Ethics code: IR.UM.REC.1401.089


Choobchian R, Raji A, Nourani H, Moghadam Jafari A, Moghtadari Esfahani A. Histopathological effects of titanium dioxide nanoparticles on the liver in rats by light and transmission electron microscopy. IJT 2024; 18 (4) :233-240
URL:
http://ijt.arakmu.ac.ir/article-1-1386-en.html
1- Department of Basic Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran
2- Department of Basic Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran , rajireza@um.ac.ir
3- Department of Pathobiology, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran
Full-Text [PDF 1699 kb]
(408 Downloads)
|
Abstract (HTML) (1072 Views)
Full-Text: (418 Views)
Introduction
Nanotechnology has given environmental engineers fresh hope for dealing with pollution by coming up with new ideas on nanomaterials [1]. However, exposure of consumers and vulnerable populations to various nanoparticles raises health concerns regarding their production and use [2]. The nanotechnology has achieved a size range of 1 to 100 nm and transitioned from micro- to nanoparticles [3]. As we reach the current size range, i.e., 1 to 100 nm, certain physical features, such as the surface-to-volume ratio, will change, thus changing the quantum effects of the materials [4]. The increase in surface-to-volume ratio enables exterior atoms to behave better than the inside ones. This behavior also influences the chemical properties of the particles [5]. Nanoparticles have a wide range of applications in various industries, particularly due to their unique optical and electrical characteristics [6, 7]. The nanoparticles of iron oxide [8, 9], titanium dioxide (TiO2) [4], and other elements are popular examples of such materials.
Titanium dioxide nanoparticles (TiO2NPs), the ninth most abundant element in the earth's crust, is a commonly utilized metal that may enter the food chain as a bleaching agent, especially in dairy products, cocoa, milk powders, soy products, and sausages. In foods and drugs, this additive is known as E171, helps define colors clearly, and can prevent the degradation of materials under UV light. It is estimated that about 300 mg of titanium is commonly consumed by people in their meals per day [10].
Titanium dioxide, or titania, a nanoparticle made of TiO2, was first developed from titanium early in 1923, and its commercial applications continued to proliferate as a mineral material. Titanium dioxide nanoparticles are classified under three categories: rutile, anatase, and brookite. Rutile and anatase molecules have tetrahedral structures, whereas brookite has an octahedral structure. Because of their unique properties in terms of interaction with biological molecules, they have attracted much interest among biologists and other scientific researchers [11].
Because of its structural features, TiO2 is an excellent photocatalyst based on semiconductor oxides, which has attracted much interest among scientists of various specialties since 1972 [12]. This molecule is easily synthesized in both industries and laboratory settings because it is easily accessed and mass-produced at low cost [13]. Titanium photocatalysts are both chemically and photochemically stable and non-toxic. These materials are resistant to both acids and bases and remain soluble in water. Most notably, TiO2 has a self-destructive function, which makes it suited for purifications and industrial applications in, for instance, water, paints, food, cosmetic products, and toothpaste, and also for environmental disinfectants [14, 15]. These nanoparticles are also used for the treatment of tumors, drug delivery, and translocating genes to cells and tissues [16].
Aim of the Study: To our knowledge, few studies have investigated the effects of titanium nanoparticles on the liver. Therefore, this study was designed to investigate the light microscopic and ultrastructural changes of rat liver and hepatocytes influenced or induced by TiO2NPs.
Materials and Methods
In this study, 40 rats were initially used and divided into four groups. They were treated every second day by gavage with 0 (control), 10, 20, or 50 mg of titanium nanoparticles per kilogram of the body weight, suspended in one milliliter of distilled water over a period of 60 days. Only 24 rats survived at the end of the treatment period (6 rats per group). Subsequently, the rats were euthanized with ether, and small samples of their liver (approximately 1 mm3) were dissected and promptly fixed in glutaraldehyde for at least 1 hour at room temperature (23°C), followed by post-fixation in osmium tetroxide. Next, the liver specimens were dehydrated in graded ethanol, followed by propylene oxide, and embedded in Epon812 prior to sectioning for microscopic examinations. The blocks were trimmed, and semi-thin sections were cut using a glass knife. Ultrathin sections were made using ultramicrotome with diamond knives and placed on copper grids, impregnated with uranyl acetate and lead citrate. The thick and ultrathin liver sections were examined under light and transmission electron microscopy (Leo Co.; Freiburg, Germany). All study procedures and protocols were reviewed and approved by the Ethics Committee of the Ferdowsi University of Mashhad, Mashhad, Iran (Approval Code: IR.UM.REC.1401.089).
Results
Histopathological Findings: Microscopic examinations of the thick sections of liver samples from the groups treated with TiO2NPs revealed dilation of hepatic sinusoids, hepatocyte degeneration, and cell necrosis. We also observed cellular swelling, cytoplasmic vacuolation, and hyperstainability of some hepatocytes, especially in periportal areas, plus loss of nuclei, nuclear pyknosis, karyorrhexis, and karyolysis. Infiltration of mononuclear inflammatory cells was observed at multiple foci in the rats’ liver samples among the treated groups (Figures 1-3). No significant difference was noted by comparing the histopathological lesions among the treatment groups.
