Introduction
After 12 months or more of consistent, unprotected sexual activity, the inability to achieve a clinical pregnancy is considered a disorder of the reproductive system known as infertility [1, 2]. This disorder affects 10%-15% of couples in their reproductive years across the globe [3]. Due to the strong correlation between sexuality, self-esteem, and the capacity to procreate, this reproductive illness exerts a marked impact on several aspects of a couple's lives [4, 5]. Even though almost 50% of all cases of childlessness worldwide are caused by male infertility, infertility is still primarily regarded as a social problem for women [6]. Diseases, psychological problems, or inherited or acquired situations that affect the normal function of reproductive organs can hinder a woman's ability to reproduce [4]. Polycystic ovarian syndrome (PCOS) is a prevalent condition that impairs the function of the female reproductive organs.
About 5%-20% of women who are of reproductive age suffer from PCOS, an endocrine and metabolic condition [7]. It is believed to be the most frequent cause of female infertility and chronic hyperandrogenic anovulation [8]. Obesity, insulin resistance, dyslipidemia, and type 2 diabetes mellitus are metabolic diseases linked to PCOS [7, 9]. Women with PCOS experience hormonal abnormalities, such as hyperandrogenism, elevated luteinizing hormone (LH), and hyperinsulinemia [9–11]. Hyperandrogenism is a significant factor in the diagnosis of PCOS essential to the onset and progression of this condition [7]. The development and progression of PCOS are significantly affected by hyperandrogenism, which is a significant requirement for PCOS diagnosis [12]. Furthermore, hyperandrogenism causes the release of insulin from pancreatic β-cells [11]. The ensuing hyperinsulinemia raises androgen and insulin-like growth factor (IGF)-1 bioavailability [13, 14]. To increase the production of androgens, LH has a more dramatic effect on theca cells in ovarian follicles when IGF-1 and insulin are present [15, 16]. In addition, high insulin and IGF-1 levels intensify the effects of LH on granulosa cells, leading to anovulation, cyst development, early differentiation, and follicle growth halt [17, 18].
The term "oxidative stress" refers to a breakdown in the equilibrium between antioxidants and oxidants, leading to an excessive production of free radicals and reactive oxygen species (ROS) at the cellular level. This can cause damage to DNA, ovarian tissue impairment, disruption of ovarian follicle development, and cell apoptosis [19, 20]. Elevated levels of inflammatory markers and serum lipid peroxidation [21-23], as well as macrophage infiltration in peripheral tissues, which results in tissue damage and compromised function, are frequently observed in PCOS patients. These factors are thought to be possible causes of PCOS and other reproductive disorders [24, 25]. It is essential to comprehend how inflammation and oxidative capability are interrelated in PCOS in order to recognize and treat this ovarian condition.
Numerous studies have examined the possible advantages of polyphenols in the treatment of reproductive diseases in recent years [26, 27]. Licorice, or Glycyrrhiza glabra, is a member of the Fabaceae family. Although this species is indigenous to the Mediterranean region, it is also found in China, India, and Iran [28]. Traditionally, licorice has been used in folk medicine and traditional medicines to treat inflammation, arthritis, dyspepsia, and gastrointestinal issues. The World Health Organization certifies the low toxicity of the aforementioned natural product by asserting that licorice is used as a demulcent in the treatment of sore throat and bronchial catarrh [29]. Proteins, amino acids, polysaccharides, simple sugars, mineral salts, tannins, phytoestrogens, phytosterols (sitosterol and stigmasterol), coumarins, and vitamins (B1, B2, B3, B5, E, and C) are all abundant in licorice [30], a few of which include antibacterial, antiviral, anti-inflammatory, and antioxidant. Licorice has also been demonstrated to possess hepatoprotective, antiproliferative, and anticancer qualities in addition to anti-ulcer effects in gastric mucosal injury, ulcerative colitis, and wounded tissue. There is also proof of other biological effects, such as skin lightening, skin depigmentation, antiaging, and cognitive improvement [31, 32]. In light of the aforementioned issues, the present study aimed to demonstrate how Glycyrrhiza glabra aqueous extract modulates hormones, inflammation, and antioxidants in response to Estradiol®induced in rats.
Materials and Methods
Chemicals
The supplier of Estradiol® was United Company for Drugs, Assuit, Egypt.
Plant materials
The Agricultural Research Center in Giza, Egypt provided the herbal licorice (Glycyrrhiza glabra L).
Preparation of Licorice (Glycyrrhiza glabra L) extract
Licorice (Glycyrrhiza glabra L) was prepared in accordance with the technique of Heshem et al. [33]. The procedure of aqueous extraction was completed. To put it briefly, 400 ml of distilled water was poured into a 100 ml round-bottom quick-fit flask containing 8 g of the powdered plant material. The mixture was filtered with qualitative Whatman filter paper No. 1 (Whatman International Ltd., Maidstone, England) after being left for 24 hours. The filtrates were put through a lypholyzation process using a freeze dryer (Snijders Scientific, Tilburg, Holland) in the Aroma and Flavoring Department of the National Research Center. The procedure was carried out at temperatures between -35°C and -41°C and pressures between 0.1 and 0.5 mbar. The dry extract was kept as quickly as possible at -20°C till additional research could be conducted.
Determination of total extract yield
After the mixed extracts were moved to a quick-fit round-bottom flask with a known weight (W1), they were freeze-dried and weighed once again (W2), and the yield was eventually computed using the formula below:
Extract yield (g/ g crude herb) = (W2 −W1)/W3
Where,
W1 is the weight of clear and dry quick-fit flask in grams,
W2 is the weight of the flask after lyophilization in grams
W3 is the weight of the crude powdered herb in grams
Determination of total phenolic content (TPC)
The estimated total phenolic component concentration of the extracts was determined according to Jayaprakasha et al. [34].
