Ethics code: IR.LUMS.REC.1402.183


1- Assistant Professor; Department of Pharmacology and Toxicology, Occupational Environment Research Center, Rafsanjan University of Medical Sciences. Rafsanjan, Iran
2- Pharmacy Student, Student Research Committee, Lorestan University of Medical Sciences. Khorramabad, Iran.
3- Department of Forensic Medicine, School of Medicine, Valiasr Hospital, Arak University of Medical Sciences. Arak, Iran.
4- Pharmacy Student; Student Research Committee, Lorestan University of Medical Sciences. Khorramabad, Iran.
5- Department of Toxicology, Faculty of Pharmacy, Razi Herbal Medicine Research Center, Lorestan University of Medical Sciences. Khorramabad, Iran. , hamidrezamohammadi65@yahoo.com
Full-Text [PDF 684 kb]
(394 Downloads)
|
Abstract (HTML) (1105 Views)
Full-Text: (440 Views)
Introduction
Rifampicin, also referred to as Rifampin (Rif), is an ansamycin antibiotic used in the treatment of various bacterial infections, such as tuberculosis, leprosy, and malaria [1, 2]. One notable drawback to this medication is its capacity to induce liver lesions, necessitating routine monitoring of hepatic function in patients who take this medicine [3]. Several studies have shown that the administration of Rif leads to notable rises in the serum levels of such hepatic parameters as alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate amino-transferase (AST), and bilirubin. It can also cause significant degenerative changes and necrosis in the central lobular regions of rat liver [4-6].
Previous studies that have investigated the side effects of Rif have demonstrated a significant rise in the malondialdehyde (MDA) levels and decreases in the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), and reductase enzymes [7, 8]. These changes may be linked to the generation of free radicals due to the interaction of Rif metabolites with oxygen, leading to the peroxidation of membrane lipids and the impairment of the body's antioxidant defense mechanisms, secondary to oxidative stress [5, 9]. This stress significantly contributes to the pathophysiological changes observed in the liver due to Rif-induced hepatotoxicity [4, 10]. Consequently, administering powerful antioxidants to support protective effects can offer significant benefits to alleviating this condition.
A recent study has shown that natural medicinal ingredients have preventive effects on Rif-induced hepatotoxicity [4]. Biologically-derived plant substances represent a modern branch of pharmacotherapy for various ailments [11]. Carvacrol is a monoterpenic phenolic compound with the chemical structure of 5-isopropyl-2-methylphenol and the molecular formula of C10H14O. This compound occurs naturally in the plant oils from thyme, oregano, and marjoram and is commonly used as culinary seasonings [12]. Numerous studies have extensively documented the antioxidant properties of Carvacrol and its potential therapeutic benefits in the management of various diseases [13]. Given that antioxidants are responsible for gathering, neutralizing, or eliminating free radicals from cells and their surrounding environment, and considering Carvacrol as a potent antioxidant [14], it is expected that it may inhibit the activity of free radicals and reduce oxidative damage as evident in various animal models.
Aim of the Study: Considering the above review, we planned this first-time study to examine the effect of varying doses of Carvacrol on Rif-induced hepatotoxicity in rats.
Materials and Methods
Chemicals: In the current study, we used Rifampin (C43H58N4O12) and Carvacrol (CH3)2CHC6H3(CH3)OH, at ≥ 98% purity, supplied from Sigma Aldrich, St. Louise, USA. All ingredients and reagents used in this work were of high quality and grades and were obtained from reputable suppliers.
Study Design: Forty-two male Wistar rats weighing 180-220 g were purchased from the animal housing of Lorestan University of Medical Sciences. The rats were fed normal food and water ad libitum and were kept under standard environmental conditions: at 24°C temperature, 12/12 dark/light cycles, and 40-70% humidity. Rats were then randomly divided into six groups of seven rats each as follows:
- Rats received normal saline (control group).
- Rats receiving only Rif (100 mg/kg/day for 28 consecutive days).
- Rats receiving Rif (100 mg/kg/day for 28 consecutive days) + Carvacrol (25 mg/kg for 28 consecutive days).
