BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijt.arakmu.ac.ir/article-1-1442-en.html
2- Department of Biology, Faculty of Mathematics and Natural Sciences, Sebelas Maret University, Surakarta, Indonesia ,
Introduction
Formalin
consists of 37-40% formaldehyde dissolved in water and 5-12% methanol as a
stabilizer
High formaldehyde concentrations induce cytotoxicity,
necrosis, and carcinogenic effects that cause inflammatory reactions, protein
denaturation, and increased free radicals. Formaldehyde and reactive oxygen
species (ROS) are involved in a mutually stimulating cycle with each other
Bidens
pilosa L. (B. pilosa L.) is an
herbaceous plant of the Asteraceae family. B. pilosa
L. plants contain 301 active compounds that include polyacetylene, phenolic
acids, terpenes, pheophytin, fatty acids, phytosterols, and flavonoids
Materials and Methods
Preparation of B. pilosa L. Leaf
Extract
Plant
material obtained from Ngoresan, Jebres,
Surakarta, then identified and authenticated at the Department of Biology, Sebelas Maret University, Indonesia (No.
159/UN27.9.6.4/Lab/2024). B. pilosa L. leaf
powder macerated with 70% ethanol (1:5, w/v) for 3×24 h. The macerate was
filtered and concentrated with a rotary evaporator at 55°C
Treatment of Animal Tests
All animal treatments approved by the Health Research
Ethics Committee, Faculty of Medicine, Universitas Muhammadiyah Surakarta,
Indonesia (4809/A.1/KEPK-FKUMS/VII/2023). A total of 25 male Wistar rats (2-3
months old, weighing 200 g) were divided into five treatment groups: control
group (Group P0), negative control (Group P1), B. pilosa
L leaf extract treatment orally with three dose variations of 25 mg/kg BW
(Group P21), 50 mg/kg BW (Group P22), and 100 mg/kg BW (Group P23). All
animals, except for those in Group P0, received per oral formalin at a dosage
of 0.2 ml/kg BW/day for 7 days. Subsequently, the B. pilosa
L leaf extract was given orally at 25 mg/kg BW (Group P21), 50 mg/kg BW (Group
P22), and 100 mg/kg BW (Group P23) for 7 days. The termination of the rats on
day 15 was conducted for renal collection. The kidneys were weighed, and
histological slides were prepared for examination.
Histology Analysis
Kidney histology was performed using the paraffin
technique with Haematoxcillin-Eosin (HE) staining.
The slides were observed at 100x and 400x magnification in five randomized
fields of view. The abnormal alterations/injuries counted included cell
atrophy/dilation, cell degeneration, cell inflammation/fibrosis, and cell
necrosis. The scoring system used was according to Table 1
|
Table 1. Kidney Profile Score |
|
|
Score |
Kidney
Profile Score |
|
1 |
Abnormal
cells <25% of the total visual field |
|
2 |
Abnormal cells 25≤50% of the total visual field |
|
3 |
|
|
4 |
Abnormal cells >75% of the total visual
field |
Molecular Docking
Molecular docking was performed on eight active
compounds that have been detected in B. Pilosa L.
Data Analysis
Quantitative
data were analyzed using the SPSS (version 25) software with a one-way ANOVA
test. If there was a significant difference between treatments, it was followed
by Least Significance Different (LSD) with a significance level of 5% (P=0.05).
Results
Effect of B. pilosa
L. Leaf Extract on Kidney Weight of Rats Due to Formalin Exposure
The
results related to kidney weight indicated that the kidney weight was not
significantly different between each treatment (Figure 1). This study revealed that administration of formalin
0.2 ml/kg BW/day for 7 days and B. pilosa L.
leaf extract did not significantly affect the kidney weight
in rats (P-value>0.05 in both kidneys).
