochrome oxidase of the electron transport chain and results in cell malfunction. This also leads to the development of various health issues, including metabolic acidosis, eye problems, and liver diseases [4].
Formic acid acts as a cytochrome C inhibitor, preventing tissue respiration and shifting the process toward anaerobic metabolism [5]. This respiration leads to the formation of lactic acid metabolites, which promote acidosis [6]. Additional effects of tissue respiration include a decrease in cell membrane activity, leading to swelling and a failure to pump calcium, which causes loss of membrane potential [7]. Damage to protein-forming organelles can also harm mitochondria and lysosomes. Consequently, the oxidative phosphorization process fails to function, lowering cellular energy production and leading to cell necrosis [8]. Early signs of methanol toxicity include central nervous system depression and Parkinsonian-like symptoms [9].
The liver is an essential organ that plays a significant role in the metabolism of xenobiotics and drugs. Consequently, when foreign substances, such as drugs, are introduced into the body, the liver becomes susceptible to various damaging issues [10]. Due to the diverse pathways in the pathophysiology of alcohol-related disease, different stages in the spectrum of this disease likely require specific therapy regimens [11]. To address this challenge, a strategy has been adopted with a focus on searching for substances that can suppress the activation of ADH in the liver and prevent detrimental metabolism [12]. This phenomenon contributed to the search for ADH inhibitors [13], such as Combretum, a plant with promising potential for liver protection. The Combretum indicum L. varr. B. is frequently used to treat liver pathological conditions.
Studies have shown that the aqueous extract of Combretum sericeum roots can protect the liver against paracetamol exposure [14]. This protective effect is associated with a decrease in the levels of alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), creatinine (CRT), urea, cholesterol, and triacylglycerol, along with boosted levels of superoxide dismutase (SOD), catalase, and thiobarbituric acid reactive substance (TBARS). A study found that the root bark of Combretum hypopilinum can protect the liver of mice from CCl4 invasion by inhibiting inflammation and free radicals [15]. These results support previous investigations, suggesting that the leaves and bark of Combretum roxburghii contain potent anti-inflammatory and antioxidant compounds [16]. Although Combretum zeyheri induces glutathione transferase (GST) activity when administered in aqueous solution in vitro, it also inhibits cytoprotective GST [17]. The genus Combretum is an ADH inhibitor with the capacity to protect the liver.
Aim of the Study: This study is unique because it is the first to test the ADH inhibition property of C. indicum varr. B., a member of the Combretum genus. Therefore, this study was planned to evaluate the therapeutic potential of C. indicum var. B. flowers and leaves for the treatment of methanol poisoning in an animal model.
Materials and Methods
Chemicals and Reagents: The materials utilized in the study included a micropipette (Eppendorf), an analytical balance (Ohaus), a spatula, a water bath, an oven, glassware, a 3 ml syringe, a 13 ml conical tube, and a microscope (Nikon). The Wistar rats used weighed between 180 and 200 g, and the reagents involved were methanol (Merck), ranitidine (Hexapharm), and CMCNa (Sigma), along with gas chromatography equipment from the BK-GC 7820 series.
Extraction: The Faculty of Mathematics and Natural Sciences at Lambung Mangkurat University conducted laboratory tests, identifying the plant as C. indicum varr. B. (Botanical I.D.: 032/LB.LABDASAR/II/2022). This study began with the extraction of 250 g of C. indicum varr. B. flowers using 12 L of 96% ethanol. Subsequently, the liquid extract was evaporated in a water bath at 60°C.
Animal Preparation: The Ethics Commission of Sari Mulia University reviewed and approved the animal tests. The study utilized Wistar rats weighing between 180 and 200 g, which were acclimatized for one week prior to the experiment. A total of 30 rats were categorized into 6 groups, each consisting of five animals: Group A served as the control, Group B as the negative control, Group C as the positive control, Group D received a dose of C. indicum flower extract at 100 mg/kg, Group E at 200 mg/kg, and Group F at 300 mg/kg.
Metanol-induced Liver Test: The animals were administered methanol orally at a dose of 3 g/kg, 4 h after the start of the experiment. Ranitidine (positive control) was administered intraperitoneally at 30 mg/kg two hours after methanol administration. Subsequently, the C. indicum flower extract was given at 100 mg/kg, 200 mg/kg, and 300 mg/kg two hours before methanol administration, and the animals were sacrificed after 8 hours.
Formaldehyde Formation Test: The positive controls consisted of methanol 7 g/kg body weight administered orally and ranitidine at 30 mg/kg body weight intraperitoneally. The experimental animals were administered concentrated extract orally or ranitidine intraperitoneally at the beginning of the experiment. Two hours later, they were given methanol orally. After six hours, the rats’ blood samples were collected to analyze the levels of formic acid.