DPPH radical scavenging activity
The ability of Glycyrrhiza glabra L. aqueous extract's antioxidants to scavenge DPPH radicals was ascertained and previously reported by Nogala-Kalucka et al. [35]
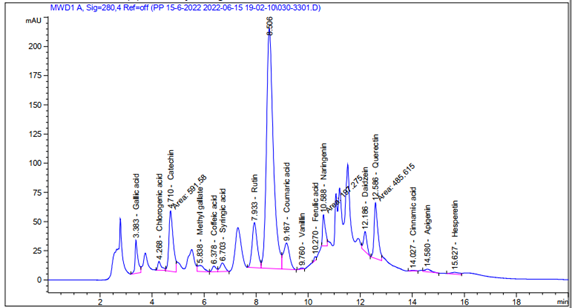
HPLC analysis of phenolic constituents
For the HPLC analysis, an Agilent 1260 series was used. Using a Kromasil C18 column (4.6 mm x 250 mm id, 5 μm), the separation was performed. One milliliter per minute of water (A) and 0.05% trifluoroacetic acid in acetonitrile (B) made up the mobile phase. The following was the sequential linear gradient programming for the mobile phase: 0 minutes (82% A), 0-5 minutes (80% A), 5-8 minutes (60% A), 8-12 minutes (60% A), 12-15 minutes (85% A), and 15-16 minutes (82% A). At 280 nm, the multi-wavelength detector was observed. For every sample solution, there was one injection volume of 10 microliters. The temperature of the column was kept constant at 35°C.
Animals
For the purpose of the study, 150-200 g adult female Wistar albino rats were acquired from the National Research Center's Animal Colony in Giza, Egypt. For acclimatization, the animals were kept in appropriate plastic cages for one week prior to the commencement of the experiment. EStandard rodent pellets and excess tap water were always available. Under the instructions of the Ethics Committee of Al-Azhar University Faculty of Science, all animals received care in accordance with the standard institutional norms for the care and use of experimental animals (Approved number AZHAR 18/2023).
Induction of Polycystic Ovary Syndrome (PCOS)
Separately made in sterile distilled water, the Estradiol® solution was administered once a day at a dose of 0.2 mg/kg of animal body weight, according to Shang et al [36][BE1] .
Experimental design
Following PCOS induction, animals in both the healthy and PCOS-modeled groups were randomly assigned to four groups, each with 10 animals, as illustrated in the following Table 1.
Table 1. Experimental animals design.
| Animals’ group |
Treatment |
| Group 1 |
For six weeks, saline was given daily to healthy animals as a control group |
| Group 2 |
For a comparable amount of time, healthy animals were given an oral dose of 100 mg/kg/day [37] of GAE |
| Group 3 |
Animals with untreated PCOS models [36] |
| Group 4 |
Animals with PCOS models that received GAE treatment (100 mg/kg/day) for a comparable amount of time. |
Blood and tissue sampling
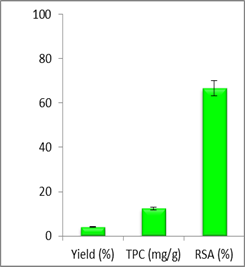 |
| Figure 1. The yield (%), total phenolic content and radical scavenging activity (%) of three replicates of aqueous extract of Glycyrrhiza glabra L dry powdered |
Following an overnight fast and weight measurement at the conclusion of the trial, blood samples (3 milliliters from each animal) were drawn under anesthesia, allowed to clot, and then cool-centrifuged. After the sera were separated, they were divided into aliquots and kept at -80°C until hormonal and biochemical testing. Following the collection of blood, the animals were quickly sacrificed by being abruptly beheaded. A portion of each animal's ovary was removed, cleaned in saline, dried, rolled in aluminum foil, and kept at -80°C for biochemical analyses. For microscopic inspection and histological processing, a different portion of the ovary was immersed in a formalin-saline (10%) buffer.
Hormonal profile
Rats' reagent ELISA kits (bought from Sunlong Biotech Co, Hang Zhou, China) were used to evaluate the hormonal profile (FSH, LH, PRL, testosterone, E2, and insulin).
Oxidative stress and antioxidant assay
Rat reagent ELISA kits from Sunlong Biotech Co., Hang Zhou, China, were used to quantify the serum levels of GSH, GPx, NO, MDA, CAT, and SOD.
Immunoinflammatory markers
Rat reagent ELISA kits (Sunlong Biotech Co., Hang Zhou, China) were used to assess the serum levels of TNF-α, IL-1β, IL-4, IL-6, and IL-10.
Biochemical determinations
Reagent kits from Biodiagnostic Co., Dokki, Giza, Egypt, were used to assess serum ASAT and ALAT activity, as well as urea, creatinine, total cholesterol, triglycerides, LDL-cholesterol, HDL-cholesterol, and glucose using spectrophotometry.
Histopathology
The histology preparations were performed in accordance with Ashry et al. [38]. Ovarian tissues were, in short, cut to a thickness of 3-4 mm, dehydrated in varying ethanol concentrations, cleaned in xylene, and stained with hematoxylin and eosin stain before the slices were inspected under a microscope.
Statistical analysis
Using statistical analysis system (SAS) program software, the acquired data were subjected to one-way ANOVA and then Duncan multiple post hoc tests at P≤0.05 [39]. Copyright (c) 1998 by SAS Institute Inc., Cary, NC, USA.
Results
Figures (1) illustrate the yield, total phenolic content, and radical scavenging activity (RSA) of the Glycyrrhiza glabra L aqueous extract (GAE). Using HPLC analysis, 16 phenolic compounds were demonstrated to be mostly present in GAE. High concentrations of Gallic acid, Catechin, Rutin, Querectin, and Naringenin were among the substances detected (Figure 2 and Table 2).
