Ethics code: IR.UM.REC.1402.169


1- Graduated Student, Doctor of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran
2- Department of Basic Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran , rajireza@um.ac.ir
3- Department of Pathobiology, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran
4- Department of Basic Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran
Full-Text [PDF 1698 kb]
(259 Downloads)
|
Abstract (HTML) (1039 Views)
Full-Text: (462 Views)
Introduction
During the past few years, nanotechnology with nanoparticlesranging in size from 1 to 100 nanometers has become increasingly intriguing to scientists, engineers, and researchers [1-3]. Nanotechnology's innovative approach to solving complex problems represents the intersection of chemistry, physics, and biology [4-6]. Nanoparticles possess several unique characteristics with variousphysical and chemical properties[7-9]. The effects of these chemicals on biological systems have received considerable attention and study [10, 11]. Various living organisms can interact with nanoparticles, increasing nanomedicine's popularity [12, 13]. Combining their surface structure and ability to transport medications makes nanoparticles effective medication transport agents [14]. Combining drugs with nanoparticles may reduce side effects and improve efficacy by delivering drugs directly to specific receptors in the body.
Nanoparticles are becoming increasingly popular for various applications; however,they raisesome concerns, e.g., such as toxicity [15]. The nanoparticles' chemical reactivity becomes more pronounced as the surface area increases compared to their volume, resulting in unpredictable interactions within biological systems. Because of their small size, they also pass through different biological barriers, including cell membranes and the blood-brain barrier [16]. As a result, concerns have been raised about their potential health risks [17,18]. Many well-known applications can be made from titanium dioxide nanoparticles (TiO2NPs) [19]. This compound is a white pigment used in paint, plastics, inks, pharmaceutical products, food, cosmetics, and toothpaste [20]. Such compoundshave a wide range of applications due to their unique properties[21]. For instance, sunscreens containing TiO2NPs provide exceptional protection against ultraviolet(UV) [22]. Chemical UV filters in conventional sunscreens do not scatter or reflect UV rays, like nanoparticles do. Their broad spectrum protection against UVA and UVB radiation makes them essential in sun protection products [23].
Titanium dioxide is used in the food industry as a whitening agent and for brightening products [24]. Candies, frostings, sweets, and confectionery products often contain such nanoparticles. Additionally, pharmaceutical companies use them in tablet coatings to render their products more consumer-friendly. In addition, Titanium dioxide is used in plastic materials to color and protect them against UV rays. Due to their widespread applications in many industries, TiO2NPs have been in high demand, and its production has increased [25-27]. Although some studies have shown that nanoparticles can improve the quality and performance of products, ingestion or exposure to these compounds is considered dangerous. As a result of these risks, researchers, governments, and consumers have all raised concerns about them.
Aim of the Study: As TiO2NPs become more prevalent, it is increasingly necessary to determine their effect on animal models, humans, and the environment. Therefore, the aim of the current study was to evaluate the TiO2NPs effects on the brain tissue of embryonic chickens.
Materials and Methods
Preparation of TiO2NPs: Preparation of TiO2NPs (From Noavaran Zist Parse, Mashhad, Iran) solution involves the following steps [28]. The sterilization process involved TiO2NPs powder for 20 minutes under UV light (Universal hood, Behdad Medical Production, Tehran, Iran). The mixture was sonicated for 30 minutes after adding 20 milliliters of normal saline using a probe sonicator. Afterward, the solution was concentrated to 12.5, 25, 50, and 100 μg/ml. The primary solution was prepared with a concentration of 20 mg in 20 ml (1 mg/ml). Through routine and standard dilution techniques, and the desired concentrations was achieved.
Injection of Nanoparticles into Fertilized Eggs: Ninety fertilized eggs were obtained from Simorgh Mashhad, Iran, and placed in an incubator (Mashhad Bargh; Mashhad, Iran) set at 37.5°C and 60% humidity. When the eggs were placed in the incubator, the authors of the current study indicated it as day zero. On the third day of fertilization, fertilized eggs were divided into five equal groups; four treatment groups and one control group. All the five groups had 14 eggs. One time injection was performed on the third day of incubation. Sterile distilled water was inoculated to the control group. 12.5 μg/ml TiO2NPs were allocated to the first group, 25 μg/ml were injected to the second group, 50μg/ml were assigned to the third group, and 100 μg/ml were assigned to the fourth group. All equipment and surfaces were sterilized using an alcohol solution of 70%. To inject the sterile water and the nanoparticle solutions into the air sac of each egg, a small hole was cut in its surface with a scalpel blade, and sterile water and the nanoparticle solutions were injected into the control and treatment groups via an insulin syringe [28]. An anti-allergenic adhesive was applied to the injection site to re-incubate the eggs. Embryonated eggs were in the incubator for the first three days. After that, injections were administered, and they were kept in the incubator until day 21 for hatching. Subsequent evaluations were then conducted.
Clinical Evaluation and Necropsy: After day 21 of incubation and hatching, the chicks were weighed using a laboratory-specific scale (manufactured in Iran). Subsequently, the brain and cerebellum were removed and weighed with a specific scale. The length and width of these parts were then measured using calipers (manufactured in Iran). The weights of the brains and chicks, the lengths and widths of the brains, and the percentage of live chicks were compared among the different groups. The hatching rate and the weights of all chicks were recorded after 21 days. After that, six chicks from each group were euthanized, and the brains were removed and placed in containers containing 10% formalin after the chicks were euthanized with CO2 gas. A refill of the formalin solution was performed after 24 hours. The samples were dehydrated, cleared, and embedded in paraffin wax after being fixed in 10% buffered formalin. After the paraffin blocks have been prepared, they are cut into 5-micron sections utilizing a microtome [29].