- Rats receiving Rif (100 mg/kg/day for 28 consecutive days) + Carvacrol (50 mg/kg for 28 consecutive days).
- Rats receiving Rif (100 mg/kg/day for 28 consecutive days) + Carvacrol (100 mg/kg for 28 consecutive days).
- Rats receiving the highest dose of Carvacrol (100 mg/kg/day for 28 consecutive days). This was considered to ensure the harmlessness of the drug dosage.
In order to reduce the risk of hypoglycemia and mortality in the Rif-induced liver injury groups, the animals were given drinking water containing 1% (w/v) dextrose throughout the study. Moreover, Carvacrol was administered via gavage one hour after the injection of Rif.
Blood and Tissue Collection: Rats were completely anesthetized using intraperitoneal injection of thiopental at 60 mg/kg. Subsequently, 5 ml of blood sample was taken from the abdominal aorta of each animal. Three ml of that blood sample was carefully transferred into a test tube to prevent blood cell lysis. The blood samples were clotted at room temperature and then centrifuged at 1000 rpm for 25 min to obtain the sera. The sera were stored at -20°C until further tests and analyses. In addition, liver tissue samples from the rats were collected for the subsequent oxidant and antioxidant activity tests.
Determination of Biochemical Parameters: After centrifuging the blood samples at 15,000 rpm for 10 min and obtaining the sera, the levels of ALP, ALT, AST, bilirubin, total protein, and albumin were determined using diagnostic kits (Pars Azmon, Tehran, Iran) based on the supplier’s instructions.
Determination of Antioxidant Activities: Following the preparation of liver tissue homogenates using a cold buffer at 4°C, the tissue levels of antioxidant enzymes, such as SOD and CAT, were quantified. These tests were performed using commercially available kits (Pars Azmoon, Tehran, Iran) based on the established protocols.
Liver Glutathione (GSH) Levels: The liver tissue samples (200 mg) were homogenized in EDTA buffer (0.02 M) at a ratio of 1:10 w/v. Then, the obtained homogenates (5 mL each) were mixed with deionized water (4 mL) and trichloroacetic acid (TCA) 50% (1 mL) and then centrifuged at 3000 × g for 15 min. Finally, the supernatants (2 mL each) were mixed well with DTNB 0.01 M (0.1 mL) and Tris buffer 0.4 M (4 mL). After shaking well for 10 min at 25°C, the absorbance was read at 734 nm wavelength, and the total GSH content was calculated based on the slope of the standard curve.
Determination of Oxidative Stress Marker: Tissue lipid peroxidation levels (MDA) were determined using the Nalondi™ (Lipid Peroxidation Assay Kit). This is based on the reaction of thiobarbituric acid (TBA) with peroxidized lipids. This reaction results in the formation of MDA from lipid peroxides, which then reacts with TBA to produce substances that are measured spectrophotometrically and compared with a standard curve.
Statistical Analyses: The study data were analyzed using SPSS statistical software, version 26.0. Following this step, the central tendency and variability measures were determined using a one-way analysis of variance (ANOVA). In cases of statistically significant results, Tukey's post hoc test was utilized in pairwise comparisons. Further, the non-normal distribution of the data was evaluated using the Kruskal-Wallis non-parametric test, and for subsequent comparisons, the corrected Bonferroni test was applied. The results were reported at a statistical significance level of P≤5%.
Results
Effect of Carvacrol on Liver Enzymes: As illustrated in Figure 1, Rif led to a notable elevation of the serum levels of AST, ALT, and ALP, indicative of liver damage, in comparison to those observed in the control group. Conversely, the administration of varying doses of Carvacrol resulted in a significant reduction in the serum levels of AST, ALT, and ALP in rats with Rif-induced liver injury.

Figure 1. Effects of different doses of carvacrol (CAR) on the serum levels of aspartate aminotransferase (AST) (A), alanine aminotransferase (ALT) (B), and alkaline phosphatase (ALP) (C) enzymes in Rif-induced liver damage in rats. The data are shown as Mean ± SD for ten animals in each group. (Carvacrol: CAR and rifampin: Rif). a = Indicates a significant increase compared to the control group (P<0.001);
* = Indicates a significant decrease compared to the group that received only Rif (P<0.05); ** = indicates a significant reduction compared to the group that received only Rif (P<0.001).