Table 2. Phenolic constituents of the aqueous extract of Glycyrrhiza glabra L using HPLC analysis
|
Area |
Conc. (µg/ml = µg/ 6.8mg) |
Conc. (µg/g) |
| Gallic acid |
211.77 |
17.34 |
1171.65 |
| Chlorogenic acid |
73.13 |
9.83 |
664.16 |
| Catechin |
591.58 |
129.51 |
8750.54 |
| Methyl gallate |
84.86 |
5.38 |
363.70 |
| Coffeic acid |
49.78 |
3.49 |
235.83 |
| Syringic acid |
94.75 |
7.51 |
507.52 |
| Pyro catechol |
0.00 |
0.00 |
0.00 |
| Rutin |
526.26 |
151.17 |
10214.49 |
| Ellagic acid |
0.00 |
0.00 |
0.00 |
| Coumaric acid |
365.48 |
9.44 |
637.75 |
| Vanillin |
8.02 |
0.26 |
17.40 |
| Ferulic acid |
17.34 |
1.03 |
69.83 |
| Naringenin |
197.27 |
14.44 |
975.80 |
| Daidzein |
151.89 |
9.41 |
635.68 |
| Querectin |
485.61 |
53.84 |
3637.94 |
| Cinnamic acid |
4.68 |
0.12 |
7.87 |
Figure 2. HPLC analysis of phenolic constituents Glycyrrhiza glabra L aqueous extract
Effect of GAE on hormonal assay
The in vivo data demonstrated a considerable decrease in progesterone and insulin levels and a large rise in FSH, LH, PRL, E2, and testosterone when comparing the PCOS group caused by Estradiol® to the control group. In comparison with PCOS rats, GAE therapy was observed to significantly increase progesterone and insulin and decrease levels of FSH, LH, PRL, E2, and testosterone, thereby increasing hormonal assay levels towards normal values (Table 3).
Table 3. Mean values of hormonal assay of control, Estradiol®-intoxicated and GAE-treated female albino rats.
| PCOS~GAE |
PCOS |
GAE |
Control |
|
| 0.20±0.04# |
0.77±0.09* |
0.14±0.01 |
0.14±0.01 |
FSH (mIU/ml) |
| 0.31±0.10# |
0.94±0.2* |
0.24±0.12 |
0.25±0.08 |
LH (mIU/ml) |
| 0.69±0.04# |
1.65±0.06* |
0.56±0.051 |
0.57±0.03 |
PRL (ng/ml) |
| 0.13±0.04# |
1.23±0.051* |
0.043±0.01 |
0.041±0.003 |
Testesteron (pg/ml) |
| 88.5±7.4# |
370.8±15.2* |
18.5±3.8 |
18.6±2.5 |
Estradiol (mIU/ml) |
| 5.56±1.2# |
0.6±0.07* |
7.16±1.2 |
7.15±0.88 |
Progesteron (mIU/ml) |
| 0.77±0.02# |
0.4±0.06* |
0.93±0.13 |
0.89±0.07 |
Insulin (ng/ml) |
Data are presented as mean ±SEM. Data were subjected to one way ANOVA followed by post hoc (Tukey) test at p≤ 0.05. * is significantly different from control group, while # is significantly different from PCOS (Polycystic Ovary Syndrome); GAE (Glycyrrhiza glabra L aqueous extract).
Effect of GAE on pro-inflammatory cytokines
Comparing the PCOS group to the control group, the data displayed a substantial increase in the levels of TNF-α, IL1β, IL-4, IL-6, and IL-10. When Estradiol® rats were given GAE, all inflammatory cytokines were shown to improve within normal levels. In particular, there was a significant decrease in TNF-α, IL1β, IL-4, IL-6, and IL-10 when compared to PCOS animals (Figure 3a-e).
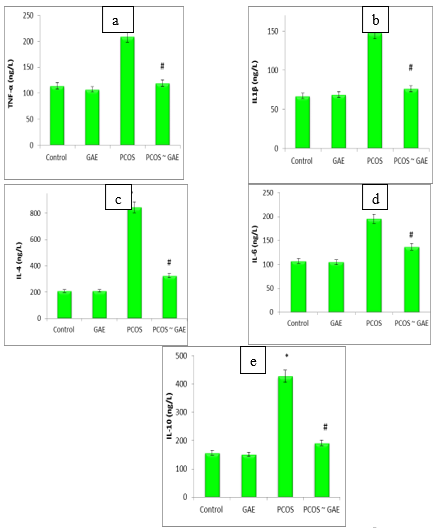 |
| Figure (3a – e). Serum TNF-α, IL1β, IL-4, IL-6, and IL-10 levels of control, Estradiol®-intoxicated and GAE-treated female albino rats. Data are presented as mean ±SEM. Data were subjected to one way ANOVA followed by post hoc (Tukey) test at p≤ 0.05. * is significantly different from control group, while # is significantly different from PCOS (Polycystic Ovary Syndrome); GAE (Glycyrrhiza glabra L aqueous extract). |
Effect of GAE on oxidant-antioxidant markers
As illustrated in Table 4, there is a considerable increase in blood MDA and NO levels together with a dramatic drop in GSH, CAT, SOD, and GPx activity during Estradiol® poisoning, indicating a severe worsening of the ovary oxidative stress status. When compared to the Estradiol®-intoxicated group, the GAE treatment of Estradiol®-intoxicated rats produced a positive shift in ovarian MDA and NO levels, as well as a noticeable increase in GSH, CAT, SOD, and GPx activities.
Table 4. Effect of Estradiol®and GAE on ovary MDA, NO, SOD, GPx, GSH and CAT
| PCOS~GAE |
PCOS |
GAE |
Control |
|
| 886±48.7# |
1230±65.3* |
701.7±29.4 |
702±40.6 |
MDA (pg/mL) |
| 10.2±0.28# |
21.7±2.3* |
8.0±0.40 |
8.22±0.40 |
NO (µmol/L) |
| 92.5±4.56# |
37.9±5.08* |
102.1±7.13 |
101.3±6.13 |
GSH (ng/mL) |
| 6.07±0.86# |
2.60±0.41* |
7.15±0.19 |
6.85±0.11 |
SOD (U/L) |
| 812.4±34.08# |
333.6±38.4* |
953.1±39.0 |
941.8±49.1 |
GPx (U/L) |
| 32.6±1.9# |
19.7±1.3* |
38.1±3.9 |
36.4±3.2 |
CAT (U/L) |
Data are presented as mean ±SEM. Data were subjected to one way ANOVA followed by post hoc (Tukey) test at p≤ 0.05. * is significantly different from control group, while # is significantly different from PCOS (Polycystic Ovary Syndrome); GAE (Glycyrrhiza glabra L aqueous extract).