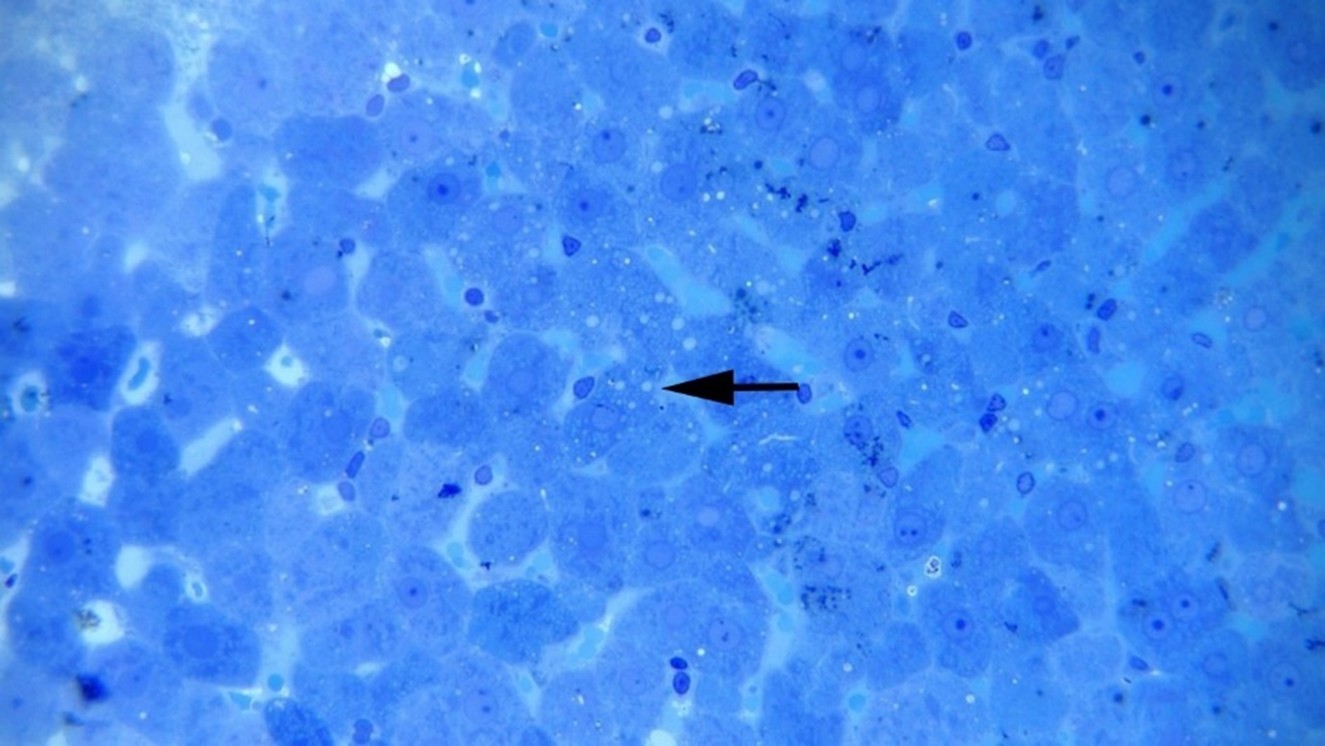
Figure 1: Hepatocellular vacuolation (arrow) and necrosis at 50 mg np, Methylene blue staining (×400).
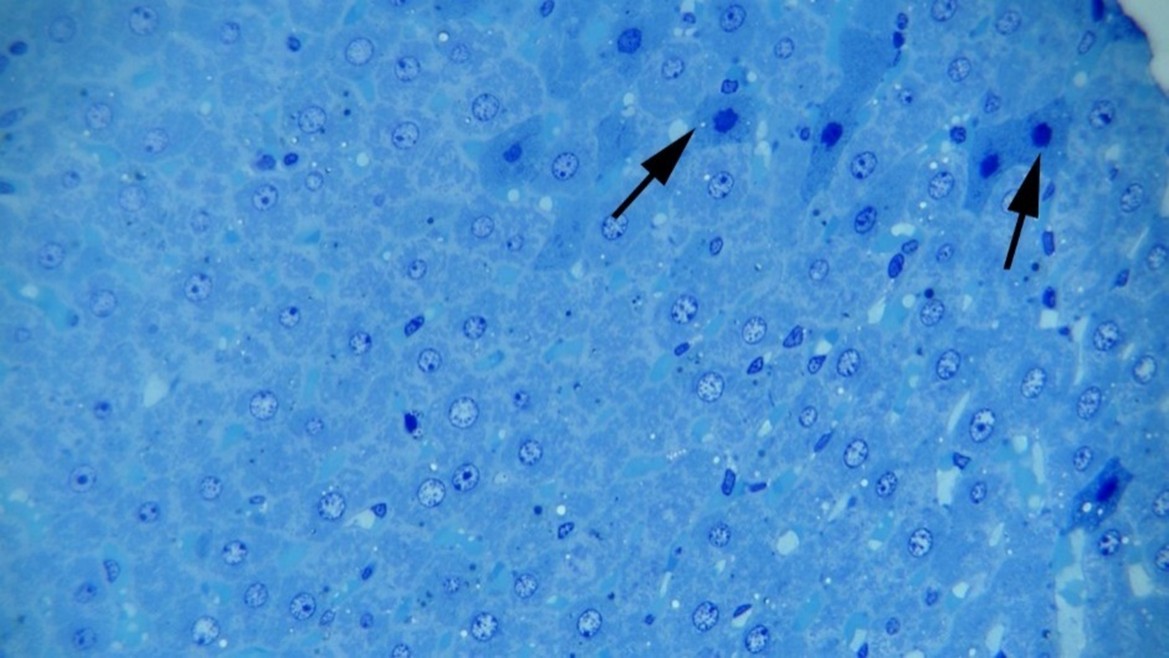
Figure 2: Hepatocellular necrosis at 10 mg np. Note cytoplasmic hyperstainability and nuclear pyknosis (arrows) of the necrotic hepatocytes, Methylene blue staining (×400).