Hematoxylin-Eosin, Cresyl Echt Violet, and Luxol Fast Blue Staining Methods: Staining with Hematoxylin and Eosin (H&E) is a widely used non-specific technique. Acidic structures are shaded by eosin, which turns them red, and basic structures are stained by hematoxylin, which turns them purple. In the cytoplasm, DNA appears purple, as does the RNA in ribosomes [29]. In contrast, the other constituents of the cytoplasm appear pink or red when stained with H&E. Myelin sheaths surrounding nerve fibers and Nissl bodies within neurons can be identified using Cresyl Echt Violet and Luxol Fast Blue staining methods [29]. In neurons, Nissl bodies are stained purple with Cresyl Echt Violet, while myelin is dyed blue with Luxol Fast Blue. There are Nissl bodies in the cytoplasm of neurons, which contain RNA. Due to their basophilicity, they can be stained with basic aniline dyes. Sections stained with CresylEcht Violet were examined to determine if myelin density varied between the cerebral cortex and cerebellum of the experimental groups.
An analysis under a light microscope was performed to determine whether brain and cerebellum tissues were affected by abnormal changes, such as degeneration, inflammatory cell infiltration, necrosis, and hemorrhage. Cresyl Echt Violet and Luxol Fast Blue staining images of the cerebral cortex and cerebellum were also examined to determine which groups possessed dense myelin. In Luxol Fast Blue Cresyl Violet staining, myelin structures appear in blue. This allows us to examine and assess the density of myelin in the brains and cerebella of chicks under different groups using a light microscope.
Histomorphometric Analysis: In this study, there was a histomorphometric measurement of the perivascular space of the cerebrum, a measure of the neurons with Nissl bodies within a 2500 square micrometer area of the cerebral cortex, the cerebellar cortex length, the width of cerebellar cortex, and the number of Purkinje cells every 100 micrometers in both the control group and the experimental group. The slides were examined using a microscope equipped with a camera (Olympus, Japan) in the histology laboratory of the School of Veterinary Medicine in Mashhad. Five fields from each slide of all groups, cortex length, and Purkinje cell count in a range of 100 micrometers.
Statistical Analyses: ANOVA and post hoc tests were used to analyze data (SPSS Software, version 25). At P-values greater than 0.05, the discrepancy was statistically significant based on the analyzed data. The data in this study are presented as means ± standard deviations (SD).
Results
Macroscopic Examinations: No statistically significant differences were observed in the weights of the brains and chicks (P>0.05) (Table 1). Survival rates decrease with the increase in TiO2NPs dose, and abnormalities such as omphalocele and impaired yolk sac absorption are observed at high doses(p<0.05, Figure 1, 2).Group one had 2 casualties and 12 live chicks, the survival rate is 85.71%. Group two had 4 casualties and 10 live ones, the survival rate is 71.42%. Group three, consisting of 5 casualties and 9 live chicks, shows a survival rate of 64.28%. Finally, group four, comprising 7 casualties and 7 live ones, has a 50% survival rate. Notably, group four with a concentration of 100 μg/ml of TiO2NPs exhibits the lowest survival rate among the groups. Four cases of omphalocele and non-absorption of the yolk sac were observed in the group treated with a concentration of 100 μg/ml. Five of the chickens from the 100 μg/ml dose group also had abnormalities such as omphaloceles, which were not seen in the low-dose group.
Table 1. Comparisonof the macroscopic parameters in groups of broiler chickens. Numbers are the means ±SD after exposure to titanium dioxide nanoparticles at 12.5, 25, 50, or 100 μg/ml.
| Parameters |
Control |
Group 1
(12.5 μg/ml) |
Group 2
(25 μg/ml) |
Group 3
(50 μg/ml) |
Group 4
(100 μg/ml) |
| Body weight (gr) |
40 ± 3.33 |
41.22 ±2.29 |
41.33 ±3.02 |
42.5 ±2.59 |
40.75 ±3.41 |
| Brain weight (gr) |
1.31 ±0.08 |
1.37 ±0.09 |
1.14 ±0.04 |
1.35 ±0.07 |
1.36 ±0.10 |
| Brain brain (cm) |
1.83 ±0.08 |
1.81 ±0.07 |
1.81 ±0.07 |
1.72 ±0.08 |
1.81 ±0.10 |
| Brain Width (cm) |
1.63 ±0.10 |
1.58 ±0.07 |
1.55 ±0.08 |
1.52 ±0.08 |
1.58 ±0.09 |
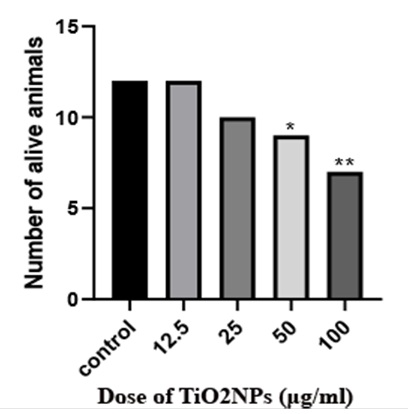
Figure 1. During the 21-day incubation period, broiler chicks were exposed to concentrations of 12.5, 25, 50, and 100 μg/ml of titanium dioxide nanoparticles until hatching. This table compares survival rates of one-day broiler chickens in different groups of the current study.