Biomarker Evaluation: The health laboratory conducted examinations on the animals to measure the levels of total protein, albumin, globulin, alkaline phosphatase (ALP), serum glutamic oxaloacetic transaminase (SGOT), and serum glutamic pyruvic transaminase (SGPT) using a reliable analysis kit.
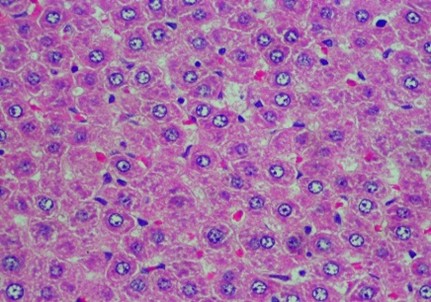
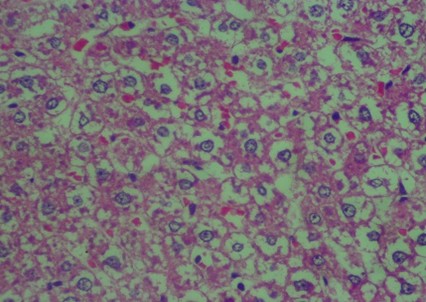
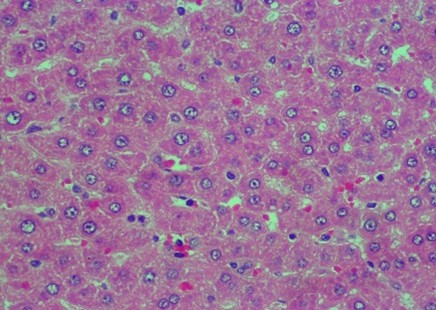
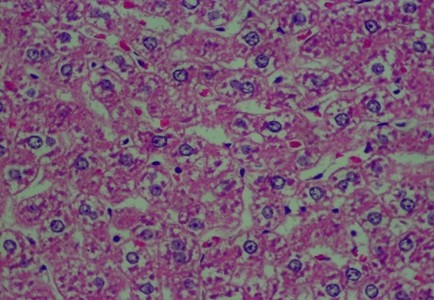
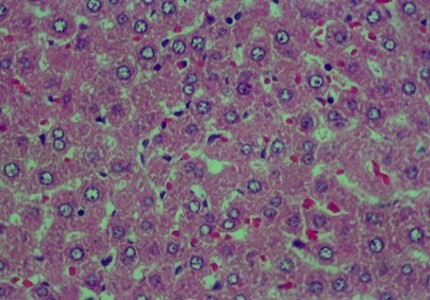
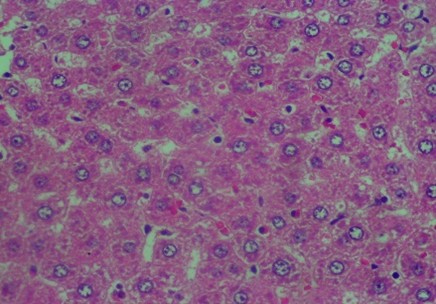
Histology Examinations: Histology examinations were carried out on the liver tissue samples to assess the effect of methanol poisoning. The cause of animal death in all cases was determined to be methanol poisoning due to hepatocellular injury. The liver tissue samples were embedded in formalin-fixed paraffin blocks and were examined microscopically at the Banjarbaru Veterinary Center. The slides were reviewed randomly by two technicians, and the prepared tissue sections were stained with hematoxylin for examination under a microscope at 1000X magnification.
To describe hepatocyte injury, microscopic features of reversible and irreversible liver cell injury in 30 subjects were considered appropriate, and any subtle pathological changes were noted in each case. This analysis was carried out to determine liver morphological changes in cases of fatal methanol poisoning in the animals, using a semiquantitative scoring system (Table 1).The histology liver preparations were analyzed using a microscope in six different parts of the field of view at 400X magnification. In each part of the observations, the number of cells was counted randomly for easy identification of each preparation between 50 and 100 cells. Subsequently, differences that occurred in cell changes were observed using the Manja Roenigk Histopathology Scoring model, and the average damage was calculated.
Table 1. Criteria for assessing the degree of histopathology of liver
cells using the Manja Roenigk Histopathology Scoring model
| Rate of Change |
Score |
| Normal |
0 |
| Parenchymatous degeneration |
1 |
| Hydropic degeneration |
2 |
| Necrosis |
3 |
Results
The extraction process carried out on flowers produced a thick extract weighing 22 g, resulting in a yield of 8.8%. As presented in Table 2, the methanol administration significantly reduced albumin, total protein, and globulin levels in the experimental rats. Methanol administration can damage the liver, disrupt the synthesis of proteins, and interfere with amino acid metabolism, which is the basic ingredient for protein synthesis. Administration of ranitidine significantly increased the total protein, albumin, and globulin; however, the indices were not comparable to those achieved under normal conditions. The administration of C. indicum leaf extract at 100, 200, or 400 mg/kg doses significantly boosted albumin, total protein, and globulin levels; nevertheless, it was not the same as those noted for ranitidine or normal controls.