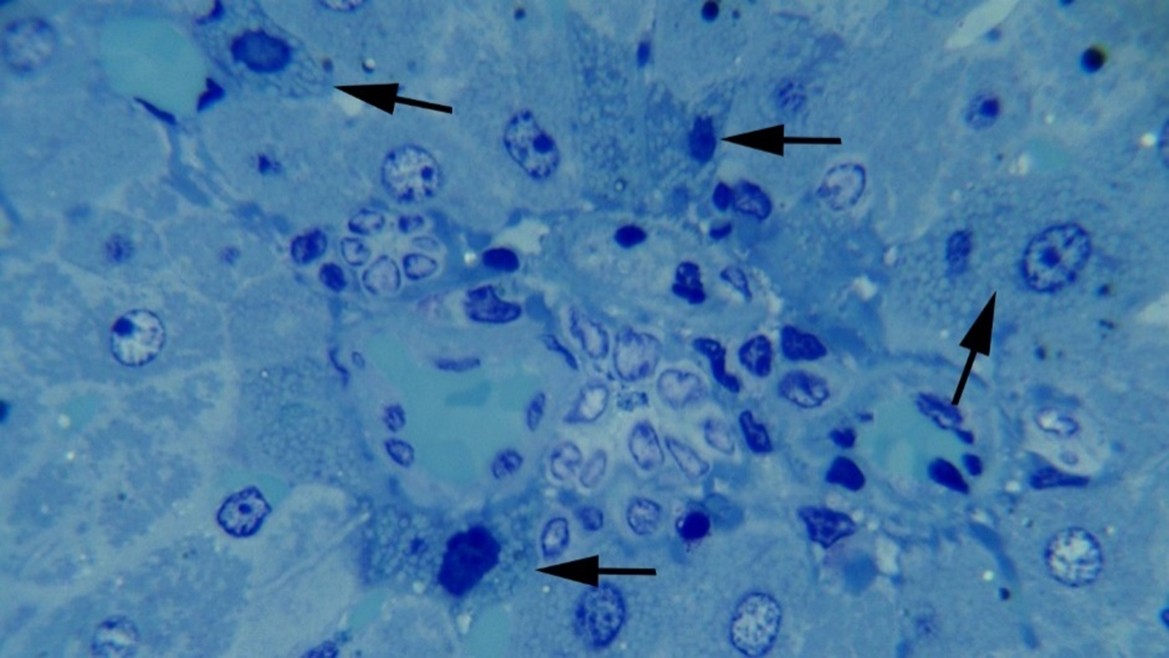
Figure 3: Hepatocellular degeneration and necrosis at 10 mg np. Note cytoplasmic vacuolation and nuclear pyknosis (arrows) of the necrotic and degenerative hepatocytes in a periportal area, Methylene blue staining (×1000).
Ultrastructural Findings: The ultrastructural sections from the control group showed intact hepatocytes with the nuclei, nucleoli, endoplasmic reticula, mitochondria, and cell membranes (Figure 4). Other findings are clearly explained in the Figures 5, 6 and 7. After that, the ultrastructural sections from the second group (10 mg/kg TiO2NP) were examined. Contrary to the analyzer's opinion and in line with the results of previous research, hepatocellular injuries were observed at low treatment doses, i.e., 10 and 20 mg. For the first time, swollen or torn mitochondrial membranes, reduced or destroyed cristae, injury to the mitochondria, increased gaps between the two mitochondrial membranes, and high-density elements were observed. See Figure 7.
Next, the ultrastructural sections from the third group (20 mg/kg TiO2NPs) were examined, and the following results were documented:
- Presence of points with high electron density
- Swelling or disappearance of cristae and endoplasmic reticula
- Destruction of mitochondria
- Presence of dense matrices in broken mitochondria
- Formation of continuous cavum (Micrographs 6).
Finally, the sections from the fourth nanoparticles treatment group (50 mg TiO2NPs) were examined microscopically. Sections in this group appeared to have adapted to the increased concentration of TiO2NPs, and to some extent, there were less harmful effects observed on the mitochondria. However, the endoplasmic reticula, the presence of electron-dense points, and the loss of membranes and cristae were evident, which confirmed the harmful effects on the mitochondria. Micrograph7 shows the microscopic findings from the fourth treatment group.
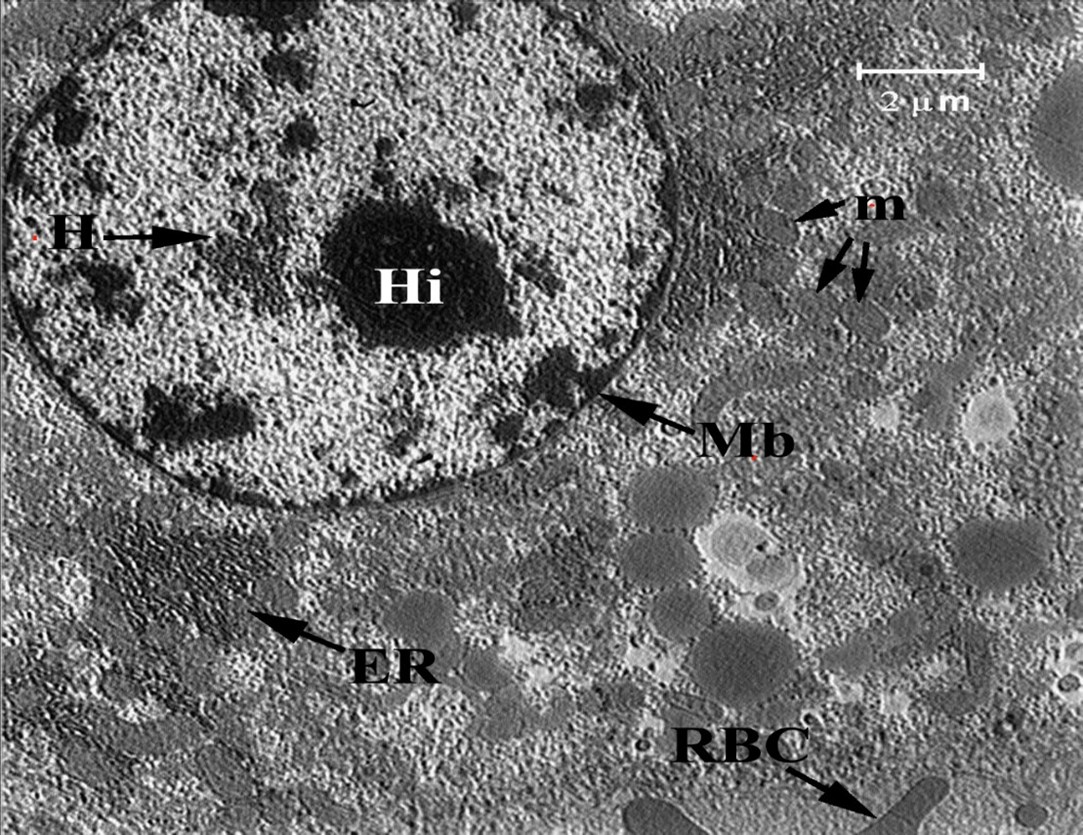
Figure 4: Ultrastructural section of liver of the control group (0 mg np): Endoplasmic Reticulum (ER), Membrane (Mb), Mitochondria (m), Heterochromatin (H), Nucleolus (Hi), Red Blood Cell (RBC).