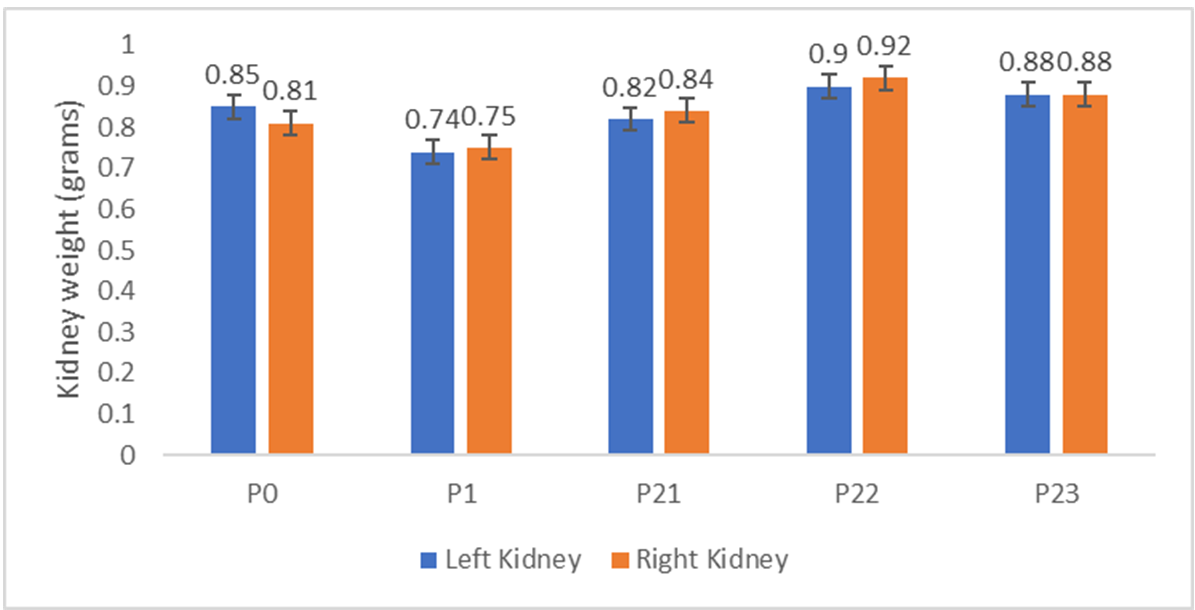
Figure 1. Comparison of the average weight of rat kidneys
after B. pilosa L. leaf extract treatment.
Blue represents the left kidney, while orange represents the right kidney
Effect of B. pilosa
L. Leaf Extract on the Histological Structure of Rat Kidney Due to Formalin
Exposure
Histological observations demonstrated cell
degeneration and necrosis in the kidney cells that received formalin.
Parenchymatous degeneration cells, hydropic
degeneration cells, dilated tubules, and necrosis cells were found in the
formalin treatment (Figure 2). The scoring
results of microscopic observation showed significant differences in the
treatments (Table 2). Formalin treatment in Group P1
increased the number of abnormal cells, as indicated by the high average score
of 2.8. Significantly decreased average scoring occurred in Group P23 or the
formalin group with B. pilosa L. extract with
a score of 1.2. In addition, the recovery effect of B. pilosa
L. extract induction is indicated in Figure 3 by decreasing the amount of
degenerated and necrotized cells.