In stages, the administration of C. indicum significantly increased albumin between the study groups. The C. indicum extract significantly increased total protein and globulin levels compared to those after methanol administration. However, doses of 200 and 400 mg/kg statistically showed no significant differences because methanol administration had already caused acute liver damage.
Table 2. Effect of C. indicum varr. B. leaf extract activity on albumin, total protein, methanol-induced globulin
| Treatment |
Albumin (g/dl) |
Total Protein (g/dl) |
Globulin (g/dl) |
| Normal control |
4.515±0.168 |
6.858±0.370 |
4.658±0.631 |
| Negative control (methanol) |
1.985±0.239a* |
2.376±0.335a* |
1.646±0.048a* |
| Positive control (ranitidine) |
3.798±0.24a*,b* |
6.588±0.184a,b* |
3.562±0.173a*,b* |
| 100 mg/kg Bw |
2.452±0.081a*,b*,c*,e*,f* |
4.586±0.116a*,b*,c*,e*,f* |
1.731±0.098a*,b,c*,e*,f* |
| 200 mg/kg Bw |
2.721±0.116a*,b*,c*,d*,f* |
5.081±0.239a*,b*,c*,d*,f |
2.741±0.022a*,b*,c*,d*,f |
| 400 mg/kg Bw |
3.306±0.167a*,b*,c*,d*,e* |
5.421±0.085a*,b*,c*,d*,e |
3.018±0.191a*,b*,c*,d*,e |
Mean ± SD aagainst normal control, bagainst control, cagainst 100 mg/kg Bw;, dagainst 200 mg/kg body weight, eagainst 400 mg/kg body weight positive;*P<0.05.
Table 3. Effect of C. indicum leaf extract on liver function and liver chemistry of Wistar rats.
| Treatment |
ALP |
SGOT/AST |
SGPT/ALT |
| Control |
62.52±5.12 |
31.37±1.16 |
33.92±1.11 |
| Methanol |
229.33±27.75 a* |
58.92±6.19a* |
63.81±4.28 a* |
| Ranitidine |
73.37±1.41a,b* |
33.82±0.56a,b* |
35.96±0.59a,b* |
| 100 mg/kg Bw |
180.37±9.45a*,b*,c*,e*,f* |
38.25±1.58a*,b*,c*,e,f |
45.28±3.34a*,b*,c*,e*,f* |
| 200 mg/kg Bw |
155.23±9.22a*,b*,c*,d*,f* |
36.53±0.62a*,b*,c,d,f |
37.96±0.63a*,b*,c,d*,f |
| 400 mg/kg Bw |
133.87±4.32a*,b*,c*,d*,e* |
35.32±0.61a,b*,c,d,e |
36.81±0.32a,b*,c,d*,e |
Mean value ± SD aagainst normal control, bagainst control, cagainst 100 mg/kg
Bw, dagainst 200 mg/kg Bw, eagainst 400 mg/kg Bw positive;*P<0.05.
The administration of methanol increased ALP, AST, and ALT values significantly, as reflected by the data in Table 3. This was a sign that the liver was responding to toxic compounds. The main purpose of releasing these three enzymes was likely to metabolize methanol into non-toxic compounds. The use of C. indicum extract significantly reduced the levels of ALP, AST, and ALT as compared to methanol. However, the decrease in the ALP level was not comparable to that of ranitidine or normal controls. The administration of C. indicum extract significantly lowered the ALP level as the extract dose was increased. The use of C. indicum leaf extract at 200 and 400 mg/kg reduced AST and ALT statistically; however, it was not significantly different from that achieved by administering ranitidine.