Effect of Carvacrol on Serum Bilirubin and Total Protein: Figure 2 illustrates the impact of the oral administration of varying doses of Carvacrol over a period of 28 days on the serum levels of bilirubin and protein in rats with Rif-induced liver damage. The results indicate that Rif caused a significant increase in the serum bilirubin (P<0.001) and total protein levels (P<0.01) compared to those of the control group. Conversely, the administration of varying doses of Carvacrol resulted in a significant reduction in the serum bilirubin and total protein levels in rats with Rif-induced liver injury.
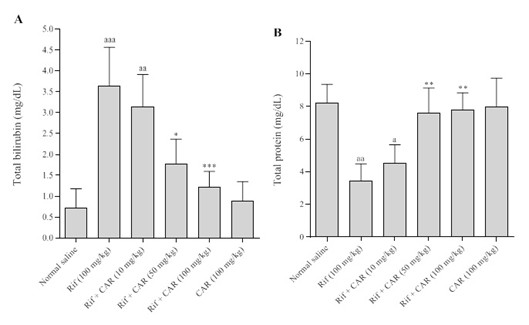
Figure 2. Effects of different doses of Carvacrol on the serum levels of bilirubin (A) and total protein (B) in Rif-induced liver damage in rats. The data are shown as Mean ± SD for ten animals in each group. (Carvacrol: CAR and rifampin: Rif). a = Indicates a significant increase compared to the control group (P<0.001); * = indicates a significant decrease compared to the group that received only Rif (P<0.05); ** = indicates a significant reduction compared to the group that received only Rif (P<0.001).
Effect of Carvacrol on Tissue Oxidative Stress Levels: Figure 3A illustrates the concentration of MDA in the liver tissue of rats across different experimental groups. The findings revealed a heightened MDA level in rats administered with Rif compared to the control group (P<0.01). Conversely, the administration of Carvacrol led to a significant decrease in MDA levels across the various experimental groups (P<0.001).
Effect of Carvacrol on Tissue Antioxidant Levels: The impact of Carvacrol on the liver levels of the antioxidants GSH, SOD, and CAT in Rif-induced liver damage in rats was depicted in Figures 3B to 3D. The results demonstrated a significant reduction in tissue levels of GSH, SOD, and CAT in rats with Rif-induced liver damage. However, the oral administration of varying doses of Carvacrol for 28 days increased the tissue levels of GSH, SOD, and CAT in rats with Rif-induced liver damage.
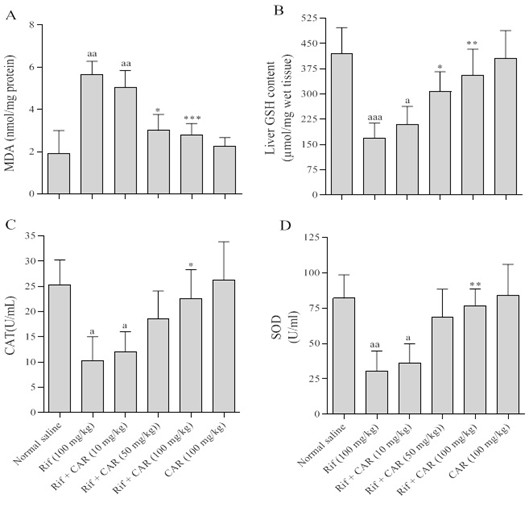
Figure 3. Effects of different doses of Carvacrol on the tissue level of Malondialdehyde (A), glutathione peroxidase (B), catalase (C), and superoxide dismutase (D) in Rif-induced liver damage in rats.
The data are shown as the means ± SD for ten animals in each group. (Carvacrol: CAR and rifampin: Rif).
a = Indicates a significant increase compared to the control group (P<0.001); * = indicates a significant decrease compared to the group that received only Rif (P<0.05); ** = indicates a significant reduction compared to the group that received only Rif (P<0.001).