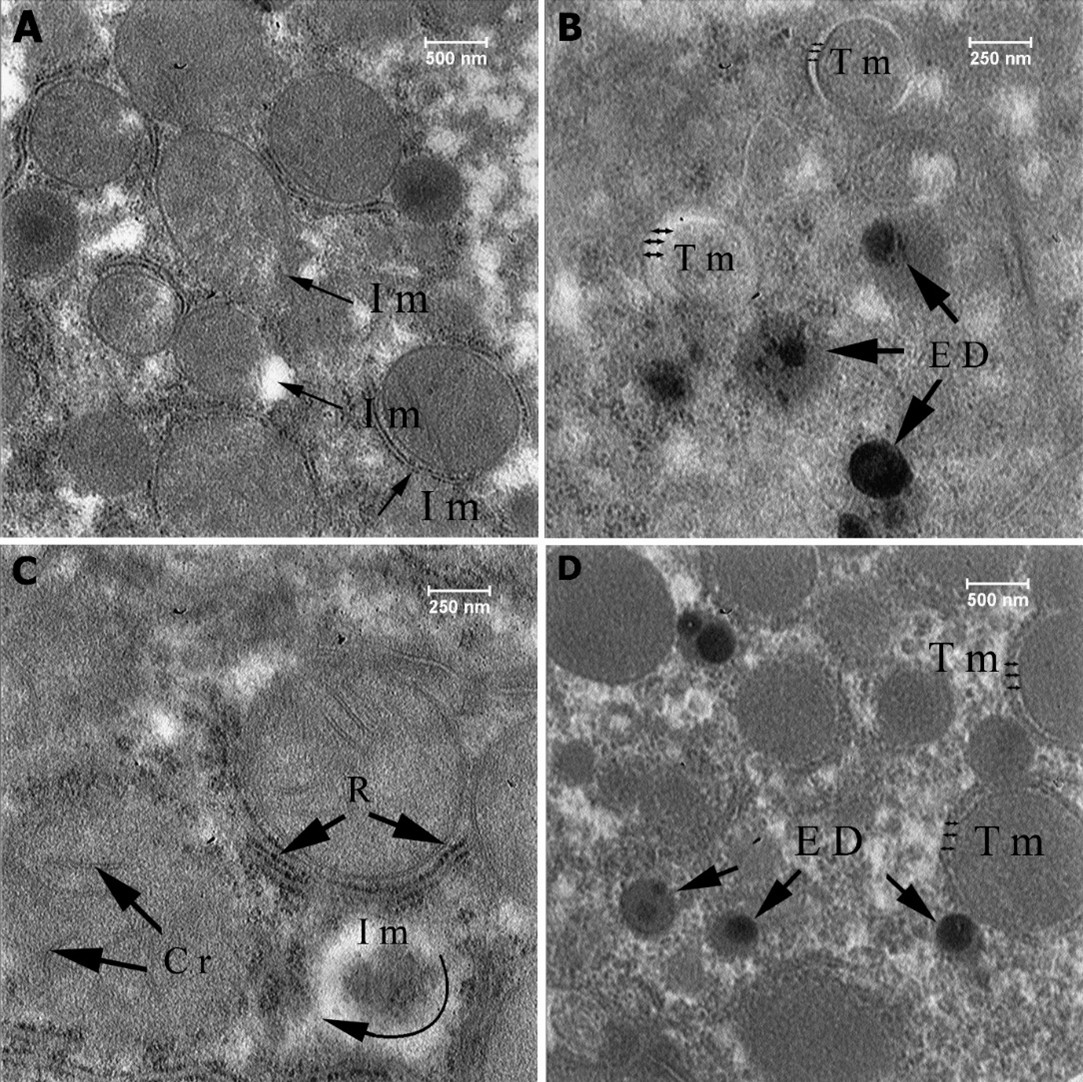
Figure 5: Ultrastructural section of liver of the first group (10 mg np), Injury to the mitochondria (Im), Torn membrane (Tm), High-density elements (ED), Cristae (Cr), Ribosome (R).