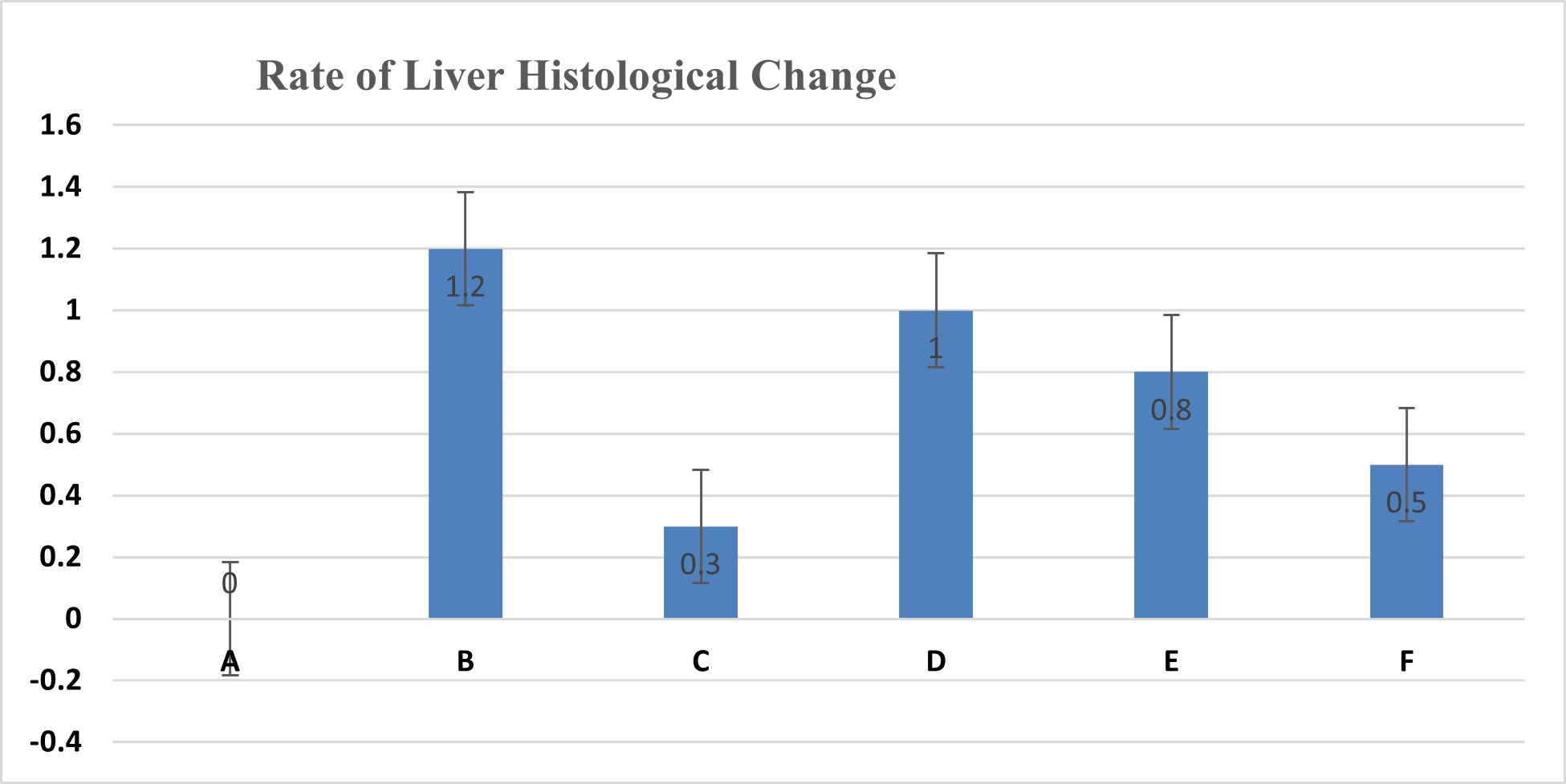
Figure 1. Average liver damage scores: 0 (no damage), 1 (mild damage), 2 (moderate damage), 3 (severe damage)
The analysis of the average liver damage scores showed that the control A scored 0, indicating no damage. After administering methanol, the positive control (C), which received ranitidine as an antidote, showed minimal liver damage. These results indicated that ranitidine effectively and significantly inhibited liver damage. The average results for group B (negative control), which did not receive antidote administration, indicated the most severe damage; however, tissue necrosis was not observed within the eight hours following treatment. The administration of C. indicum extracts at varying doses (D, E, and F groups) showed average improvements that were directly proportional to the extract dosage. Compared to ranitidine, these levels of liver improvement demonstrated less effectiveness in Figure 1. Several factors, including the type of toxic substance, dosage, duration of exposure, and the tissue response (i.e., acute, subchronic, or chronic), influenced the levels of liver damage due to toxic substances. The concentration of the toxic substance or its metabolite played a crucial role in the extent of liver damage. Generally, liver damage may occur immediately or gradually over several months in various forms, such as hepatocyte necrosis, cholestasis, and/or other disorders.
The data in Figure 2 show clear changes between groups, indicating liver damage associated with bleeding or liver acinus problems. Further, there were also abnormal changes in the hepatocyte arrangement, where liver cells were deposited, leading to structural changes.