*,**P<0.05
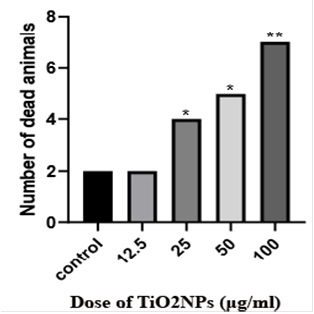
Figure 2. During the 21-day incubation period, broiler chicks were exposed to concentrations of 12.5, 25, 50, and 100 μg/ml of titanium dioxide nanoparticles until hatching. This table compares one-day broiler chicken mortality rates in different groups of the current study. *,**P<0.05
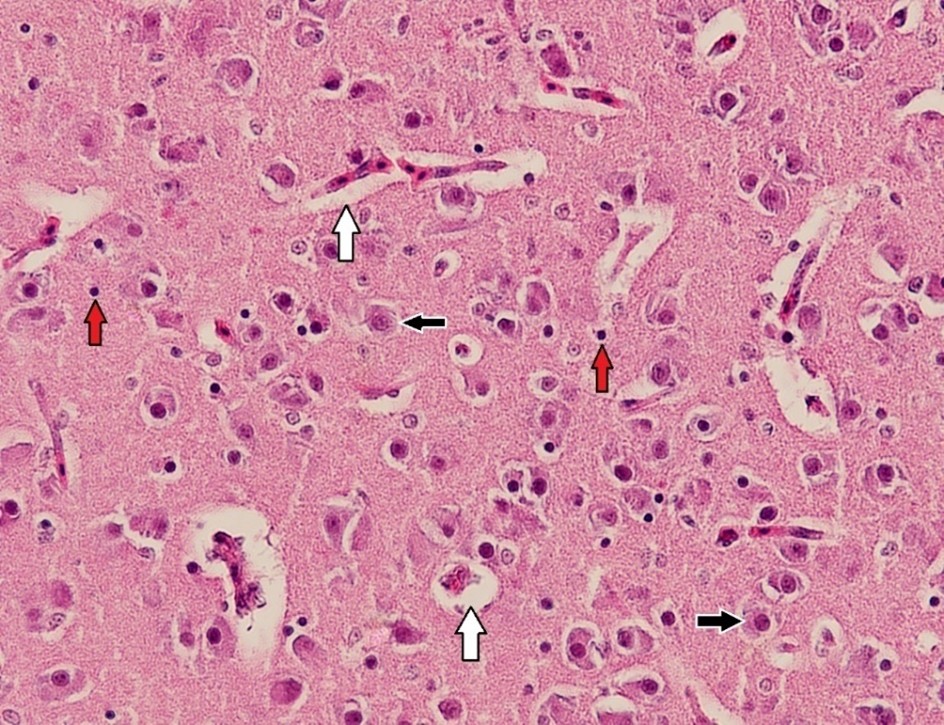
Figure 3. Histopathological section of the brain in the control group of broiler chicks. The white arrow indicates perivascular space, the black arrow represents neurons, and the red arrow identifies glial cells (H&E stain, × 400)
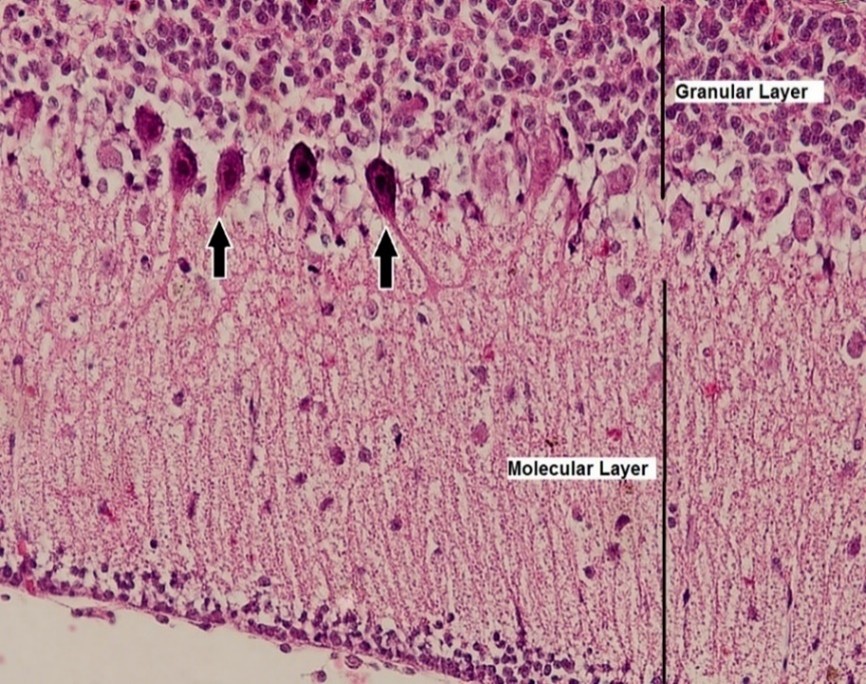
Figure 4. Histopathological section of the cerebellum in the control group of broiler chicks. The black arrow points to Purkinje cells (H&E stain, × 400×).