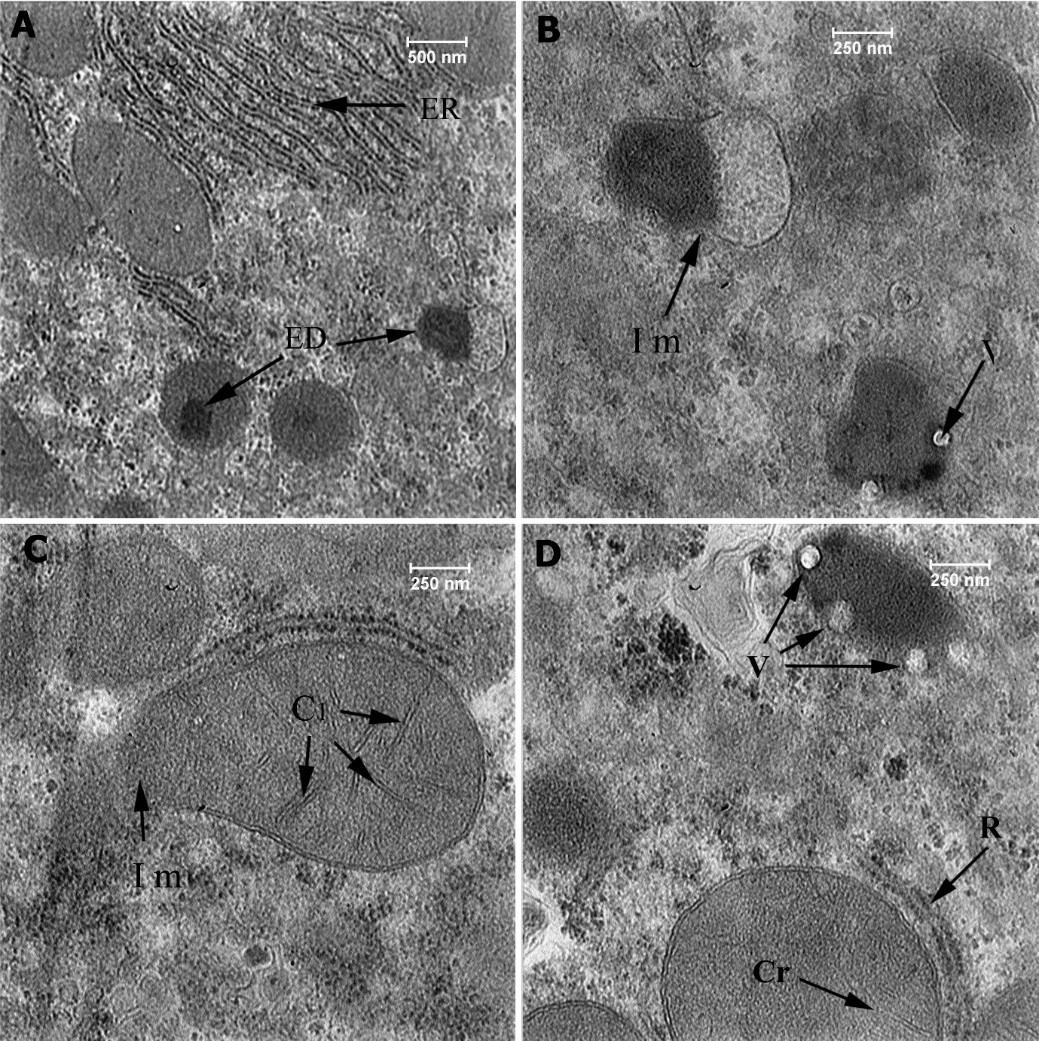
Figure 6: Ultrastructural section of liver of the second group (20 mg np), Endoplasmic Reticulum (ER), Injury to the mitochondria (Im), High-density elements (ED), Cristae (Cr), Ribosome (R), High-density elements matrix (EDM), Vacuole (V).
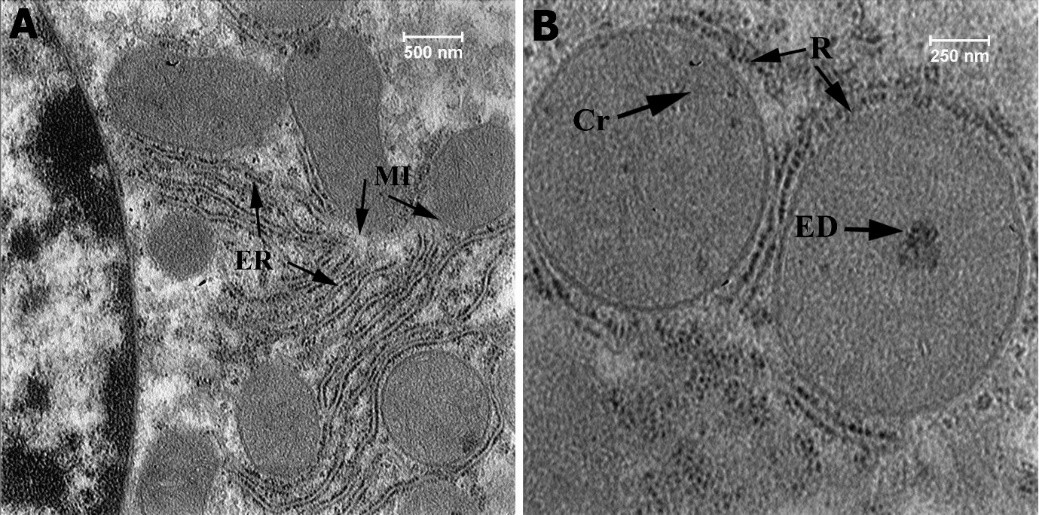
Figure 7: Ultrastructural section of liver of the fourth group (50 mg np), High-density elements (ED), Cristae (Cr), Ribosome (R), Membrane (Mb), Endoplasmic Reticulum (ER).
Discussion
The current study investigated the toxic effects of TiO2NPs since this compound has a wide application in various industries. We compared our study findings with those of other studies on hepatocytes from adult rat liver tissue. Given the vital role of the liver as the most important purifying organ of the body, the effect of nanoparticles, such as TiO2, on this organ is equally of great importance.