As tabulated in Table 4, the 7 tested species of Combretum showed hepatoprotective effects, specifically the roots and leaf extract. These results strengthened the theory that the Combretum species had a promising potential to inhibit liver damage induced by exposure to toxic substances.
Figure 2. Rat liver histology: (A) Control; (B) negative control; (C) positive control; (D) 100 mg/kg C. indicum extract; (E) 200 mg/kg C. indicum extract; (F) 300 mg/kg C. indicum extract
Table 4. Combretum species with hepatoprotective effect
| No. |
Species |
Plant Part |
Test |
Reference |
| 1 |
C. quadrangulare |
Leaves |
Induced D-GalN/TNF-alpha |
Banskota dkk., 2000 [37] |
| 2 |
C. dolichopentalum |
Leaves |
Exposure CCl4 |
Ujowundu dkk., 2011 |
| 3 |
C. sericeum |
Roots |
Exposure paracetamol |
M. Sini dkk., 2017 |
| 4 |
C. albidum |
All Parts |
Exposure CCl4 |
Rajalingam dkk., 2016 |
| 5 |
C. micranthum |
Leaves |
Induced parasetamol |
Adebisi & Ugwah‐Oguejiofor, 2021 |
| 6 |
C. hypopilinum |
Roots |
Induced CCl4 |
Idoh dkk., 2023 |
| 7 |
C. zeyheri |
Leaves |
Inhibits GST |
Gweshelo dkk., 2016 |
Discussion
Methanol poisoning is particularly uncommon but presents a potential health risk due to its volatile nature. This study aimed to investigate the toxic effect of C. indicum extract on the liver of Wistar rats administered methanol. The microscopic examinations were conducted to assess the histopathological effects in Wistar rats’ liver, comparing several doses of the extract versus methanol. The principle process began with the presence of the liver enzyme ADH in the stomach mucosa, followed by methanol metabolism primarily occurring in the liver through the action of alcohol and ALDH [18].
Ethanol, ranitidine, and 4-MP are substances that are known to have efficacy against methanol toxicity. In this study, ranitidine was more effective in lowering the levels of formate metabolites and showed no significant difference from 4-MP [19]. Ranitidine enhances the bioavailability of ethanol, thereby preventing the activity of the liver's ALD and stomach's ADH enzymes [20]. Due to its fewer side effects and ease of blood level determination, ranitidine is preferable to ethanol [12]. Further, the use of alcohol as an antidote required supervision and was infrequently successful [21]. The prolonged use of ethanol can also cause hypoglycemia [22], which contributed to the use of ranitidine as a positive control in this study.
The study showed that the extract of C. indicum leaves stopped the metabolism of methanol by lowering the levels of formate in the blood significantly compared to the negative control. However, it did not reduce formic acid as strongly as ranitidine. The malondialdehyde levels in methanol-poisoned rats increased, indicating impaired antioxidant defense mechanisms [23]. Using ranitidine as a positive control improved this situation by inhibiting neutrophil activation either directly or by blocking the action of TNFα. This response plays an inflammatory role during oxidative stress [24].
Alcohol dehydrogenase inhibitors can directly affect the activity of the lactate dehydrogenase enzyme, thereby lowering the lactic acid levels in the blood, as demonstrated by in vitro studies [25]. High amounts of antioxidant enzymes, such as SOD, catalase, and glutathione peroxidase, and low amounts of lipid peroxidase can help protect the liver [26]. Rises in globulin, albumin, and total protein levels after the administration of C. indicum leaf extract demonstrated these changes, consistent with the hepatoprotective effect of Combretum abyssinica extract and fractions [27]. Similar to the research performed on the Combretum sericeum root extract [14], giving 400 mg/kg of C. indicum extract decreased liver biomarkers of toxicity, such as ALP, AST, and ALT. This effect is likely due to the fact that C. indicum leaf extract blocks alcohol dehydrogenase, acting like a potent antioxidant. However, further clinical studies are necessary to validate this effect.
We tested how well the C. indicum extract and ranitidine worked to protect rats from the harmful effects of methanol by scoring the damage to the liver and looking at the liver's histology. The animals previously ingested methanol at a dose of 3 gr/kg, a level expected to cause liver damage due to its harmful metabolites and by decreasing the osmolar gap and increasing the anion gap. Ashurst and Nappe also noted that these events stopped mitochondrial respiration in cells by formate and cytochrome oxidase, disrupting oxidative phosphorylation in liver cells [28].
To determine the potency and efficacy, three different concentrations of C. indicum extract were prepared and used in rats. Comparing the liver damage scores from the rats, it was indicated that the extract at 300 mg/kg yielded the best value compared to other extract doses. Ranitidine showed efficacy at a slightly lower concentration than that of the extract. Based on the results, C. indicum was considered nearly as potent as ranitidine. The histopathology of the rats’ liver showed similar outcomes when the extract was compared with the positive and the negative controls. The treatment of rats with the extract at 300 mg/kg provided clinical results that were almost identical to those from ranitidine treatment.