Effect of GAE on liver, kidney, and lipid profile
When rats were given GAE alone, the results in Table 5 demonstrate that serum levels of ASAT, ALAT, urea, creatinine, cholesterol, triglycerides, HDL-c, and LDL-c were not affected. Nevertheless, when rats received an injection of estradiol®, there was a significant increase in serum levels of ASAT, ALAT, urea, creatinine, cholesterol, triglycerides, and LDL-c, along with a significant decrease in HDL-c, when both groups were compared to the corresponding values of the control group. Positively, co-ingesting GAE in accordance with Estradiol® injection greatly reduced the declines in the aforementioned parameters brought on by Estradiol®
Table 5. Effect of Estradiol®and GAE on liver enzymes, kidney function and lipid profile
| |
PCOS~GAE |
PCOS |
GAE |
Control |
|
| 78.9±3.7# |
146.3±16.6* |
53.7±8.4 |
54.4±4.2 |
ALAT (U/L) |
| 139.1±10.5# |
212±15.1* |
132.6±5.2 |
136.2±4.1 |
ASAT (U/L) |
| 37.7±2.46# |
51.75±1.13* |
33.2±2.43 |
33.5±2.07 |
Urea (mg/dl) |
| 0.69±0.09# |
1.37±0.05* |
0.51±0.05 |
0.52±0.06 |
Creatinine (mg/dl) |
| 167.3±6.5# |
235.2±10* |
154.6±17.6 |
157.2±13.5 |
Cholesterol (mg/dl) |
| 82.5±4.2# |
210±7.7* |
64.3±4.9 |
65.8±4.4 |
Triglycerides (mg/dl) |
| 43.2±1.58# |
34.2±0.91* |
46.5±1.2 |
46.2±1.3 |
HDL-C (mg/dl) |
| 107.6±5.1# |
159.4±7.4* |
95.2±4.1 |
97.8±3.6 |
LDL-C (mg/dl) |
|
|
|
|
|
|
Data are presented as mean ±SEM. Data were subjected to one way ANOVA followed by post hoc (Tukey) test at p≤ 0.05. * is significantly different from control group, while # is significantly different from PCOS (Polycystic Ovary Syndrome); GAE (Glycyrrhiza glabra L aqueous extract).
Histopathological examination
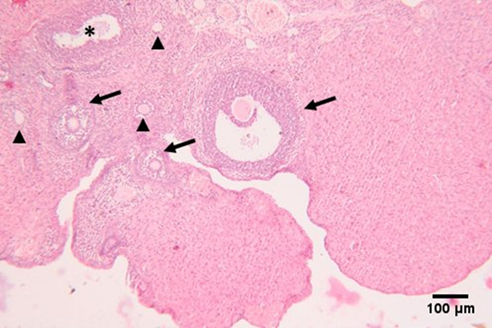
Under a light microscope, ovarian sections from the control group stained with hematoxylin and eosin revealed the ovary's typical histological structure complete with main and secondary follicles, as well as normal cortical and medullary regions (Figure 4). The results of the GAE groups were nearly identical to those of the standard control group (Figure 5). Large cystic ovarian follicles, ovarian follicles with a thin granulosa layer and cell debris within the follicular cavity, and atretic deteriorated follicles were all observed in the group of women with PCOS treated with Estradiol® (Figure 6). The histological structure of the ovaries, where different phases of ovarian follicles were noted, revealed a noteworthy improvement in both PCOS treated with GAE and estradiol-induced PCOS, with the GAE group showing an advantage that was almost equivalent to the control group (Figure 7).
 |
 |
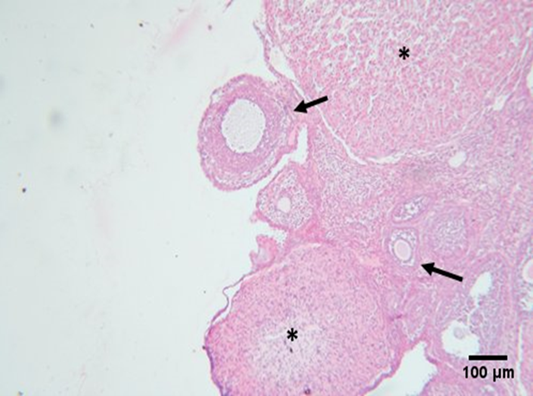
| Figure 4. Photomicrograph of an ovarian section of adult female albino rat of the control group showing normal histological structure of the ovary, consisting of outer cortex and inner medulla, primary follicles (arrow head), secondary follicles at different stages of growth including Graafian follicle (arrow), and atretic follicle (*). |
Figure 5. Photomicrograph of an ovarian section of adult female albino rat of the GAE showing regular histological architecture of the ovary, including growing secondary follicle (arrow), and corpus luteum. |
 |
 |
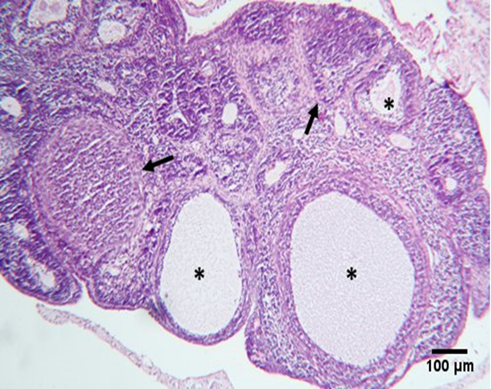
| Figure 6. Photomicrograph of an ovarian section of adult female albino rat of the Estradiol® induced PCOS group showing atypical histological structure of the ovary, including atretic degenerating follicles (arrow), and cystic follicles (*). |
Figure 7. Photomicrograph of an ovarian section of adult female albino rat of the Estradiol® induced PCOS~GAE treated group showing remarkable improvement of the histological structure of the ovary, including reduced atretic degenerating follicles, and improved growing follicles (arrow), and corpus luteum (*). |
Discussion
The prevalence of ovarian dysfunction and the ensuing infertility among PCOS women have been on the rise recently in both industrialized and developing countries [40]. Even among young couples, this has resulted in divorce, emotional stress, and socioeconomic pressure. The complex pathophysiology and uncertain etiology of PCOS are unsurprisingly linked to this. This underscores the need for additional research on the pathophysiological pathways and etiopathology of this disease in order to improve PCOS diagnosis, management, and treatment. Therefore, the purpose of the current investigation was to assess how Glycyrrhiza glabra L. aqueous extract affected various metabolic and reproductive problems linked to PCOS in female Wistar rats. As we previously described, PCOS can be caused by the reversible aromatase inhibitor Estradiol® [41]. In line with our earlier findings [42], a PCOS rat model with similar metabolic (overweight and dyslipidemia) and reproductive (hyperandrogenism, high LH and FSH levels, ovarian cysts, and infertility) disorders as observed in PCOS women was created by daily administration of a dose of 0.2 mg/kg of Estradiol® for 21 consecutive days. Estradiol® works by preventing the conversion of androgen to estrogens [42]. The pituitary gland releases excessive amounts of LH and FSH in response to excess androgens [43] and encourages weight gain, anovulation, ovarian cyst formation, and ovarian androgen production, either directly or via an insulin-mediated mechanism of action [44, 45]. It was also discovered that insulin inhibits the function of AMP-activated protein kinase (AMPK), or adenosine monophosphate-activated protein kinase [46]. The manufacture of fatty acids, or lipogenesis is encouraged by low AMPK activation [47] and high cholesterol [48].