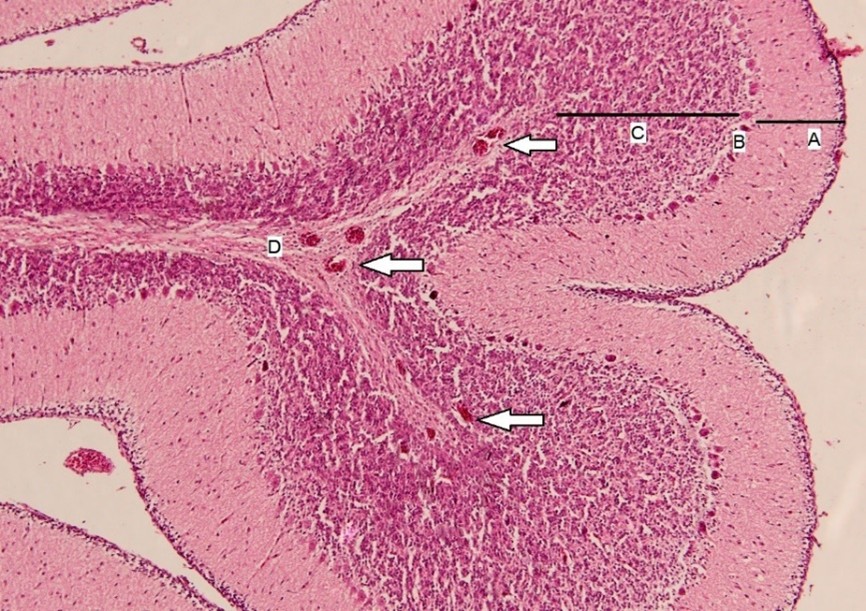
Figure 5. Histopathological section of the cerebellum in group 4, of broiler chicks. In the central portion, the identified components are as follows: A: Molecular layer, B: Purkinje cells, C: Granular layer, and D: White matter. White arrows indicate areas of congestion (H&E stain, × 40).
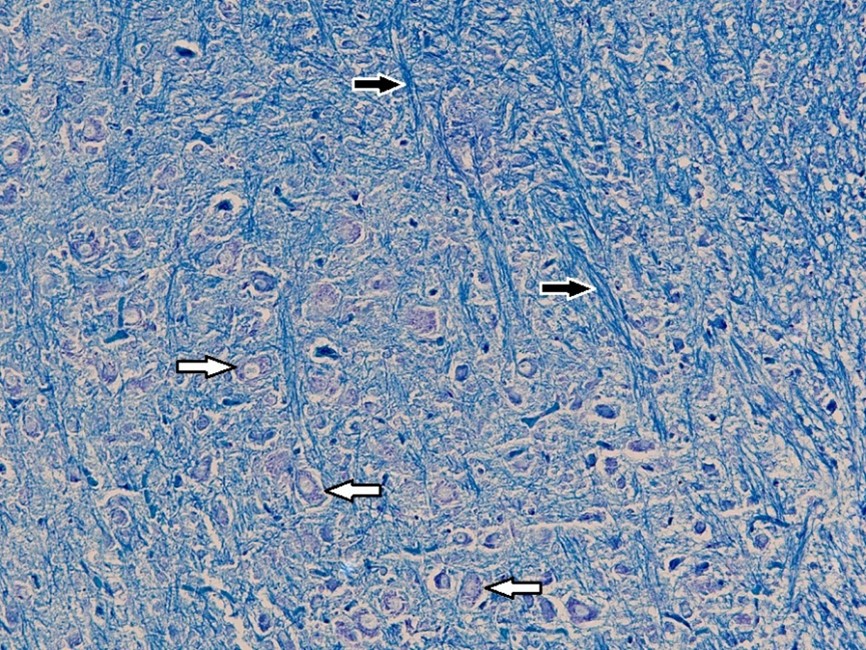
Figure 6. Histopathological section of the brain in the control group of broiler chicks. The white arrow indicates neurons containing Nissl bodies, and the black arrow represents myelinated fibers (Luxol Fast Blue stain, × 40).
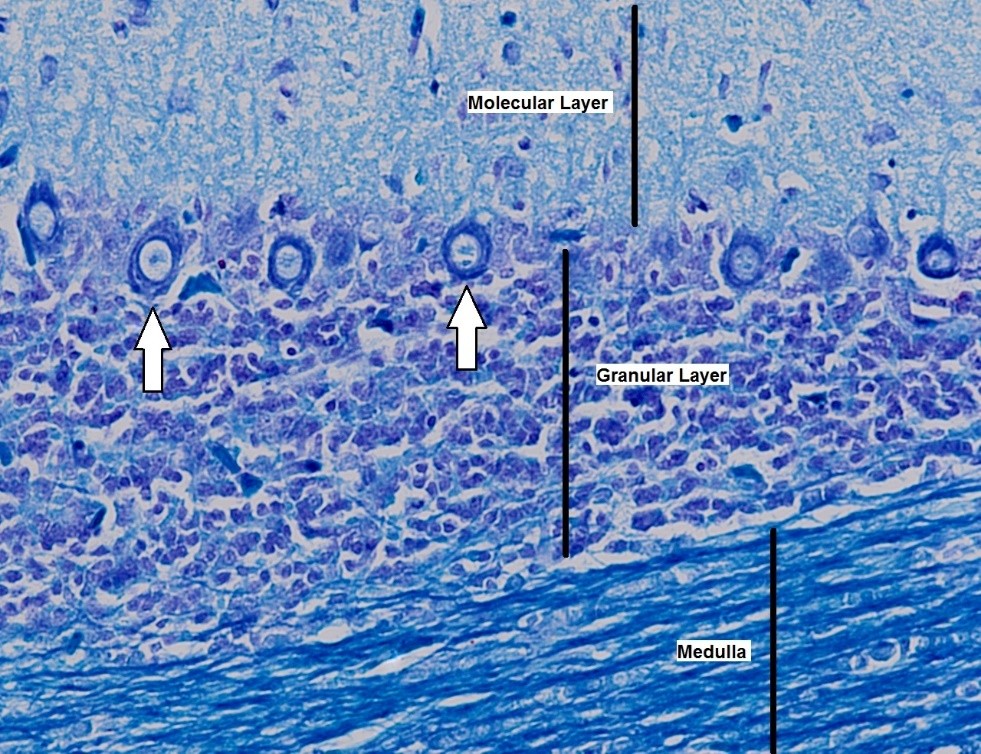
Figure 7. Histopathological section of the cerebellum in the control group of broiler chicks. The white arrow points to Purkinje cells (Luxol Fast Blue stain, × 400).