The current study's findings align with previous studies that used ranitidine to treat alcohol intoxication. It is known that the treatment stops the activity of alcohol-dehydrogenase enzymes in the stomach and liver [29]. Patients with increased stomach acid secretion often use ranitidine, particularly H2 blockers, to treat gastritis and damage from methanol ingestion. Scholten recommends that ranitidine not be used in individuals younger than 12 years old [30]. Therefore, alternative therapies are needed for methanol toxicity, which makes C. indicum extract a potential candidate as an antidote for methanol poisoning [31].
Previous studies have suggested that erythrophyllic acid in C. indicum interacts with ADH. This substance is believed to be an anti-inflammatory and hepatoprotective agent and has shown protective effects against preventing liver damage [32]. Liver damage may result in various pathological outcomes, including an innate immune response, the release of pro-inflammatory cytokines and chemokines, and damage to the mitochondria and microtubules in the liver cells [33]. Heating the triterpenoid cycloartan produces erythrophyllic acid, a naturally occurring substance. Mass spectrometry (MS) testing specifically indicates the presence of erythrophyllic acid in the leaves and stems of the Terminalia macroptera plant extract used in hepatoprotective studies [34].
Despite the promising research findings in the literature, the mechanism of action of erythrophyllic acid is not clearly known. The cycloartan triterpenoid group, with its immunomodulatory effect, contributes to its value as an anti-inflammatory and antioxidant agent [35]. The anti-inflammatory effect inhibits pro-inflammatory enzymes and cytokines, thereby reducing inflammation and relieving conditions associated with chronic inflammation. This antioxidant activity aids in shielding cells and tissues from oxidative damages that contribute to the pathology of various diseases [36]. Therefore, C. indicum extract can provide hepatoprotective effects and be used to treat methanol toxicity, which otherwise leads to major liver disease.
Conclusions
In conclusion, this study showed that the leaves of the C. indicum plant can lower the amount of formic acid and formates in the blood and protect the liver from the harmful effects of drinking methanol. Thus, at a dose of 300 mg/kg, the C. indicum extract inhibits liver damage with an average damage score of 0.5, whereas ranitidine only shows an average damage score of 0.3.
Conflict of Interests
No potential conflict of interest was reported by the authors.
Funding
The authors are grateful to the Directorate General of Higher Education, Research, and Technology for the main contract number: 056/E5/PG.02.00.PL/2024 and derivative contract number: 1029/UN8.2/PG/2024.
Acknowledgement
The authors are grateful to the DRTPM for their support of this study.
Compliance with Ethical Guidelines
This research has received ethical legislation number: No. 922/KEP-UNISM/VIII/2023
Authors' Contributions
SH methodology, data analysis and interpretation of results. SH and DS article structuring and writing. PV revision and supervision. KN and DA analysis and scoring of the liver damages. All authors have read and approved the manuscript prior to submission for publication.
References
- McMartin K, Jacobsen D, ovda KE. Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol [Internet]. 2016;81:505–15. [doi:10.1111/bcp.12824][pmid: 26551875]
- Viinamäki J, Rasanen I, Vuori E, Ojanperä I. Elevated formic acid concentrations in putrefied post-mortem blood and urine samples. Forensic Sci Int. 2011;208:42–6. [doi:10.1016/j.forsciint.2010.10.