Concerning the reproductive disorders linked to PCOS (hyperandrogenism, elevated serum levels of LH, obstruction of the estrous cycle during the diestrus phase, anovulation, ovarian cysts, and infertility), our findings demonstrated that the aqueous extract of GAE induced ovarian dynamics to resume the estrous cycle, reduced serum levels of LH and testosterone, and marginally elevated serum levels of estradiol. After PCOS-related symptoms, such as thickening of the theca layer, thinning of the granulosa layer of antral follicles, reduction of the number of antral follicles, and induction of follicular cysts were eliminated, the LH and FSH levels significantly decreased after treatment with GAE [49]. In particular, 18β-glycyrrhetinic acid and glycyrrhizic acid are thought to reduce inflammation via promoting immune activity and modifying proinflammatory cytokines [50]. In order for glycyrrhizic acid and 18β-glycyrrhetinic acid to activate the glucocorticoid receptor (GR) signaling by binding to the GR and blocking corticosteroid function, PI3K may be involved in this regulation [51]. The phytosterols stigmasterol and sitosterol have molecular similarities to animal cholesterol. More precisely, stigmasterol serves as a precursor in the synthesis of synthetic progesterone, whereas sitosterol is mostly recognized and utilized for its cholesterol-like characteristic. According to reports, the aforementioned phytosterols have a significant physiological role in the tissue-rebuilding and regulating processes associated with the actions of estrogen [51].
TNF-𝛼, IL-1β, IL-4, IL-6, and IL-10 levels were significantly elevated in the current investigation on PCOS caused by Estradiol®. These results are consistent with the findings of Olaniyi and Areloegbe et al. [40] and Dadkhah [41]. On the other hand, the results obtained indicated that pro-inflammatory cytokines (TNF-𝛼, IL-1β, IL-4, IL-6, and IL-10 levels) were decreased in rats with PCOS treated with GAE. These results conflict with the discovery of Bisht et al. [52] and Rizvi et al. [53]. PCOS is associated with abnormal androgen hormone levels, chronic inflammation, and dysfunction in endocrine metabolism [54]. Although the majority of research has been on the effects of hyperandrogenism on the pathological processes of PCOS, including follicular dysplasia and anovulation, inflammation is one of the key risk factors that contribute most to the pathophysiology of PCOS [55, 56]. Numerous investigations have demonstrated a correlation between PCOS and an increasing degree of inflammation in the ovarian follicles as well as a reduction in ovarian function [57, 58]. Ovarian follicle necrosis or apoptosis has been linked to TNF-𝛼, IL-1β, IL-4, IL-6, and IL-10 levels, which have been identified as key inflammatory response mediators [59]. Our PCOS experiment results also showed that oxidative stress, which is marked by high ROS production and antioxidant breakdown, is a key factor in the etiology of PCOS. Oxidative stress status and inflammatory response have been linked in preclinical animal models and PCOS-affected women [60, 61]. Furthermore, new research has shown how herbal remedies and complementary therapies can effectively reduce the symptoms of PCOS, especially when it comes to oxidative stress and inflammation [62, 63]. In this instance, we evaluated in a rat PCOS model the impact of GAE as a preventive agent against PCOS damage on these markers. Numerous studies have shown that GAE has significant potential as an anti-inflammatory and antioxidant factor because it contains phytochemical components (such as flavonoids and trans-anethole) that give this plant its therapeutic qualities [52].
In the current investigation, TNF-𝛼, IL-1β, IL-4, IL-6, and IL-10 levels were shown to be lowered in PCOS rats treated with GAE, as well as in the amount of these parameters in ovarian tissue. Furthermore, in PCOS rats, ovarian MDA levels rose while SOD and GPX activity dropped. As far as we are aware, no research has been released that looks at how GAE treatment affects oxidative stress biomarkers and inflammatory responses in PCOS rat ovarian tissue. Our findings are in line with reports of reduced inflammation and improved oxidant/antioxidant state in other organs following GAE therapy in rat models of other diseases [54]. Moreover, "Effects of Natural Products on PCOS: From Traditional Medicine to Modern Drug Discovery" describes the hepato-nephroprotective benefits of natural sources in PCOS [64].
Conclusions
In summary, the findings of this study demonstrated that dose-dependent GAE administration restored antioxidant enzymes in PCOS rats and decreased the generation of androgenic hormones, pro-inflammatory cytokines, and oxidative stress linked to PCOS. These results lend credence to the use of Glycyrrhiza glabra L as an adjuvant therapy for PCOS suppression.
Conflict of Interests
The authors declared no conflict of interest.
Funding
No funding
Acknowledgement
Not applicable.