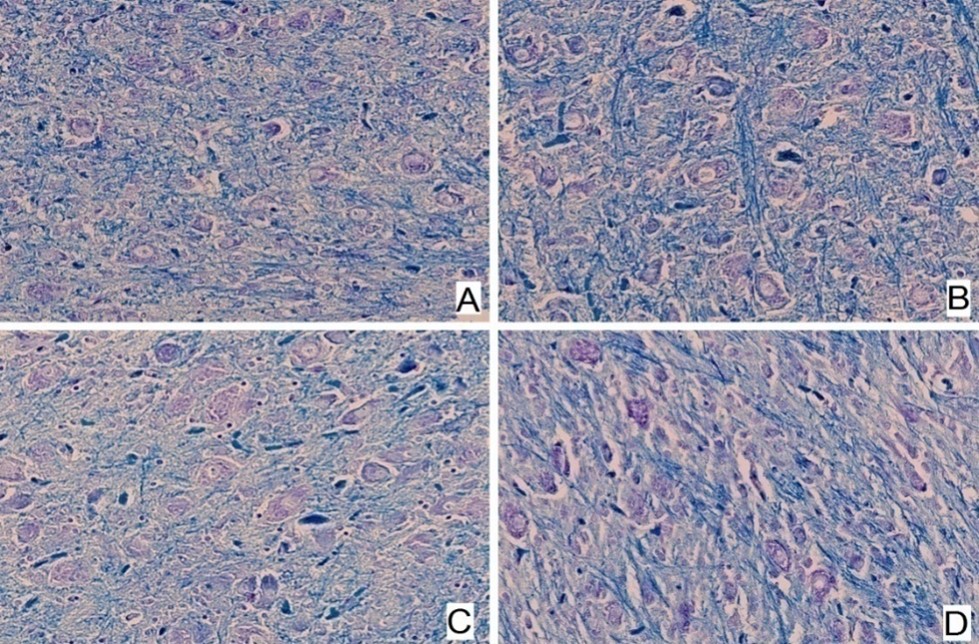
Figure 8. Central section of the cerebellum of broiler chicks. Different groups are indicated: A: Group 1, B: Group 2, C: Group 3, D: Group 4 (Luxol Fast Blue, × 400). No significant differences were observed among the various chicken groups.
Discussion
The current experiment involved injecting TiO2NPs into fertilized chicken eggs at 12.5, 25, 50, and 100 μg/ml. Incubation of the eggs began as soon as they were laid and continued until day 21 when the chicks hatched. Six one-day-old chicks from each group were examined macroscopically, histomorphometrically and histopathologically. Using TiO2NPs was significantly linked to a group injected with 100 μg/ml mortality rate, and the mortality amount was dose-dependent. There was a significant increase in mortality with an increase in nanoparticle dose at the highest dose, indicating developmental issues. Additionally, in the group 12.5, μg/ml of TiO2NPs did not result in a significant increase in mortality.
These findings show a similar pattern to those found in Jia, et al.'s 2017 study, which examined how TiO2NPs affected the fetal brains of mice during pregnancy [30]. Additionally, they observed visible anomalies and growth disruptions at high nanoparticle doses. As a result of oxidative stress and DNA damage caused by TiO2NPs, high amounts of TiO2NPs adversely affect the growth and development of animals. It was also reported that high TiO2NPs doses produced excessive nitric oxide production by cNOS and iNOS.
Acetylcholinesterase activity, as well as glutamic acid activity, were reduced in the brain. Acetylcholinesterase is an enzyme that regulates acetylcholine levels in the peripheral and central nervous systems. As a result, high doses of TiO2NPs nanoparticles can be toxic to the central nervous system and affect brain tissue abnormally, causing abnormalities in the tissue.
Further, Yamashita et al. in 2011, and Karimipour, et al. in 2018 reported a reduced number of births in mice following 100 mg/ml administration of TiO2NPs, consistent with the current study findings[31, 32].TiO2NPs cause toxicity, functional changes, and eventually cell death when they enter living cells, triggering inflammatory and apoptotic pathways and generating excessive reactive oxygen species. The effects of TiO2NPs on the development and health of pregnant women and fetuses have also been demonstrated to be harmful.