026][ pmid: 21112705]
- Marumo M, Wakabayashi I. Effects of methanol and formic acid on human platelet aggregation. Environ Health Prev Med. 2017;22:81. [doi:10.1186/s12199-017-0687-7][pmid: 29246106]
- Osna NA, Rasineni K, Ganesan M, Donohue TMJ, Kharbanda KK. Pathogenesis of Alcohol-Associated Liver Disease. J Clin Exp Hepatol. 2022;12:1492–513. [doi:10.1016/j.jceh.2022.05.004][pmid: 27373608]
- Tran S, Nowicki M, Facciol A, Chatterjee D, Gerlai R. Ethanol-Induced ADH Activity in Zebrafish: Differential Concentration-Dependent Effects on High- Versus Low-Affinity ADH Enzymes. Zebrafish. 2016;13:75-78. [doi:10.1089/zeb.2015.1173][pmid: 26741829]
- Ghorbani H, Nezami A, Sheikholeslami B, Hedjazi A, Ahmadimanesh M. Simultaneous measurement of formic acid, methanol and ethanol in vitreous and blood samples of postmortem by headspace GC-FID. J Occup Med Toxicol. 2018;13:1. [doi:10.1186/s12995-017-0184-3][pmid: 29321805]
- Lao YE, Heyerdahl F, Jacobsen D, Hovda KE. An enzymatic assay with formate oxidase for point-of-care diagnosis of methanol poisoning. Basic Clin Pharmacol Toxicol. 2022;131:547-554. [doi:10.1111/bcpt.13789][pmid: 36083569]
- Teschke R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines. 2018;6(4):106. [doi:10.3390/biomedicines60401
06][pmid: 30424581]
- Teschke R. Alcoholic Liver Disease: Current Mechanistic Aspects with Focus on Their Clinical Relevance. Biomedicines. 2019;7(3):68. [doi:10.3390/biomedicines7030068][pmid: 31491888]
- Yan C, Hu W, Tu J, Li J, Liang Q, Han S. Pathogenic mechanisms and regulatory factors involved in alcoholic liver disease. J Transl Med. 2023;21:300. [doi:10.1186/s12967-023-04166-8][pmid: 37143126]
- Pohanka M. Toxicology and the biological role of methanol and ethanol: Current view. Biomed Pap Med Fac Univ Palacky, Olomouc, Czechoslov. 2016;160:54–63. [doi:10.5507/bp.2015.023][pmid: 26006090]
- Jelski W, Wolszczak-Biedrzycka B, Zasimowicz-Majewska E, Orywal K, Lapinski TW, Szmitkowski M. Alcohol Dehydrogenase Isoenzymes and Aldehyde Dehydrogenase Activity in the Serum of Patients with Non-alcoholic Fatty Liver Disease. Anticancer Res. 2018;38:4005–9. [doi:10.21873/anticanres.12688][pmid: 29970524]
- Miaffo D, Wansi SL, Ntchapda F, Kamanyi A. Chronic oral safety study of the aqueous extract of Combretum molle twigs on biochemical, haematological and antioxidant parameters of Wistar rats. BMC Complement Med Ther. 2020;20:106. [doi:10.1186/
s12906-020-02896-6][pmid: 32248808]
- Sini M, Nwodo OF., Alumanah EO. Hepatoprotective activity of aqueous extract of Combretum sericeum roots against paracetamol induced hepatic damage in rats. J Sci Res Stud [Internet]. 2017;4:40-46. [Link]
- Idoh K, Dosseh K, Gautam M, Kpatcha T, Manikandan N, Agbonon A, et al. Combretum hypopilinum exhibited anti-inflammatory activity in LPS-stimulated RAW 264.7 murine macrophages through inhibition of inflammatory mediators and apoptosis. J Appl Pharm Sci. 2023;13:193–8. [doi:10.7324/JAPS.
2023.119177]
- Bhatnagar S, Sahoo SK, Mohapatra AK, Behera DR. Phytochemical analysis, Antioxidant and Cytotoxic activity ofmedicinal plant Combretum roxburghii (Family: Combretaceae). Int J Drug Dev Res. 2012;4. [Link]
- Gweshelo D, Muswe R, Mukanganyama S. In vivo and in vitro inhibition of rat liver glutathione transferases activity by extracts from Combretum zeyheri (Combretaceae) and Parinari curatellifolia (Chrysobalanaceae). BMC Complement Altern Med. 2016;16:238. [doi:10.1186/s12906-016-1235-5]
- Zakharov S, Pelclova D, Diblik P, Urban P, Kuthan P, Nurieva O, et al. Long-term visual damage after acute methanol poisonings: Longitudinal cross-sectional study in 50 patients. Clin Toxicol (Phila). 2015;53:884–92. [doi:10.3109/15563650.2015.1086488][pmid: 26
364866]
- El-Bakary AA, El-Dakrory SA, Attalla SM, Hasanein NA, Malek HA. Ranitidine as an alcohol dehydrogenase inhibitor in acute methanol toxicity in rats. Hum Exp Toxicol. 2010;29:93-101. [doi:10.1177/0960327109353777][pmid: 20026516]