Compliance with Ethical Guidelines
Faculty of Science, Al-Azhar University, Assuit; approved number (AZHAR, 23/2023)
The study was conducted according to the guidelines approved by the Ethics Committee of the Faculty of Science, Al-Azhar University, Assuit (AZHAR, 23/2023).
Authors' Contributions
Conceptualization: Mahmoud Ashry; Walaa A. Kandeel, Seham A. Farag and Hend M. Ali Methodology, Investigation, Writing-original draft; All authors; Supervision and Writing-review & editing Mahmoud Ashry; Walaa A. Kandeel, Seham A. Farag and Hend M. Ali.
References
- Borght MV and Wyns C. Fertility and infertility: definition and epidemiology. Clinical Biochemistry, 2018; 62: 2–10. [doi: 10.1016/j.clinbiochem.2018.03.012] [pmid:29555319]
- Szamatowicz M and Szamatowicz J. Proven and unproven methods for diagnosis and treatment of infertility. Advances in Medical Sciences, 2020; 65: 93–96. [doi: 10.1016/j.advms.2019.12.008] [pmid: 31923772]
- Tamrakar SR and Bastakoti R. Determinants of infertility in couples. Journal of Nepal Health research Council, 2019; 17: 85–89. [doi: 10.33314/jnhrc.1827] [pmid: 31110383]
- Starc A, Trampus M, Pavan Jukic D, Rotim C, Jukic T, and Polona Mivsek A. Infertility and sexual dysfunctions: a systematic literature review. Peer Reviewed General Medical Journal, 2019; 58: 508–515. [doi: 10.20471/acc.2019.58.03.15] [pmid: 31969764]
- Lei A, You H, Luo B and Ren J. The associations between infertility-related stress, family adaptability and family cohesion in infertile couples. Scienti1c Reports, 2021; 11: 24220. [doi: 10.1038/s41598-021-03715] [pmid: 34930989]
- Inhorn MC and Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Human Reproduction Update, 2015; 21(4): 411–426. [doi: 10.1093/humupd/dmv016] [pmid: 25801630]
- Ye W, Xie T, Song Y and Zhou L. The role of androgen and its related signals in PCOS. Journal of Cellular and Molecular Medicine, 2021; 25: 1825–1837. [doi: 10.1111/jcmm.16205] [pmid: 33369146]
- Palomba S, Piltonen TT, and Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Human Reproduction Update, 2021; 27(3): 584–618. [doi: 10.1093/humupd/dmaa051] [pmid: 33302299]
- Anagnostis P, Tarlatzis BC and Kau3man RP. Polycystic ovarian syndrome (PCOS): long-term metabolic consequences. Metabolism 2018; 86: 33–43. [doi:10.1016/j.metabol.2017.09.016] [pmid: 29024702]
- Azziz R, Carmina E, Chen Z et al. Polycystic ovary syndrome. Nature Reviews Disease Primers. 2016; 2: 160570. [doi: 10.1038/nrdp.2016.57] [pmid:27510637]
- Xu W, Morford J and Mauvais-Jarvis F. Emerging role of testosterone in pancreatic β cell function and insulin secretion. Journal of Endocrinology, 2019; JOE-18-0573.R1. [doi: 10.1530/JOE-18-0573] [pmid: 30601759]
- Chaudhari N, Dawalbhakta M and Nampoothiri L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reproductive Biology and Endocrinology, 2018; 16(1): 37. [doi: 10.1186/s12958-018-0354-x] [pmid: 29642911]
- Karakas SE. New biomarkers for diagnosis and management of polycystic ovary syndrome. International Journal of Clinical Chemistry, 2017; 471: 248–253. [doi:10.1016/j.cca.2017.06.009] [pmid: 28624501]
- Qu X and Donnelly R. Sex hormone-binding globulin (SHBG) as an early biomarker and therapeutic target in polycystic ovary syndrome. International Journal of Molecular Sciences, 2020; 21(21): 8191. [doi: 10.3390/ijms21218191] [pmid: 33139661]
- Cara JF and Rosenfeld RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal interstitial cells. Endocrinology, 1988; 123: 733–739. [doi: 10.1210/endo-123-2-733] [pmid: 2969325]
- Bergh C, Carlsson B, Olsson JH, Selleskog U and Hillensjo T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertility and Sterility, 1993; 59(2); 323–331. [doi: 10.1016/s0015-0282(16)55675-1] [pmid: 8425626]
- Allahbadia GN and Merchant R. Polycystic ovary syndrome and impact on health. Middle East Fertility Society Journal, 2011; 16(1): 19–37. [doi: 10.1016/j.mefs.2010.10.002]
- Dou L, Zheng Y, Li L et al. The effect of cinnamon on polycystic ovary syndrome in a mouse model. Reproductive Biology and Endocrinology, 2018; 16(1): 99. [doi: 10.1186/s12958-018-0418-y] [pmid: 30340496]
- Murri M, Luque-Ramirez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Human Reproduction Update. 2013;19(3):268–288. [doi: 10.1093/humupd/dms059] [pmid: 23303572]
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reproductive Biology and Endocrinology. 2012; 10: 49. [doi: 10.1186/1477-7827-10-49] [pmid: 22748101]
- Mancini A, Bruno C, Vergani E, d’Abate C, Giacchi E, Silvestrini A. Oxidative Stress and Low-Grade Inflammation in Polycystic Ovary Syndrome: Controversies and New Insights. International Journal of Molecular Sciences. 2021; 22(4):1667. [doi: 10.3390/ijms22041667] [pmid: 33562271]
- Ghowsi M, Khazali H, Sisakhtnezhad S. The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: An experimental study. International Journal of Reproductive Biomedicine. 2018; 16(3):149–58. [pmid: 29766146]
- Spritzer PM, Lecke SB, Satler F, Morsch DM. Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction. 2015;149(5): R219–27. [doi: 10.1530/REP-14-0435] [pmid: 25628442]