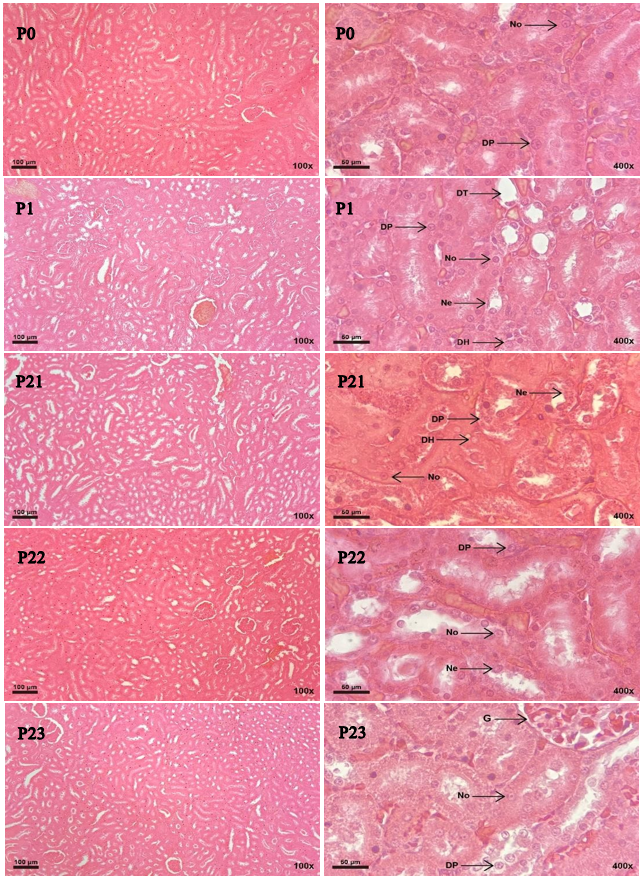
Figure 2. Histology of white rat kidney after treatment with HE stains at 100x and
400x magnification. G: Glomerulus, No: normal kidney cells, DP: parenchymatous
degeneration, DH: hydropic degeneration, DT: tubule dilatation, and Ne:
necrosis cells
|
Table 2. Scoring results of kidney cell
damage in each treatment. |
||
|
Group |
Mean Percentage of Abnormal Cells
(%) |
Mean Score |
|
P0 |
2.1±0.01 |
1±0.00a |
|
P1 |
56.5±0.16 |
2.8±0.45b |
|
P21 |
54±0.26 |
2.6±0.89bc |
|
P22 |
38±0.29 |
2.2±1.00c |
|
P23 |
20.5±0.26 |
1.2±0.89a |
|
Note: a, b, and bc superscripts of the same letter indicate no
significant difference from the LSD test results with a significance of
P<0.05. |
||
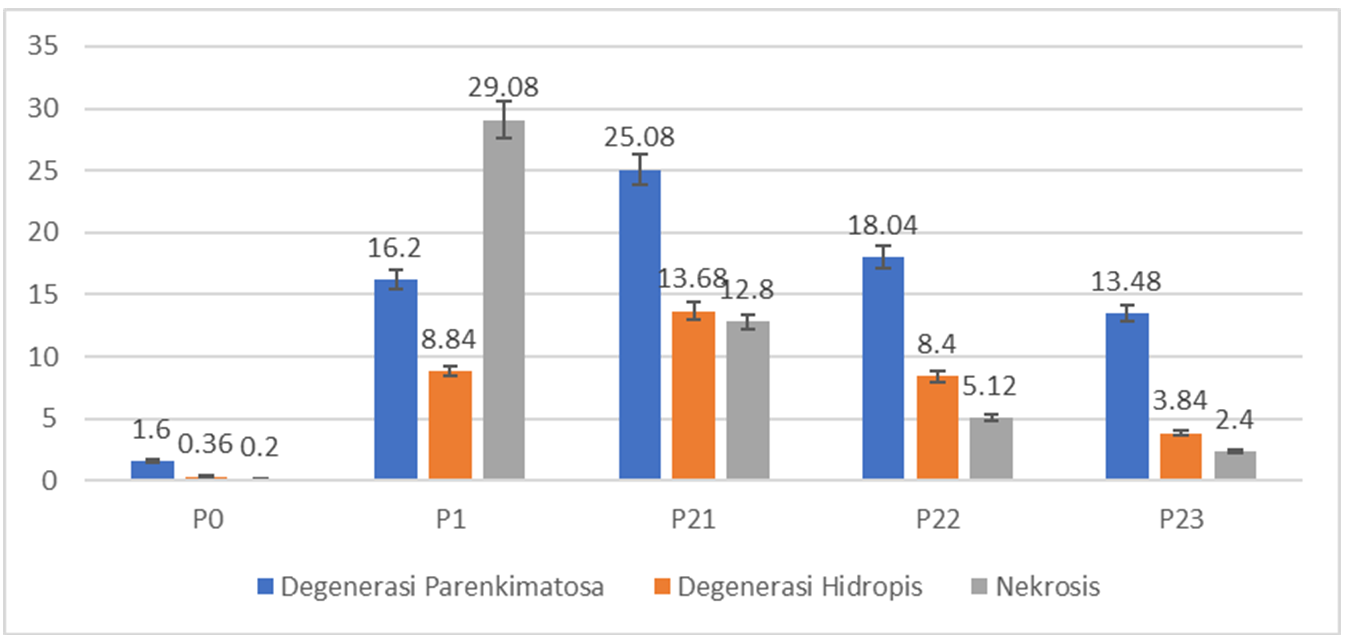
Figure 3. Comparison of the average number of abnormal cells in formalin-induced rat
kidneys after treatment with B. pilosa L. leaf
extract
Molecular Docking of B. pilosa
L. Secondary Metabolite Compounds
The
results of molecular docking demonstrated the interaction between compounds
contained in B. pilosa L. with Kelch-like
ECH-associated protein (KEAP1) so that these compounds had antioxidant activity
(Table 3).