Discussion
Acute liver failure can be caused under various conditions, including viral hepatitis, exposure to toxins and drugs, and due to ischemia [15]. Rifampin, a commonly prescribed antibiotic drug to treat tuberculosis, is known for its hepatotoxic side effects [3]. Based on the findings of the current study, the administration of Rif for four consecutive weeks led to significant liver damage in rats, which was manifested by significant elevation in serum levels of AST, ALT, lactate dehydrogenase, bilirubin, and oxidative stress markers. The findings of this study are consistent with the conclusions reached by other researchers [16-18].
However, the exact mechanism by which Rif causes liver damage is not fully understood. Several studies have suggested that Rif induces lipid peroxidation and reduces the levels of the GSH antioxidant and free radical scavenging enzymes, leading to oxidative damage [4, 7]. Nevertheless, Rif is known for its strong ability to stimulate the mixed oxidase system [19]. It is known to be a powerful inducer of the cytochrome P450 system, leading to the production of free radicals and their subsequent covalent attachment to hepatic macromolecules [20-22].
Research has shown that oxidative stress is the main mechanism of Rif causing toxicity to liver tissue [4, 18]. The findings of the current study confirmed the above mechanism. This is because in the liver tissue of rats receiving Rif, a significant rise of lipid peroxidation was observed, plus significant decreases in the antioxidant enzymes. The free radicals resulted in the peroxidation of membrane lipids, leading to the formation of lipid peroxidation and ending in the rupture of the hepatocyte membrane and other liver tissue damages. In other words, the rise in the liver MDA due to Rif administration indicates an increase in lipid peroxidation that leads to liver tissue damage. These events lead to the insufficiency of the liver antioxidant defense mechanisms and prevention of indiscriminate formation of free radicals.
The administration of Rif in this study resulted in a significant increase in MDA levels and a decrease in the activity of SOD and CAT. These findings align with the results reported earlier by Tasduq, et al. [18]. Further, the administration of Rif led to a significant increase in the serum levels of liver enzymes and total bilirubin. In the evaluation of liver lesions, the levels of such enzymes as ALP, ALT, and AST were measured [23]. The release of these enzymes into the bloodstream is commonly triggered by necrosis or damage to the cell membranes. The ALT, which facilitates the conversion of alanine to pyruvate and glutamate, is more specific to the liver and serves as a more appropriate parameter for the clinical diagnosis of liver damage. The elevated levels of these enzymes in the serum indicate cellular sedimentation and suggest damage to cell structures and membrane dysfunction in the liver [23, 24].
Investigation of natural products that offer hepatoprotective properties is of particular clinical significance [11, 25]. Due to their accessibility, minimal side effects, and cost-effectiveness, natural products have long been considered viable alternatives to synthetic drugs, attracting increased attention from researchers in recent decades [26, 27]. The search for an effective medication to reduce Rif-induced liver damage remains important and requires continued research. In this study, Carvacrol demonstrated a positive effect on the changes in liver enzyme levels induced by Rif. The findings of this study are consistent with those of Aristatile, et al. (2009) research, which examined the hepatoprotective properties of Carvacrol against the harmful effects of D-galactosamine in relation to the impact on ALP, AST, and ALT levels [28]. The capacity of Carvacrol to return elevated serum enzymes to normal levels after liver damage caused by Rif may be attributed to its role in preventing intracellular enzyme leakage by maintaining cell membrane integrity and stability or by promoting the regeneration of damaged liver cells.
Following the use of Rif in this study, there was a significant decrease in the activity of SOD and CAT in the liver, which can lead to an increase in the level of superoxide radicals in the liver cells. The SOD, CAT, and glutathione peroxidase are antioxidant enzymes that form a defense system against reactive oxygen species [29]. The decreased activities of SOD and CAT are sensitive indicators of cellular lesions [23]. The enzyme SOD is one of the most important elements in the enzymatic antioxidant defense system. This enzyme cleans the superoxide anion by converting it to hydrogen peroxide and thus reduces its toxic effects [30]. The use of Carvacrol, along with Rif, restores the SOD activities.