- Gonzalez F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. Journal of Clinical Endocrinology Metabolism. 2006; 91(1):336–40. [doi: 10.1210/jc.2005-1696] [pmid:16249279]
- Pruett JE, Everman SJ, Hoang NH, Salau F, Taylor LC, Edwards KS, et al. Mitochondrial function and oxidative stress in white adipose tissue in a rat model of PCOS: effect of SGLT2 inhibition. Biology of Sex Differences. 2022;13(1):45. [doi:10.1186/s13293-022-00455-x] [pmid:35986388]
- Zaychenko G, Stryga O, Sinitsyna O, Doroshenko A, Sulaieva O, Falalyeyeva T, et al. Resveratrol Effects on the Reproductive System in Ovariectomized Rats: Deciphering Possible Mechanisms. Molecules, 2022;27(15):4916. [doi: 10.3390/molecules27154916] [pmid: 35956866]
- Aitken RJ, Muscio L, Whiting S, Connaughton HS, Fraser BA, Nixon B, et al. Analysis of the effects of polyphenols on human spermatozoa reveals unexpected impacts on mitochondrial membrane potential, oxidative stress and DNA integrity; implications for assisted reproductive technology. Biochemical Pharmacology. 2016; 121:78–96. [doi: 10.1016/j.bcp.2016.09.015] [pmid: 27659810]
- Kao TC, Wu CH, Yen GC. Bioactivity and potential health benefits of licorice, Journal Agricultural and Food Chemistry. 2014; 62 (3): 542–553. [doi:10.1021/jf404939f] [pmid:24377378]
- Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MBPP. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review, Phytotherapy Research. 2018; 32 (12): 2323–2339. [doi: 10.1002/ptr.6178] [pmid: 30117204]
- Xiaoying W, Han Z, Yu W. 14 - Glycyrrhiza glabra (licorice): ethnobotany and health benefits, Sustained Energy for Enhanced Human Functions and Activity.2017; 231–250. [doi: 10.1016/B978-0-12-805413-0.00014-4]
- Wahab S et al. Glycyrrhiza glabra (licorice): a comprehensive review on its phytochemistry, biological activities, clinical evidence and toxicology, Plants. 2021; 10 (12): 2751. [doi: 10.3390/plants10122751] [pmid: 34961221]
- Mamedov NA and Egamberdieva D. Phytochemical constituents and pharmacological effects of licorice: a review, Plant and Human Health, Volume 3, 2019;3: 1–21. [doi: 10.1007/978-3-030-04408-4_1]
- Hesham A, Karami Z, Emam-Djomeh Z and Mirzaee H. Optimization of microwave assisted extraction (MAE) and soxhlet extraction of phenolic compound from licorice root, J. Science gov., 2017; (9):50-58.[BE2]
- Jayaprakasha GK and Rao LJ. Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.) Hale and their antioxidant activity. Journal of Biosciences. 2000; 55(11-12): 1018-1022. [doi:10.1515/znc-2000-11-1227] [pmid:11204179]
- Nogala-Kalucka M, Korczak J, Dratwia M, Lampart-Szczapa E, Siger A, et al. Buchowski M. Changes in antioxidant activity and free radical scavenging potential of rosemary extract and tocopherols in isolated rapeseed oil triacylglycerols during accelerated tests. Food Chemistry. 2005; 93(2):227–235. [doi: 10.1016/j.foodchem.2004.09.021]
- Shang H, Cao S, Wang J, Zheng H and Putheti R. Glabridin from Chinese herb licorice inhibits fatigue in mice, African Journal of Traditional, Complementary and Alternative Medicines. 2010; 7(1): 17–23. [doi: 10.4314/ajtcam.v7i1.57225] [pmid: 21304608]
- Shamsi M, Nejati V, Najafi Gh, Khajeh Pour S. “Protective effects of licorice extract on ovarian morphology, oocyte maturation, and embryo development in PCOS-induced mice: An experimental study, International Journal of Reproductive BioMedicine (IJRM). 2020; 18(10): 865–876. [doi:10.18502/ijrm. v13i10.7771]
- Ashry M, Askar H, Obiedallah MM, Elankily AH, Galal El-Sahra D, et al. Hormonal and inflammatory modulatory effects of hesperidin in hyperthyroidism-modeled rats. Frontiers in Immunology. 2023; 14:1087397. [doi:10.3389/fimmu.2023.1087397]
- Steel RG, Torrie GH: Principles and Procedures of Statistics: A Biometrical Approach. New York: McGraw‑ Hill. 1980; p 633. [link]
- Olaniyi KS and Areloegbe SE. Acetate ameliorates ovarian mitochondrial dysfunction in letrozole-induced polycystic ovarian syndrome rat model by improving mitofusin-2. The Journal of Physiological Sciences. 2024; 74(1):22. [doi: 10.1186/s12576-024-00908-5] [pmid: 38561673]
- Puspitasari, V.D., Lestari, E.S., Pramono, N. Propolis effects on dysbiosis of polycystic ovarian syndrome (PCOS) induced Wistar rats. Bali Medical Journal. 2024:13(1):924-929. [doi:10.15562/bmj. v13i1.5147]
- DadkhahM, Gholizadeh N, Azgomi RND, Hosseinzadeh S, Hamedeyazdan S, Haghighat K, Afshari S, Salimi M and Jazani AM. Therapeutic Effects of Pimpinella anisum Fruit Extract on Polycystic Ovary Syndrome in a Rat Model: Emerging Role of Inflammatory Responses and Oxidative Stress. Iran Journal of Pharmaceutical Research. 2024; 23(1): e143290. [doi: 10.5812/ijpr-143290] [pmid: 39005731]
- Saiyed A, Jahan N, Makbul SAA, Ansari M, Bano H and Habib SH. Effect of combination of Withania somnifera Dunal and Tribulus terrestris Linn on letrozole induced polycystic ovarian syndrome in rats, Integrative Medicine Research. 2016; 5(4): 293–300. [ doi: 10.1016/j.imr.2016.10.002] [ pmid: 28462131]