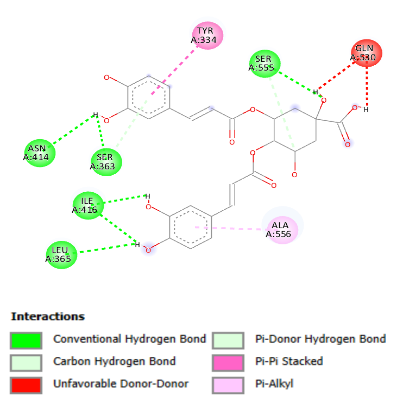
The compound with the strongest interaction with KEAP1 protein was isochlorogenic acid, followed by luteolin. Conventional
hydrogen bonds involved in the interaction are found at Ser 555, Ser 363, Asn 414, Ile 416, and Leu 365 (Figure 4A).
Compounds in B. pilosa
L. also had the potential for anti-inflammatory response. It was observed that these
compounds have the ability to interact with TNF-α, thereby inhibiting the
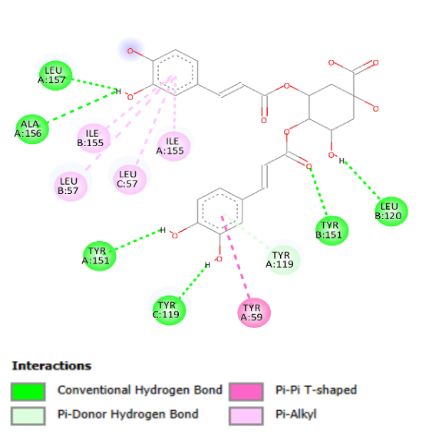
activity of this protein. The isochlorogenic acid
compound had the strongest bond with a binding affinity value of -8.9 kcal/mol
(Table 3), involving hydrogen bonds with
amino acid residues Leu157, Ala 156, and Tyr 151 in chain A, Leu 120 and Tyr
151 in chain B, as well as Tyr 199 in chain C (Figure 4B).
|
Table 3. Binding
affinity values of compounds with target proteins |
||
|
Compound |
Binding Affinity (Kcal/mol) |
|
|
KEAP1 |
TNF-α |
|
|
Native
ligan |
-11.9 |
-12.1 |
|
Isoclorogenic acid |
-9.9 |
-8.9 |
|
Luteolin |
-8.9 |
-8 |
|
Chlorogenic
acid |
-8.7 |
-8.1 |
|
Quercetin |
-8.3 |
-7.7 |
|
Caffeic
acid |
-6.4 |
-7 |
|
Ferulic
acid |
-6.1 |
-7.3 |
|
P-coumaric
acid |
-5.9 |
-7.3 |
|
Gallic
acid |
-6.1 |
-6.2 |
|
|
|
|
Figure 4. Interaction
of isochlorogenic acid compounds on (A) KEAP1 and
(B) TNF-α. |
|
Discussion
Formaldehyde
is a toxicant to the urinary system, including the kidneys [12]. Intraperitoneal exposure to
formalin 10 mg/kg BW for 14 days indicated a significant decrease in kidney
weight
Histological
kidney damage showed that the formalin-induced group had a higher damage score
than the other groups. The damage included parenchymatous degeneration cells,
hydropic degeneration, tubular dilatation, and necrosis. This result is
consistent with the research conducted by George et al. (2017), who found
tubular dilatation and hydropic degeneration of renal epithelial tubular cells
exposed to formaldehyde
Kidney
histology damage that occurs in formaldehyde-induced groups is a form of cell
defense response to formaldehyde exposure and metabolism. Mechanisms exist in
healthy cells to rigorously maintain formaldehyde homeostasis through
S-adenosylmethionine biosynthesis and one-carbon metabolism
Formic
acid has inhibited mitochondrial cytochrome c oxidase, thereby reducing ATP
synthesis. Acidosis due to formic acid can increase the formation of superoxide
anions and hydroxyl radicals, which results in membrane damage, lipid
peroxidation, and mitochondrial damage. The decrease in pH also allows calcium
to be influx into the mitochondria, which causes mitochondrial dysfunction and
cell death
The
components of B. pilosa L. that are extracted