Carvacrol is a monoterpenic phenolic compound that is abundant in natural oils extracted from oregano, thyme, and coconut. The antioxidant properties of Carvacrol have been abundantly mentioned in studies [14, 31]. Considering that the function of antioxidants is to collect, neutralize, or remove the radicals within the cells and the extracellular environment, and since Carvacrol is a strong and potential antioxidant, it is expected that it can reduce liver damage by inhibiting free radicals and oxidative damage caused in different animal models [12]. In another study, Khalaf, et al. (2021) investigated the antioxidant role of Carvacrol against propiconazole-induced toxicity in the rats’ kidneys and liver [32].
The findings of this study demonstrated that exposure to propiconazole led to oxidative stress and lipid peroxidation, which was evident by a significant decline in the glutathione content and CAT activity, plus a significant rise in the MDA levels in the liver and kidneys. These toxic effects were also confirmed by histopathological studies. Moreover, due to its antioxidant properties, Carvacrol was able to reduce the harmful effects caused by propiconazole and improve liver and kidney tissue damage [32]. In another study conducted by Samarkandian, et al. in 2016, the protective effect of Carvacrol against oxidative damage in rat liver was demonstrated. That study showed that the amount of liver damage in 20-month-old rats was higher than that of 10-month-old rats, and the amount of lipid peroxidation was also higher. On the other hand, the administration of Carvacrol through inhibiting oxidative stress and increasing the antioxidant defense system was a candidate for inhibiting the progression of liver damage caused by aging [33]. It has also been reported that Carvacrol can significantly increase the activity of three antioxidant enzymes, namely CAT, SOD, and glutathione peroxidase, which are decreased in fungal infections caused by Candida auris [34]. The results of the above-mentioned studies are consistent with those reported by the current research.
Conclusions
In general, the findings of this study confirm the hepatoprotective properties of Carvacrol in mitigating liver damages induced by Rif. Carvacrol can reduce Rif-induced oxidative damage by decreasing the levels of reactive oxygen species and enhancing the activity of CAT and SOD. As a result, Carvacrol may be a viable option for treating individuals who take Rif to mitigate the potential risk of liver damage. Nevertheless, the comprehensive understanding of this compound, precise determination of its mechanisms of action, and the specific pharmacological benefits necessitate additional future research.
Conflict of Interests
The authors declare no competing interests with any internal or external entities.
Funding
The current study was funded by Lorestan University of Medical Sciences (Grant #: 2986). The authors received no extramural funding for the study.
Acknowledgement
The authors of this manuscript wish to express their gratitude to Lorestan University of Medical Sciences, Khorramabad, Iran.
Compliance with Ethical Guidelines
This study was approved by the Ethical Committee of Lorestan University of Medical Sciences (ethics code: IR.LUMS.REC.1402.183).
Authors' Contributions
HM and AGB conceived and designed the experiment. SH collected the data. HM performed the statistical analyses. MJ and AA cooperated in the interpretation of the results. AGB wrote the manuscript’s first draft. HM, MJ, and AA participated in finalizing the draft manuscript and scientific editions. All authors actively cooperated in writing the final manuscript.
References
- Oliveira, M., P. Chellini, and T. Amorim, Simultaneous determination of rifampicin, isoniazid, pyrazinamide and ethambutol in fixed-dose combination antituberculosis pharmaceutical formulations: a review. Analytical methods, 2018. 10(10): 1103-1116. [doi:10.1039/
C7AY02686B]
- Baltz, R.H., Spontaneous and induced mutations to rifampicin, streptomycin and spectinomycin resistances in actinomycetes: mutagenic mechanisms and applications for strain improvement. The Journal of antibiotics, 2014. 67(9): 619-624.[doi: 10.1038/ja.2014.105]
- Abulfathi, A.A., et al., Clinical pharmacokinetics and pharma-codynamics of rifampicin in human tuberculosis. Clinical pharmacokinetics, 2019. 58: 1103-1129.[doi: 10.1007/s40262-019-00764-2] [pmid: 31049868]
- Zhuang, X., et al., Mechanisms of isoniazid and rifampicin-induced liver injury and the effects of natural medicinal ingredients: A review. Frontiers in Pharmacology, 2022. 13: 1037814.[doi: 10.3389/fphar.