To achieve the study’s goal of investigating the effect of TiO2NPs on the liver cells, we used rats as a laboratory model similar to humans, allowing for the potential generalization of results to human health. In this study, we used TiO2NPs because both humans and animals are at risk of exposure to this compound. This methodology allows for the measurement of the amount of nanoparticles ingested and enables an assessment of their effects. Based on the Food and Drug Administration standards, which permit this compound to be used at 1% in pharmaceutical and food products, this study investigated chronic nanoparticle use similarly to that of actual daily human consumption. Thus, we administered the nanoparticle at four doses of 0, 10, 20, and 50 mg/kg of the rat's body weight. To demonstrate the chronic form of these nanoparticles, the treatment was given every second day over 60 days.
Based on the current study findings, TiO2NPs affected the mitochondria of rat hepatocytes and damaged these organelles that have a vital role in cellular respiration. We demonstrated that mitochondria were destroyed due to cristae swelling and membrane rupture, formation of vacuoles, and electron-dense spots after exposure to TiO2NPs.
Noori, et al. examined tissue injuries in mice liver after intraperitoneal injection of nanoparticles, and their results were in line with those of the current research [17]. However, the findings reported by Yahyaei and Abbasi differed from those of the present study and those of Noori et al., as they did not observe tissue damage caused by nanoparticles in their study [17]. Jalili et al. had results similar to those of Yahyaei and Abbasi. They did not detect any differences, implying that their findings contradicted those of the current study [17, 18]. Giselle Domingo, et al. discovered that only 5 nm particles reduced the activity of superoxide dismutase and catalase in the liver. They observed no changes in the oxidative metabolism of the kidneys in any of the animal groups exposed to TiO2NPs [19]. Based on our findings, nanoparticles have a greater effect on liver tissue than on kidneys. These are similar to the results of Shaltout, et al., who demonstrated that nanoparticles affected the kidneys and intestines in addition to the liver. The findings reported by these two studies are consistent with those of the current study [20].
Sarikhani, et al. discovered that TiO2NPs harm cell growth since they damage the cellular DNA, causing them to stop multiplying, restarting the process, and promoting aging. As a result, nanoparticles can influence cellular DNA in addition to the mitochondria [21]. The review articles by Baranowska-Wójcik, et al. and Wang, et al. have confirmed that nanoparticles negatively affect the gastrointestinal tract [22, 23]. The liver, as a vital gland in the digestive system, is no exception to this rule. However, damages have been caused in the epithelia of the small intestinal villi, and increases in the cellular vacuoles have been observed in the villi [24]. As suggested by Baykose, et al. [24] , nanoparticles lower the overall energy of the liver, which can lead to liver damage. Thus, the results of the latter study are consistent with those of the current research [24].
Studies conducted on other organs have shown that nanoparticles have no destructive effect on the heart and lungs [24]. However, Dianová, et al. have found that nanoparticles can reduce sperm motility and cause histopathological alterations in testicular epithelia together with infiltration of inflammatory cells in the epididymis [24]. The female reproductive system is also affected by nanoparticles, as they cause polycystic ovary syndrome and follicular atresia [24]. These findings suggest that the effects of nanoparticles on the heart and lungs may vary depending on the applied dosage. Further, the findings of Dianová, et al. have indicated that genital organs are susceptible to nanoparticles [24]. Fatemi and Noori have also demonstrated that exposure to silver nanoparticles during breastfeeding might have toxic effects on the infant’s liver [25]. Based on their findings, the exposure of mothers to nanoparticles can pass harmful effects to their babies through breastfeeding [25].
In studies conducted on fish and rabbits, it has been suggested that the destructive effects of nanoparticles on all organisms are very high [26]. Moreover, the data from earlier studies on serum samples and liver enzymes have shown a rise in the number of white blood cells and liver enzymes, indicative of damage to the liver tissue. These findings [27-33] are also consistent with those obtained by the current study. Other studies have emphasized that
pre-exposure to TiO2NPs at doses of up to 200 mg/kg can potentially promote the development of syndromes similar to alcoholic liver diseases.
Lastly, this study provides new insights into the evaluation of the safety of TiO2Nps [34]. However, treatments with silymarin and nano silymarin [34], melatonin [35], and Lactobacillus rhamnosus GG are capable of mitigating the effects of nanoparticles [36]. Based on the findings of the current study, it can be concluded that long-term exposure to titanium nanoparticles can have harmful effects on human and animal health.
Conclusions
This study has demonstrated that the continuous use of TiO2NPs can cause histopathological damage to the liver and disrupt the function of this vital organ.
Microscopic examinations of the liver samples from the groups treated with TiO2NPs revealed dilation of hepatic sinusoids, hepatocytes degeneration, cell necrosis, cellular swelling, cytoplasmic vacuolation, and hyper stainability of some hepatocytes. The ultrastructural sections from the third group (20 mg/kg TiO2NPs) were examined, and the following results were documented: Presence of points with high electron density, Swelling or disappearance of cristae, and endoplasmic reticula, Destruction of mitochondria, Presence of dense matrices in broken mitochondria, Formation of continuous cavum. Consequently, it is recommended the use of this ingredient be limited and controlled in industrial, sanitary, and cosmetic products.
Conflict of Interests
The authors have declared that there is no conflict of interest.
Funding
Ferdowsi University of Mashhad: Grant NO. 51713.
Acknowledgement
We thank the Research Deputy of Ferdowsi University of Mashhad for supporting this study financially.
Compliance with Ethical Guidelines
All procedures and protocols for this study were reviewed and approved by the Ethics Committee of Ferdowsi University, Mashhad. Mashhad, Iran (Approval code: IR.UM.REC.1401.089).
Authors' Contributions
Ahmadreza Raji played a pivotal role in the conceptualization, laying the foundation for the study. The methodology was a collective endeavor with fairly equal contributions from all authors. The formal data analysis and investigation phase also saw the active involvement of all authors, demonstrating a shared commitment to thorough examination and robust results. Rozbeh Choobchian took the lead in writing the original draft, bringing coherence and clarity to the research findings. The writing process, encompassing both the original draft and the subsequent review and editing, engaged the entire authorship, attesting to the collaborative nature of this scholarly work. Lastly, Ahmadreza Raji provided supervision, offering guidance and expertise throughout this research. All authors reviewed and approved the final version of the manuscript for publication in this journal.