- Xu W, Morford J and Mauvais-Jarvis F. Emerging role of testosterone in pancreatic β cell function and insulin secretion. Journal of Endocrinology, 2019; R97–R105[BE3] . [doi: 10.1530/JOE-18-0573] [pmid: 30601759]
- Schiffer L, Arlt W and O’Reilly MW. Understanding the role of androgen action in female adipose tissue, Frontiers of Hormone Research, 2019; 53: 33–49. [doi: 10.1159/000494901] [pmid: 31499495]
- Valentine RJ, Coughlan KA, Ruderman NB and Saha K. Insulin inhibits AMPK activity and phosphorylates AMPK Ser485/491 through Akt in hepatocytes, myotubes and incubated rat skeletal muscle, Archives of Biochemistry and Biophysics, 2014; 562:62–69. [doi: 10.1016/j.abb.2014.08.013] [pmid: 25172224]
- Wang B, Cheng KKY. Hypothalamic AMPK as a mediator of hormonal regulation of energy balance. International Journal of Molecular Sciences. 2018; 19: 3552. [doi: 10.3390/ijms19113552] [pmid: 30423881]
- Loh K, Tam S, Murray-Segal L. et al. Inhibition of adenosine monophosphate-activated protein kinase-3-hydroxy-3-methylglutaryl coenzyme A reductase signaling leads to hypercholesterolemia and promotes hepatic steatosis and insulin resistance, Hepatology Communications. 2018; 3(1): 84–98. [doi: 10.1002/hep4.1279] [pmid: 30619997]
- Yang H, Kim HJ, Pyun BJ, Lee HW. Licorice ethanol extract improves symptoms of polycytic ovary syndrome in Letrozole-induced female rats, Integrative Medicine Research. 2018; 7(3): 264–270. [doi: 10.1016/j.imr.2018.05.003] [pmid: 30271715]
- Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus, Lancet. 2003;361(9374):2045–2046. [doi: 10.1016/S0140-6736(03)13615-X] [pmid: 12814717]
- Kao TC, Shyu MH and Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation, J Journal of Agricultural and Food Chemistry. 2010; 58 (15): 8623–8629. [ doi: 10.1021/jf101841r] [pmid: 20681651]
- Bisht D, Rashid M, Arya RKK, Kumar D, Chaudhary SK, Rana VS, Sethiya NK. Revisiting liquorice (Glycyrrhiza glabra L.) as anti-inflammatory, antivirals and immunomodulators: Potential pharmacological applications with mechanistic insight. Phytomedicine Plus 2022; 2(1): 100206. [doi: 10.1016/j.phyplu.2021.100206] [pmid: 35403088]
- Rizvi ZA, Babele P, Sadhu S, Madan U, Tripathy MR, Goswami S, Mani S, Kumar S, Awasthi A and Dikshit M. Prophylactic treatment of Glycyrrhiza glabra mitigates COVID-19 pathology through inhibition of pro-inflammatory cytokines in the hamster model and NETosis. Frontiers in Immunology. 2022; 13: 945583. [doi: 10.3389/fimmu.2022.945583] [pmid: 36238303]
- Goorani S, Zhaleh M, Zangeneh A, Koohi MK, Rashidi5 K, Moradi R and Zangeneh MM. The aqueous extract of Glycyrrhiza glabra effectively prevents induced gastroduodenal ulcers: experimental study on Wistar rats. Comparative Clinical Pathology. 2019; 28(1):339–347. [doi:10.1007/s00580-018-2852-9]
- De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reproductive Biology and Endocrinology . 2016;14 (1):38. [doi: 10.1186/s12958-016-0173-x] [pmid: 27423183]
- Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Human Reproduction Update. 2011 ; 17(1) :17–33. [doi: 10.1093/humupd/dmq032] [pmid: 20639519]
- Xiang Y, Wang H, Ding H, Xu T, Liu X, Huang Z, et al. Hyperandrogenism drives ovarian inflammation and pyroptosis: A possible pathogenesis of PCOS follicular dysplasia. International Immunopharmacoogyl. 2023; 125 (Pt A):111141. [doi: 10.1016/j.intimp.2023.111141] [pmid:37918087]
- Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM, et al. Chronic Low-Grade Inflammation in Pathogenesis of PCOS. International Journal of Molecular Sciences. 2021; 22 (7):3789. [doi: 10.3390/ijms22073789] [pmid: 33917519]
- Shabbir S, Khurram E, Moorthi VS, Eissa YTH, Kamal MA, Butler AE. The interplay between androgens and the immune response in polycystic ovary syndrome. Journal of Translational Medicine. 2023; 21(1):259. [doi: 10.1186/s12967-023-04116-4] [pmid: 37062827]
- Zuo T, Zhu M, Xu W. Roles of Oxidative Stress in Polycystic Ovary Syndrome and Cancers. Oxidative Medicine Cellular Longevity. 2016; 2016:8589318. [doi: 10.1155/2016/8589318] [pmid: 26770659]
- Sandhu JK, Waqar A, Jain A, Joseph C, Srivastava K, Ochuba O, et al. Oxidative Stress in Polycystic Ovarian Syndrome and the Effect of Antioxidant N-Acetylcysteine on Ovulation and Pregnancy Rate. Cureus. 2021 ; 13(9) : e17887. [doi: 10.7759/cureus.17887] [pmid: 34660086]
- Wang Z, Zhai D, Zhang D, Bai L, Yao R, Yu J, et al. Quercetin Decreases Insulin Resistance in a Polycystic Ovary Syndrome Rat Model by Improving Inflammatory Microenvironment. Reproductive Sciences. 2017; 24 (5):682–90. [doi:10.1177/1933719116667218] [pmid: 27634381]
- Zuo T, Zhu M, Xu W, Wang Z, Song H. Iridoids with Genipin Stem Nucleus Inhibit Lipopolysaccharide-Induced Inflammation and Oxidative Stress by Blocking the NF-kappaB Pathway in Polycystic Ovary Syndrome. Cellular Physiology Biochemistry. 2017; 43(5):1855–65. [doi: 10.1159/000484074] [pmid: 29049992]
- Jung W, Choi H, Kim J, Kim J, Kim W, Nurkolis F, et al. Effects of natural products on polycystic ovary syndrome: From traditional medicine to modern drug discovery. Heliyon. 2023; 9 (10). e20889. [doi: 10.1016/j.heliyon.2023.e20889] [pmid: 37867816]