2022.1037814][pmid: 36299895]
- Kim, J.-H., et al., Mechanism investigation of rifampicin-induced liver injury using comparative toxicoproteomics in mice. International journal of molecular sciences, 2017. 18(7): 1417. [doi: 10.3390/
ijms18071417] [pmid: 28671602]
- Fountain, F.F., et al., Rifampin hepatotoxicity associated with treatment of latent tuberculosis infection. The American journal of the medical sciences, 2009. 337(5): 317-320. [doi: 10.1097/MAJ.0b013
e31818c0134]
- Sodhi, C., et al., Study of oxidative-stress in isoniazid-rifampicin induced hepatic injury in young rats. Drug and chemical toxicology, 1997. 20(3): p. 255-269.[doi: 10.3109/01480549709003881][pmid: 9292280]
- Bharti, U., N.R. Kumar, and J. Kaur, Bee pollen attenuates rifampicin and isoniazid in combination induced oxidative stress in testis of SD rats. Research Journal of Pharmacy and Technology, 2018. 11(3): p. 1159-1163.[doi: 10.5958/0974-360X.2018.00216.0]
- Madhavan, S., J. Rajkumar, and S. Dorai, Evaluation of polyherbal formulation against isoniazid and rifampicin induced oxidative stress in rat kidney. Indian Journal of Traditional Knowledge (IJTK), 2021. 20(3): p. 671-678.[doi: 10.56042/ijtk.v20i3.28376]
- Yue, J., et al., Effects of rifampin on CYP2E1-dependent hepatotoxicity of isoniazid in rats. Pharmacological research, 2009. 59(2):112-119. [doi: 10.1016/j.phrs.2008.10.006] [pmid:
19013243]
- Nasim, N., I.S. Sandeep, and S. Mohanty, Plant-derived natural products for drug discovery: current approaches and prospects. The Nucleus, 2022. 65(3): 399-411.[ doi: 10.1007/s13237-022-00405-3] [pmid: 36276225]
- Sharifi‐Rad, M., et al., Carvacrol and human health: A comprehensive review. Phytotherapy Research, 2018. 32(9):.1675-1687.[ doi: 10.1002/ptr.6103][pmid:29744941]
- Imran, M., et al., Therapeutic application of carvacrol: A comprehensive review. Food Science & Nutrition, 2022. 10(11): 3544-3561. [doi: 10.1002/fsn3.2994 ][pmid: 36348778]
- de Carvalho, F.O., et al., Anti‐inflammatory and antioxidant activity of carvacrol in the respiratory system: A systematic review and meta‐analysis. Phytotherapy research, 2020. 34(9) 2214-2229. [doi: 10.1002/ptr.6688][ pmid: 32249518]
- Gill, R.Q. and R.K. Sterling, Acute liver failure. Journal of clinical gastroenterology, 2001. 33(3): 191-198.[ doi: 10.1097/00004836-200109000-00005]
- Santhosh, S., et al., Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. European Journal of Pharmacology, 2007. 572(1): 69-73. [doi: 10.1016/j.ejphar.2007.05.059] [pmid:17612523]
- Tasduq, S.A., et al., Biochemical manifestations of anti-tuberculosis drugs induced hepatotoxicity and the effect of silymarin. Hepatology research, 2005. 31(3): 132-135. [doi: 10.1016/j.hepres.2005.01.005] [pmid: 15777701]
- Tasduq, S.A., et al., Potentiation of isoniazid‐induced liver toxicity by rifampicin in a combinational therapy of antitubercular drugs (rifampicin, isoniazid and pyrazinamide) in Wistar rats: A toxicity profile study. Hepatology Research, 2007. 37(10): 845-853.[doi: 10.1111/j.1872-034X.2007.00129.x]
- Piriou, A., et al., Enzyme induction with high doses of rifampicin in Wistar rats. Toxicology letters, 1983. 17(3-4): 301-306.[doi: 10.1016/0378-4274(83)90242-4] [pmid: 6137887]