- Nugrahanti MP, Armalina D, Partiningrum DL, Fulyani F. The effect of ranitidine administration in graded dosage to the degree of liver damage: A study on Wistar rats with acute methanol intoxication. Hum Exp Toxicol. 2021;40:497–503. [doi: 10.1177/
0960327120954529][pmid: 32909853]
- Contreras-Zentella ML, Villalobos-García D, Hernández-Muñoz R. Ethanol Metabolism in the Liver, the Induction of Oxidant Stress, and the Antioxidant Defense System. Antioxidants (Basel, Switzerland). 2022; 11(7):1258. [doi:10.3390/antiox11071258][pmid: 35883749]
- DiPadova C, Roine R, Frezza M, Gentry RT, Baraona E, Lieber CS. Effects of ranitidine on blood alcohol levels after ethanol ingestion. Comparison with other H2-receptor antagonists. JAMA [Internet]. 1992;267:83-86. [pmid:1727201]
- Skrzydlewska E, Farbiszewski R. Protective effect of N-acetylcysteine on reduced glutathione, reduced glutathione-related enzymes and lipid peroxidation in methanol intoxication. Drug Alcohol Depend. 1999;57:61–7. [doi:10.1016/s0376-8716(99)00040-x]
- Okajima K, Harada N, Uchiba M. Ranitidine reduces ischemia/reperfusion-induced liver injury in rats by inhibiting neutrophil activation. J Pharmacol Exp Ther. 2002;301:1157-1165. [doi:10.1124/jpet.301.3.1157][pmid:12023551]
- Dudka J, Burdan F, Korobowicz A, Klepacz R, Korobowicz E. Human skeletal muscle lactate dehydrogenase activity in the presence of some alcohol dehydrogenase inhibitors. Basic Clin Pharmacol Toxicol. 2004;95(1):38–42. [doi:10.1111/j.1742-7843.2004.pto950108.x][pmid:15245575]
- Saoudi M, Jebahi S, Jamoussi K, Ben Salah G, Kallel C, El Feki A. Haematological and biochemical toxicity induced by methanol in rats: ameliorative effects of Opuntia vulgaris fruit extract. Hum Exp Toxicol. 2011;30:1963–71. [doi:10.1177/0960327111
403175][pmid: 21422078]
- Meharie BG, Amare GG, Belayneh YM. Evaluation of Hepatoprotective Activity of the Crude Extract and Solvent Fractions of Clutia abyssinica (Euphorbiaceae) Leaf Against CCl(4)-Induced Hepatotoxicity in Mice. J Exp Pharmacol. 2020;12:137–50. [doi:10.2147/JEP.S248677]
- Ashurst JV, Nappe TM. Methanol Toxicity. [Updated 2023 Jun 12]. In: Stat Pearls. Treasure Island (FL): Stat Pearls Publishing; 2024. Available from: [Link]
- Moody DE. The inhibition of first-pass metabolism of ethanol by H2-receptor antagonists: a tabulated review. Expert Opin Drug Saf. 2018;17:917-934. [doi:10.1080/14740338.2018.1512969][pmid: 30
117350]
- Scholten T. Long-term management of gastroesophageal reflux disease with pantoprazole. Ther Clin Risk Manag. 2007;3:231–43. [doi: 10.2147/tcrm.2007.3.2.231]
- Khairunnisa A, Hadi S, Sari SO. Determination of Antioxidant Activity of Ethanol Extract of Round Type Ceguk Flowers (Combretum Indicum L.) in Several Regions in South Kalimantan. J Pharmascience. 2022;9:319. [doi:10.20527/jps.
v9i2.13859]
- Haidara M, Denou A, Haddad M, Camara A, Traoré K, Aubouy A, et al. Evaluation of Anti-inflammatory, Anti-pyretic, Analgesic, and Hepatoprotective Properties of Terminalia macroptera. Planta Medica Int Open. 2020;07:e58–67. [doi:10.1055/a-1142-7072]
- Kong L-Z, Chandimali N, Han Y-H, Lee D-H, Kim J-S, Kim S-U, et al. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int J Mol Sci. 2019;20. [doi:10.3390/
ijms20112712][pmid: 31159489]
- Soliman GA, Ansari MN, Alqarni MH, Foudah AI, Alam A, Salkini MA, et al. Analgesic, antipyretic, anti-inflammatory, and hepatoprotective activities of Pulicaria crispa (Forssk.) Oliv. (Asteraceae). Brazilian J Pharm Sci. 2022;58:1–14. [doi:10.
1590/s2175-97902022e18851]
- De Morais Lima GR, de Sales IRP, Caldas Filho MRD, de Jesus NZT, de Sousa Falcão H, Barbosa-Filho JM, et al. Bioactivities of the genus Combretum (Combretaceae): a review. Molecules. 2012;17:9142–206. [doi:10.3390/molecules17089142]
- Renda G, Gökkaya İ, Şöhretoğlu D. Immunomodulatory properties of triterpenes. Phytochem Rev. 2022;21:537–63. [doi:10.1007/s11101-021-09785-x][pmid: 34812259]
- Banskota A, Tezuka Y, Adnyana IK, XIONG Q, Hase K, TRAN K, et al. Hepatoprotective Effect of Combretum quadrangulare and Its Constituents. Biol Pharm Bull. 2000;23(4):456-460. [doi:10.
1248/bpb.23.456][pmid:10784427]
Type of Study:
Research |
Subject:
General