The results of the current study indicate that the dose of TiO2NPs does not significantly affect the histomorphometry measurements of the one-day-old chick brain. There was no significant difference observed in the number of neurons in the cerebral cortex area measuring 2500 square micrometers, the length of the cortex, the cerebellum, the number of Purkinje cells in 100 micrometers, and the perivascular space between the different groups in this study.
Additionally, Latifi et al. 2019, injected TiO2NPs under the skin of male rats[33]. With an increase in the injected dose, cellular changes increased, such as changes in neuronal shape and size, axonal dystrophy, increased spongiosis, and local hemorrhages, all of which they attributed to free radical production. In their study, Latifi et al. used considerably higher doses of nanoparticles and applied a different injection technique, which may explain the differences in results.
Yamano, et al. 2022, evaluated the carcinogenic potential of TiO2NPs in a 26-week exposure study using (rasH2) mice[34]. A major objective of this study was to assess the effect of TiO2NPs on lung tumors and their associated endpoints. Various concentrations of TiO2NPs (2, 8, or 32 mg/m3) were administered to male and female rasH2 mice over 26 weeks, 6 hours per day, 5 days a week. Results showed that particles were deposited in the lungs in a dose-dependent manner. There was no increase in lung tumor incidence, nor were there any pre-neoplastic lesions or pulmonary fibrosis observed despite exposure to these agents. Further, the study examined the proliferation of alveolar epithelial type 2 cells and found no evidence of an increase associated with TiO2NPs exposure. TiO2NPs were exposed at 32 mg/m3, which is considered a high concentration. In this study conducted on rasH2 mice,26-week inhalation of TiO2NPs results in inconclusive for pulmonary fibrogenicity and carcinogenesis.
Li, et al. examined the combined effects of TiO2NPs and CYP on reproductive toxicities using male rats exposed to testosterone and cypermethrin (CYP) over 90 days [35]. As a result of the combination of TiO2NPs and CYP, glutathione peroxidase and catalase activities in testicular tissue were decreased, while malondialdehyde and lactate dehydrogenase activities increased. Rats exposed to a combination of chemicals showed testicular cell apoptosis and DNA damage, suggesting detrimental effects on reproduction. It highlights concerns regarding the reproductive safety of simultaneous exposure to TiO2NPs and CYP, substances commonly used in agriculture, industry, and medicine.
Rolo, et al. were specifically interested in identifying the mechanism underlying the adverse effects of oral TiO2NP exposure [36]. In the review, oxidative stress, cytotoxicity, apoptosis, cell death, inflammation, cellular and systemic uptake, genotoxicity, and carcinogenicity were highlighted as key endpoints. It is well known that these agents can trigger many adverse outcomes, including colorectal cancer, liver injury, reproductive toxicity, cardiac and kidney damage, and hematological complications.
The toxicity of silver nanoparticles and TiO2NPs on mitochondrial dynamics in the testicles of mice was investigated by Arslan et al. [37]. At 3-day intervals, mice received intravenous nanoparticles administered in the form of TiO2 NP, Ag NP, and control groups. The AgNPs treatment did not significantly affect testicular parameters, despite both types of nanoparticles entering the testis and accumulating. In contrast, TiO2NPs (anatase, 25 nm) decreased sperm motility and caused sperm tail swelling. An analysis of mitochondrial dynamics revealed that TiO2NPs disrupt mitochondrial dynamics by increasing mitochondrial fission, as evidenced by increased expression of the Drp1 gene and the DRP1 protein. The study demonstrates for the first time that nanoparticle exposure to TiO2NPs can adversely affect mitochondrial dynamics in testicular cells, emphasizing the potential reproductive consequences.
The distribution of inhaled TiO2NPs in a pregnant rat model was investigated by D'Errico et al. 2022 [38]. Rats were exposed to either filtered air or TiO2NPs aerosols while they were pregnant. An analysis of maternal tissue on gestational day 20 revealed the extrapulmonary distribution of titanium, particularly in different placental zones. The presence of nanoparticles within the cellular organelles of the placenta was confirmed by transmission electron microscopy. In light of the systemic distribution and placental accumulation of TiO2NPs, these findings raise concerns about the potential effects of poor air quality during pregnancy and the association between poor air quality and problematic pregnancy outcomes.
Conclusions
Titanium dioxide nanoparticles demonstrated dose-dependent teratogenic effects on chicken brain development. While macroscopic and histomorphometric analyses did not show significant differences, higher TiO2NP concentrations increased the mortality rates of omphaloceles. The absence of notable variations in the weights of brain tissues and chickens, supported by histopathological findings, may suggest limited immediate impacts on these parameters. However, impaired yolk sac absorption at higher doses raises concerns about the long-term effects. The dose-dependent nature of the observed outcomes underscores the significance of careful consideration in using and regulating titanium dioxide nanoparticles, particularly in embryonic tissue development. These findings contributed valuable insights into the potential hazards associated with TiO2NPs exposure, emphasizing the need for further research and regulatory measures to ensure the safe utilization of these nanoparticles in various industries.
Conflict of Interests
The authors declare no conflict of interest with any entity.
Funding
This study was supported by a research grant provided by Ferdowsi University of Mashhad (Grant #:53082).
Acknowledgement
We thank the research deputy of the Ferdowsi University of Mashhad for supporting us and providing financial provisions.
Compliance with Ethical Guidelines
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study procedure has been approved by the ethical committee of the Animal Welfare Committee at the Ferdowsi University of Mashhad. (Approval #: IR.UM.