References
- Vasantharaja D, Ramalingam V, Reddy GA. Oral toxic exposure of titanium dioxide nanoparticles on serum biochemical changes in adult male Wistar rats. Nanomedicine journal. 2015;2(1). [doi: 10.7508/nmj.2015.01.005]
- Hong F, Wang L, Yu X, Zhou Y, Hong J, Sheng L. Toxicological effect of TiO 2 nanoparticle-induced myocarditis in mice. Nanoscale research letters. 2015;10:1-11. [doi: 10.1186/s11671-015-1029-6] [pmid :26269254]
- Karshenas R, Noori A, Shahbazi F. Effect of copper oxide nanoparticles on toxicity, enzymatic changes and tissue structure of rat liver. Journal of Gorgan University of Medical Sciences. 2020;22(2):18-25. [Link]
- Sheydaei P, Bayrami A, Arbabi S. Evaluation the toxicity of Titanium dioxide nanoparticles on hematological and biochemical parameters in mice. Journal of Animal Research (Iranian Journal of Biology). 2019;31(4):396-404.[Link]
- Rezaei-Zarchi S, Taghavi-Foumani MH, Sheshdeh S, Negahdary M, Rahimi G. The effect of silver nano-particles on blood cells in rats. Journal of Biology. 2012;1(1):17-22. [Link]
- Faddah L, Abdel Baky N, Al-Rasheed NM, Al-Rasheed NM. Biochemical responses of nanosize titanium dioxide in the heart of rats following administration of idepenone and quercetin. Afr J Pharm Pharmacol. 2013;7(38):2639-51.[ doi:10.5897/AJPP2013.3426]
- Chang Y-N, Zhang M, Xia L, Zhang J, Xing G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials. 2012;5(12):2850-71.[ doi: 10.3390/ma5122850]
- Tamjid M, Mahmoudi F, Abdolmaleki A, Mirzaee S. Preparation of Omega-3 Coated Iron Oxide Nanoparticles and Their Effect on Hepatic, Renal, and Splenic Function in Rats: An Experimental Study. Journal of Rafsanjan University of Medical Sciences. 2021;20(8):879-90. [doi:10.52547/jrums.20.8.879]
- Hajirahimi A, Farokhi F, Tokmehchi A. Effects of Iron oxide and zinc nanoparticles on the liver and muscles in rainbow trout (Oncorhynchus mykiss). Journal of Animal Research (Iranian Journal of Biology). 2015;28(3):293-306. [Link]
- Mansouri A, Fathi M, Mansouri B, Azadi NA. Coexisting of titanium dioxide nanoparticles and diazinon on histopathology of common carp (Cyprinus carpio). Comparative Clinical Pathology. 2016;25:1227-36.[ doi:10.1007/s00580-016-2333-y]
- Thamaphat K, Limsuwan P, Ngotawornchai B. Phase characterization of TiO2 powder by XRD and TEM. Agriculture and Natural Resources. 2008;42(5):357-61. [Link]
- Rozhkova EA, Ulasov I, Lai B, Dimitrijevic NM, Lesniak MS, Rajh T. A high-performance nanobio photocatalyst for targeted brain cancer therapy. Nano letters. 2009;9(9):3337-42. [doi: 10.1021/
nl901610f] [pmid: 19640002]
- Dastani Z, Absalan Y, Gholizadeh M, Kovalchukova O. A composite of 2-aminoterephthalic acid coupled with TiF3@ TiO2/polyvinyl alcohol with enhanced visible-light photocatalytic reactivity; investigation of the photocatalytic mechanism. Journal of Materials Research and Technology. 2021;15:7158-73. [doi:10.1016/j.jmrt.2021.11.139]
- Pawar M, Topcu Sendoğdular S, Gouma P. A brief overview of TiO2 Photocatalyst for organic dye remediation: case study of reaction mechanisms involved in Ce‐TiO2 Photocatalysts system. Journal of Nanomaterials. 2018;2018(1):5953609. [doi:10.1155/2018/5953609]
- Hamzeh M, Sunahara GI. In vitro cytotoxicity and genotoxicity studies of titanium dioxide (TiO2) nanoparticles in Chinese hamster lung fibroblast cells. Toxicology in Vitro. 2013;27(2):864-73. [doi: 10.1016/j.tiv.2012.12.018] [pmid:23274916]
- Zucker R, Massaro E, Sanders K, Degn L, Boyes W. Detection of TiO2 nanoparticles in cells by flow cytometry. Cytometry Part A. 2010;77(7):677-85. [doi: 10.1002/cyto.a.20927] [pmid: 20564539]
- Yahyaei B, Abbasi S. Evaluation of accumulation and tissue effects of biological magnetic iron nanoparticles in response to the electromagnetic field by Inductively Coupled Plasma and histopathological methods in liver tissue of wistar rats. Cell and Tissue Journal. 2021;12(4):220-232. [doi: 10.52547/JCT.12.4.220]
- Jalili P, Krause B-C, Lanceleur R, Burel A, Jungnickel H, Lampen A, et al. Chronic effects of two rutile TiO2 nanomaterials in human intestinal and hepatic cell lines. Particle and Fibre Toxicology. 2022;19(1):37. [doi: 10.1186/s12989-022-00470-1] [pmid: 35578293]
- Domingo MG, Kurtz M, Maglione G, Martin M, Brites F, Tasat DR, Olmedo DG. Systemic effect of TiO2 micro‐and nanoparticles after acute exposure in a murine model. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2022;110(7):1563-72.[ doi:10.1002/jbm.b.35017]