with 70% ethanol maceration include flavonoids (e.g., luteolin and quercetin),
aromatics (e.g., gallic acid), and phenylpropanoids (e.g., p-coumaric acid,
ferulic acid, caffeic acid, chlorogenic acid, and isochlorogenic
acid)
Conclusions
In
conclusion, oral administration of B. pilosa
L. leaf extract, at the most optimal dose of 100 mg/kg BW, reduced the damage
of rat kidney cells caused by formalin exposure. Isochlorogenic
acid and luteolin are two secondary metabolite compounds from B. pilosa L. that potentially could turn on NRF2 signaling
for antioxidant expression and stop inflammation by blocking NF-κB signaling.
Conflict of Interests
The authors declare no conflicts of
interest.
Funding
The research was funded by
Universitas Sebelas Maret (No. 194,2/UN27,22/PT.01.03/2024).
Acknowledgement
This study supported by
Universitas Sebelas Maret.
Compliance with Ethical Guidelines
Compliance with ethical guidelines: All animal
treatments approved by the Health Research Ethics Committee, Faculty of
Medicine, Universitas Muhammadiyah Surakarta (4809/A.1/KEPK-FKUMS/VII/2023).
Authors' Contributions
MFA methodology, data analysis and interpretation
of results. OPA and WMR article structuring and writing. OPA revision and
supervision. MFA and SL analysis and scoring of the kidney damages. All authors
have read and approved the manuscript prior to submission for publication.
References
1.
Pandey CK, Agarwal A, Baronia A, Singh N. Toxicity of ingested
formalin and its management. Hum Exp Toxicol. 2000;19(6):360-366.
[doi:10.1191/096032700678815954] [pmid: 10962510]
2.
Rahman MB, Hussain M, Kabiraz MP, Nordin
N, Siddiqui SA, Bhowmik S, et al. An update on formaldehyde adulteration in
food: sources, detection, mechanisms, and risk assessment. Food Chem. 2023; 427:136761.
[doi: 10.1016/j.foodchem.2023.136761]
[pmid: 37406446]
3.
Ungureanu LB, Ghiciuc CM, Amalinei C, Ungureanu C, Petrovici CG, Stănescu
RȘ. Antioxidants as protection against reactive oxygen stress induced by
formaldehyde (FA) exposure: A systematic review. Biomedicines. 2024;12(8):1820.
[doi:10.3390/biomedicines12081820] [pmid: 39200284]
4.
Reingruber H, Pontel LB. Formaldehyde metabolism and its impact on
human health. Curr Opin Toxicol.
2018; 9:28-34. [doi:10.1016/j.cotox.2018.07.001]
5.
Dorokhov YL, Shindyapina AV, Sheshukova EV, Komarova TV. Metabolic methanol: molecular
pathways and physiological roles. Physiol Rev. 2015;95(2):603-644.
[doi: 10.1152/physrev.00034.2014] [pmid: 25834233]
6.
Aydemir S, Akgun SG, Beceren A, et al.
Melatonin ameliorates oxidative DNA damage and protects against
formaldehyde-induced oxidative stress in rats. Int J Clin Exp Med. 2017;10(4):6250-6261.
[Link]
7.