REC.1402.169).
Authors' Contributions
Ahmadreza Raji played a pivotal role in conceptualization, laying the foundation for the study. The methodology was a collective endeavor with contributions from all authors, ensuring a comprehensive and diverse approach. The formal analysis and investigation phase also saw the active involvement of all authors, demonstrating a shared commitment to thorough examination and robust results. MatinehDelrobaei took the lead in crafting the original draft, bringing coherence and clarity to the research findings. The writing process, encompassing both original draft preparation and subsequent review and editing, engaged the entire authorship, attesting to the collaborative nature of the scholarly work. Lastly, Ahmadreza Raji provided supervision, offered guidance and expertise throughout this research project. All authors reviewed and approved the final version of the manuscript prior to submission for publication to the present journal.
References
- Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules. 2019;25(1):112. [doi: 10.3390/molecules2501011] [pmid: 31892180]
- Joseph TM, Kar Mahapatra D, Esmaeili A, Piszczyk Ł, Hasanin MS, Kattali M, et al. Nanoparticles: Taking a unique position in medicine. Nanomaterials. 2023;13(3):574. [doi: 10.3390/nano13030574] [pmid: 36770535]
- Abbasi-Oshaghi E, Mirzaei F, Pourjafar M. NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: in vivo and in vitro study. International journal of nanomedicine. 2019: 14:1919-36. [doi: 10.2147/IJN.S192382] [pmid: 30936694]
- Bharatia R, Kumar S, Yadav V. Nano Technology-A Comprehensive Review. International Journal of Pharma Professional’s Research (IJPPR). 2023;14(3):94-105. [doi: 10.48165/ijppronline.2023.
14309]
- Saritha GNG, Anju T, Kumar A. Nanotechnology-Big impact: How nanotechnology is changing the future of agriculture? Journal of Agriculture and Food Research. 2022:100457. [doi: 10.1016/j.jafr.2022.100457]
- Sadr S, Poorjafari Jafroodi P, Haratizadeh MJ, Ghasemi Z, Borji H, Hajjafari A. Current status of nano‐vaccinology in veterinary medicine science. Veterinary Medicine and Science. 2023;9(5):2294-308.[ doi: 10.1002/vms3.1221] [pmid: 37487030]
- Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as Drug Delivery Systems: A Review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15(7):1596. [doi: 10.3390/polym15071596] [pmid: 37050210]
- Sadr S, Lotfalizadeh N, Ghafouri SA, Delrobaei M, Komeili N, Hajjafari A. Nanotechnology innovations for increasing the productivity of poultry and the prospective of nanobiosensors. Veterinary Medicine and Science. 2023;9(5):2118-31. [doi: 10.1002/vms3.1193] [pmid: 37433046]
- Sadr S, Lotfalizadeh N, Abbasi AM, Soleymani N, Hajjafari A, Roohbaksh Amooli Moghadam E, et al. Challenges and Prospective of Enhancing Hydatid Cyst Chemotherapy by Nanotechnology and the Future of Nanobiosensors for Diagnosis. Tropical Medicine and Infectious Disease. 2023;8(11):494. [doi: 10.3390/tropicalmed8110494] [pmid: 37999613]
- Negrescu AM, Killian MS, Raghu SN, Schmuki P, Mazare A, Cimpean A. Metal Oxide Nanoparticles: Review of Synthesis, Characterization and Biological Effects. Journal of Functional Biomaterials. 2022;13(4):274.[ doi: 10.3390/jfb13040274] [pmid: 36547533]
- Chandrakala V, Aruna V, Angajala G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Materials. 2022;5(6):1593-615. [doi: 10.1007/s42247-021-00335-x] [pmid: 35005431]
- Staroń A, Długosz O, Pulit-Prociak J, Banach M. Analysis of the Exposure of Organisms to the Action of Nanomaterials. Materials. 2020;13(2):349. [doi: 10.3390/ma13020349] [pmid: 31940903]
- Prasad RD, Charmode N, Shrivastav OP, Prasad SR, Moghe A, Sarvalkar PD, et al. A review on concept of nanotechnology in veterinary medicine. ES Food & Agroforestry. 2021;4:28-60. [doi:10.30919/esfaf481]
- Liu R, Luo C, Pang Z, Zhang J, Ruan S, Wu M, et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chinese chemical letters. 2023;34(2):107518. [doi: 10.1016/j.cclet.2022.05.032]
- Egbuna C, Parmar VK, Jeevanandam J, Ezzat SM, Patrick-Iwuanyanwu KC, Adetunji CO, et al. Toxicity of nanoparticles in biomedical application: nanotoxicology. Journal of Toxicology. 2021;2021:1-21. [doi: 10.1155/2021/9954443][ pmid: 34422042]
- Długosz O, Szostak K, Staroń A, Pulit-Prociak J, Banach M. Methods for reducing the toxicity of metal and metal oxide NPs as biomedicine. Materials. 2020;13(2):279. [doi: 10.3390/ma13020279] [pmid: 31936311]
- Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, Ansari MJ, et al. Nanotechnology in cosmetics and cosmeceuticals—A review of latest advancements. Gels. 2022;8(3):173. [doi: 10.3390/gels8030173] [pmid: 35323286]