- Shaltout E, Makboul R, Abdellah N, Ebrahem N. Possible Multi-Organ Toxicity in Rats after Chronic Oral Administration of Titanium Dioxide: Biochemical and Histopathological Study. The Egyptian Journal of Forensic Sciences and Applied Toxicology. 2022;22(2):127-37. [doi: 10.21608/ejfsat.2021.78014.1202]
- Sarikhani M, Vaghefi Moghaddam S, Firouzamandi M, Hejazy M, Rahimi B, Moeini H, Alizadeh E. Harnessing rat derived model cells to assess the toxicity of TiO2 nanoparticles. Journal of Materials Science: Materials in Medicine. 2022;33(5):41. [doi: 10.1007/s10856-022-06662-7] [pmid: 35507219]
- Baranowska-Wójcik E, Szwajgier D, Winiarska-Mieczan A. A review of research on the impact of E171/TiO2 NPs on the digestive tract. Journal of Trace Elements in Medicine and Biology. 2022;72:126988.[doi: 10.1016/j.jtemb.2022.126988] [pmid: 35561571]
- Wang S, Alenius H, El-Nezami H, Karisola P. A new look at the effects of engineered ZnO and TiO2 nanoparticles: evidence from transcriptomics studies. Nanomaterials. 2022;12(8):1247. [doi: 10.3390/nano12081247][pmid: 35457956]
- Rezaei Zarchi S. Effect of titanium dioxide nanoparticles on the amount of blood cells and liver enzymes in Wistar rats. Shahid Sadoughi University of medical Sciences Journals. 2011;19(5):
618-26. [Link]
- Fatemi M, Noori A. The effect of maternal exposure to silver nanoparticles during lactation on the liver of newborn rats. Shahid Sadoughi University of medical Sciences Journals. 2015;23(5):
440-51. [Link]
- Ostadhadi S, Bakhtiarian A, Azizi Y, Nikoui V. Respiratory and blood responses following intratracheal instillation of titanium dioxide nanoparticles in rabbits. Tehran University Medical Journal. 2013;71(1). [Link]
- Arbabi S, Bayrami A, Sheidaii P. An Investigation of the toxicity of zinc oxide and titanium oxide nanoparticles on some liver enzymes in male mice. Journal of Rafsanjan University of Medical Sciences. 2017;16(7):633-44. [Link]
- Hosseini S, Moshrefi A, Amani R, Razavimehr S, Aghajanikhah M. Biochemical and histopathological study of the toxicity of Zinc oxide nanoparticles on liver in Rat. 2016. [Link]
- Hamedi M, Fatahian Dehkordi RA, Heidarnejad MS, Mobini Dehkordi M. Effect of Zinc Oxide Nanoparticles on Inflammatory Factor TNF-β, Value of serum LDH and Tissue changes in the Mice Liver. Journal of Animal Research (Iranian Journal of Biology). 2016;29(1):37-45. [Link]
- Honarvar F, Vaezi G, Nourani M, Kamrani A, Sadeghnezhad E. Oxidant/Antioxidant index evaluation in the rat embryo induced by Nano-silver particle. New Cellular and Molecular Biotechnology Journal. 2016;6(23):53-60. [Link]
- Seyedalipour SB, Fattahi R, Khanbabaee R, Abdullahpour R. The effect of MgO nanoparticles on histopathological and biomarker changes of liver injuries (ALT, ALP, and AST) in pregnant NMRI mice. Journal of Advances in Medical and Biomedical Research. 2016;24(102):44-56. [Link]
- Mazaheri N, Karimi A, Salavati H, Rezaei Zarchi S, Khalilian S, Rezaei Ranjbar Sardari R. Investigating the effect of intraperitoneal injection of magnesium oxide nanoparticles on the liver and kidney function of rat in vivo. Shahid Sadoughi University of medical Sciences Journals. 2014;22(4):1430-8. [Link]
- Ghaedi S, Hassanpour-Ezatti M, Naji T, Rahmanifar MS. Comparison of tissue damages resulting from chronic administration of manganese dioxide nano-and microparticles on the liver, kidneys and testes of rats. Pathobiology Research. 2014;16(4):67-81. [Link]
- Liu S, Zhao Y, Liu Y, Tang Y, Xu X, Wang M, et al. Pre-exposure to TiO2-NPs aggravates alcohol-related liver injury by inducing intestinal barrier damage in mice. Toxicological Sciences. 2022;185(1):28-37. [doi: 10.1093/toxsci/kfab127][ pmid: 34718815]
- Rajaiah SG, Nadoor P, Rao S, Thimmaiah RP, Hanumanthu PB, Gowda L, et al. Sub-Chronic Exposure (90 Days) to Titanium Dioxide Nanoparticles (TiO2 NPs) Induce Alterations in Hematology and Serum Biochemistry in Male Wistar Rats: Effect of Exogenous Melatonin Supplementation. Letters in Applied NanoBioScience. 2022; 12(1):7. [doi: 10.33263/LIA
NBS121.007]
- Nie P, Wang M, Zhao Y, Liu S, Chen L, Xu H. Protective effect of Lactobacillus rhamnosus GG on TiO2 nanoparticles-induced oxidative stress damage in the liver of young rats. Nanomaterials. 2021;11(3):803. [doi: 10.3390/nano11030803] [pmid: 33801059]
Type of Study:
Research |
Subject:
Special









































 , Ahmadreza Raji *2
, Ahmadreza Raji *2 

 , Hossein Nourani3
, Hossein Nourani3 
 , Amir Moghadam Jafari1
, Amir Moghadam Jafari1 
 , Arezo Moghtadari Esfahani1
, Arezo Moghtadari Esfahani1 