- Burk, O., et al., The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (PXR) and constitutively activated receptor (CAR). Journal of Biological Chemistry, 2004. 279(37): 38379-38385.[ doi: 10.1074/jbc.M404949200]
- Brewer, C.T. and T. Chen, PXR variants: the impact on drug metabolism and therapeutic responses. Acta Pharmaceutica Sinica B, 2016. 6(5): 441-449.[ doi: 10.1016/j.apsb.2016.07.002] [pmid: 27709012]
- Sousa, M., A. Pozniak, and M. Boffito, Pharmacokinetics and pharmacodynamics of drug interactions involving rifampicin, rifabutin and antimalarial drugs. Journal of Antimicrobial Chemotherapy, 2008. 62(5): 872-878.[doi: 10.1093/jac/dkn330]
- Ozer, J., et al., The current state of serum biomarkers of hepatotoxicity. Toxicology, 2008. 245(3): p. 194-205.[doi: 10.1016/j.tox.2007.11.021] [pmid: 18291570]
- Ghaffarian-Bahraman, A., et al., Protective effect of magnesium and selenium on cadmium toxicity in the isolated perfused rat liver system. Acta Medica Iranica, 2014: p. 872-878. [Link]
- Datta, S., et al., Hepatoprotective effects of natural drugs: Current trends, scope, relevance and future perspectives. Phytomedicine, 2023: p. 155100.[ doi: 10.1016/j.phymed.2023.155100] [pmid: 37801892]
- Bachar, S.C., et al., Hepatoprotective natural products. Annual reports in medicinal chemistry, 2020. 55: 207-249.[doi: 10.1016/bs.armc.2020.06.003]
- Haghani, F., et al., Aloe vera and streptozotocin-induced diabetes mellitus. Revista Brasileira de Farmacognosia, 2022. 32(2): p. 174-187.[doi: 10.1007/s43450-022-00231-3][pmid: 35287334]
- Aristatile, B., et al., Effect of carvacrol on hepatic marker enzymes and antioxidant status in d‐galactosamine‐induced hepatotoxicity in rats. Fundamental & clinical pharmacology, 2009. 23(6): 757-765.[doi: 10.1111/j.1472-8206.2009.00721.x] [pmid: 19650854]
- Matés, J.M., C. Pérez-Gómez, and I.N. De Castro, Antioxidant enzymes and human diseases. Clinical biochemistry, 1999. 32(8): 595-603.[doi: 10.1016/s0009-9120(99)00075-2][pmid: 10638941]
- Rosa, A.C., et al., Superoxide dismutase administration: A review of proposed human uses. Molecules, 2021. 26(7): 1844. [doi:10.3390/
molecules26071844][ pmid: 33805942]
- Rathod, N.B., et al., Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends in Food Science & Technology, 2021. 116: 733-748.[doi:10.1016/
j.tifs.2021.08.023]
- Khalaf, A.A.A., et al., Antioxidant role of carvacrol against hepatotoxicity and nephrotoxicity induced by propiconazole in rats. Revista Brasileira de Farmacognosia, 2021. 31: 67-74.[ doi: 10.
1007/s43450-021-00127-8]
- Samarghandian, S., M. Azimi-Nezhad, and T. Farkhondeh, Preventive effect of carvacrol against oxidative damage in aged rat liver. Int. J. Vitam. Nutr. Res, 2016: 1-8.[ doi: 10.1024/0300-9831/a000393][ pmid: 27866466]
- Najafizadeh, A., et al., The protective effect of carvacrol on acetaminophen-induced renal damage in male rats. Molecular Biology Reports, 2022. 49(3): p. 1763-1771.[doi: 10.1007/s11
033-021-06985-8] [pmid: 35020122]
Type of Study:
Research |
Subject:
Special










































 , Salma Heidari2
, Salma Heidari2 

 , Mohammad Jamalian3
, Mohammad Jamalian3 

 , Anahita Esmaeili4
, Anahita Esmaeili4 

 , Hamidreza Mohammadi *5
, Hamidreza Mohammadi *5 