- Ettlinger R, Lächelt U, Gref R, Horcajada P, Lammers T, Serre C, et al. Toxicity of metal–organic framework nanoparticles: from essential analyses to potential applications. Chemical Society Reviews. 2022;51(2):464-84. [doi: 10.1039/D1CS00918D]
- Ziental D, Czarczynska-Goslinska B, Mlynarczyk DT, Glowacka-Sobotta A, Stanisz B, Goslinski T, et al. Titanium dioxide nanoparticles: prospects and applications in medicine. Nanomaterials. 2020;10(2):387. [doi: 10.3390/nano10020387] [pmid: 32102185]
- Akakuru OU, Iqbal ZM, Wu A. TiO2 nanoparticles: properties and applications. TiO2 Nanoparticles: Applications in Nanobiotechnology and Nanomedicine. 2020:1-66. [Link]
- Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Particle and fibre toxicology. 2013;10:1-33.[doi: 10.1186/1743-8977-10-15] [pmid: 23587290]
- Ghamarpoor R, Fallah A, Jamshidi M. Investigating the use of titanium dioxide (TiO2) nanoparticles on the amount of protection against UV irradiation. Scientific Reports. 2023;13(1):9793. [doi: 10.1038/s41598-023-37057-5] [pmid: 37328531]
- Musial J, Krakowiak R, Mlynarczyk DT, Goslinski T, Stanisz BJ. Titanium dioxide nanoparticles in food and personal care products—What do we know about their safety? Nanomaterials. 2020;10(6):
1110.[ doi: 10.3390/nano10061110] [pmid: 32512703]
- Barad DL, Chotaliya UJ, Patel NK. A brief review on titanium dioxide. Asian Journal of Pharmaceutical Analysis. 2022;12(3):187-96. [doi: 10.52711/2231-5675.2022.00032]
- Irshad MA, Nawaz R, ur Rehman MZ, Adrees M, Rizwan M, Ali S, et al. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicology and environmental safety. 2021;212:111978. [doi: 10.1016/j.ecoenv.2021.111978] [pmid: 33561774]
- Kang X, Liu S, Dai Z, He Y, Song X, Tan Z. Titanium dioxide: From engineering to applications. Catalysts. 2019;9(2):191. [doi: 10.3390/
catal9020191]
- Waghmode MS, Gunjal AB, Mulla JA, Patil NN, Nawani NN. Studies on the titanium dioxide nanoparticles: Biosynthesis, applications and remediation. SN Applied Sciences. 2019;1(4):310. [doi:10.1007/
s42452-019-0337-3]
- Patel S, Jana S, Chetty R, Thakore S, Singh M, Devkar R. TiO2 nanoparticles induce omphalocele in chicken embryo by disrupting Wnt signaling pathway. Scientific Reports. 2018;8(1):4756. [doi: 10.1038/s41598-018-23215-7] [pmid: 29555972]
- Luna LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology. Armed Forces Institute of Pathology1968. [Link]
- Jia X, Wang S, Zhou L, Sun L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale research letters. 2017;12:1-14. [doi: 10.1186/s11671-017-2242-2] [pmid: 28774157]
- Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nature nanotechnology. 2011;6(5):321-8.[ doi: 10.1038/nnano.2011.41]
- Karimipour M, Javanmard MZ, Ahmadi A, Jafari A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. International Journal of Reproductive BioMedicine. 2018;16(6):
397. [pmid:30123868]
- Latif MA, Jabeen F, Ali M, Rasul A, Naz S, Akram M. Neurotoxic effects of titanium dioxide nanoparticles on the brain of male sprague dawley rats. Pak J Pharm Sci. 2019;32(5):2311-6. [pmid: 31894060]
- Yamano S, Takeda T, Goto Y, Hirai S, Furukawa Y, Kikuchi Y, et al. No evidence for carcinogenicity of titanium dioxide nanoparticles in 26-week inhalation study in rasH2 mouse model. Scientific Reports. 2022;12(1):14969. [doi: 10.1038/s41598-022-19139-y] [pmid: 36056156]
- Li Y, Zhong M, He X, Zhang R, Fu Y, You R, et al. The combined effect of titanium dioxide nanoparticles and cypermethrin on male reproductive toxicity in rats. Environmental Science and Pollution Research. 2023;30(9):22176-87. [doi: 10.1007/s11356-022-23796-x] [pmid: 36282392]
- Rolo D, Assunção R, Ventura C, Alvito P, Gonçalves L, Martins C, et al. Adverse Outcome Pathways Associated with the Ingestion of Titanium Dioxide Nanoparticles—A Systematic Review. Nanomaterials. 2022;12(19):3275. [doi: 10.3390/nano121932
75][pmid: 36234403]
- Arslan NP, Keles ON, Gonul-Baltaci N. Effect of Titanium Dioxide and Silver Nanoparticles on Mitochondrial Dynamics in Mouse Testis Tissue. Biological Trace Element Research. 2022;200(4):1650-1658. [doi: 10.1007/s12011-021-02763-6] [pmid: 34105085]
- D'Errico JN, Doherty C, Reyes George JJ, Buckley B, Stapleton PA. Maternal, placental, and fetal distribution of titanium after repeated titanium dioxide nanoparticle inhalation through pregnancy. Placenta. 2022;121:99-108.[doi: 10.1016/j.placenta.
2022.03.008] [pmid: 35305398]
Type of Study:
Research |
Subject:
Special
















