Xuan TD, Khanh TD. Chemistry and pharmacology of Bidens pilosa: an overview. J Pharm Investig.
2016;46(2):91-132. [doi: 10.1007/s40005-016-0231-6]
[pmid: 32226639]
8.
Bartolome AP, Villaseñor IM, Yang WC. Bidens pilosa L. (Asteraceae): Botanical properties, traditional
uses, phytochemistry, and pharmacology. Evid Based Complement Alternat Med.
2013; 2013:340215. [doi: 10.1155/2013/340215] [pmid: 23935661]
9.
Pegoraro CMR, Nai GA, Garcia LA, Serra FM, Alves JA, Chagas PHN,
et al. Protective effects of Bidens pilosa on
hepatoxicity and nephrotoxicity induced by carbon tetrachloride in rats. Drug
Chem Toxicol. 2021;44(1):64-74. [doi:
10.1080/01480545.2018.1526182] [pmid: 30394117]
10.
Falowo AB, Muchenje V, Hugo CJ, Charimba G. In vitro antimicrobial activities of Bidens pilosa and Moringa oleifera leaf extracts and their effects
on ground beef quality during cold storage. CyTA
- J Food. 2016;14(4):541-546. [doi:10.1080/19476337.2016.1162847]
11.
Hao X, Luan J, Jiao C, Ma C, Feng Z, Zhu L, et al.
LNA-anti-miR-150 alleviates renal interstitial fibrosis by reducing
pro-inflammatory M1/M2 macrophage polarization. Front Immunol. 2022;
13:913007. [doi:10.3389/fimmu.2022.913007] [pmid: 35990680]
12.
Inci M, Zararsız I, Davarci M, Gorur S. Toxic effects
of formaldehyde on the urinary system. Türk J Urol. 2013;39(1):48-52. [doi: 10.5152/tud.2013.010] [pmid: 26328078]
13.
Abdel Hamid W, ElMoslemany A, Zeima N. The potential protective effects of aqueous
extracts of some herbs on renal toxicity induced by formaldehyde in
experimental rats. Bulletin of the National Nutrition Institute of the Arab
Republic of Egypt. 2022;60(2):1-31. [doi:10.21608/bnni.2022.255461]
14.
Shoyaib AA, Archie SR, Karamyan
VT. Intraperitoneal route of drug administration: should it be used in
experimental animal studies? Pharm Res. 2020;37(1):12. [doi:10.1007/s11095-019-2745-x] [pmid: 31873819]
15.
George S, Yassa H, Hussein H, El Refaiy
A. Protective effect of L- carnitine against formaldehyde-induced kidney, liver
and testicular damage in rabbits, a histopathological study. Mansoura J
Forensic Med Clin Toxicol. 2017;25(2):13-24. [doi:10.21608/mjfmct.2018.47239]
16.
Pham VN, Bruemmer KJ, Toh JDW, Ge EJ, Tenney L, Ward CC, et al.
Formaldehyde regulates S -adenosylmethionine biosynthesis and one-carbon
metabolism. Science. 2023;382(6670): eabp9201. [doi: 10.1126/science.abp9201] [pmid: 37917677]
17.
Nakamura J, Holley DW, Kawamoto T, Bultman SJ. The failure of two
major formaldehyde catabolism enzymes (ADH5 and ALDH2) leads to partial
synthetic lethality in C57BL/6 mice. Genes Environ. 2020;42(1):21. [doi: 10.1186/s41021-020-00160-4]
[pmid: 32514323]
18.
Umansky C, Morellato AE, Rieckher M, Scheidegger MA, Martinefski
MR, Fernández GA, et al. Endogenous formaldehyde scavenges cellular
glutathione resulting in redox disruption and cytotoxicity. Nat Commun. 2022;13(1):745.
[doi:10.1038/s41467-022-28242-7] [pmid: 35136057]
19.
Kou Y, Zhao H, Cui D, Han H, Tong Z. Formaldehyde toxicity in
age-related neurological dementia. Ageing Res Rev. 2022; 73:101512. [doi: 10.1016/j.arr.2021.101512]
[pmid: 34798299]
20.
Liesivuori J, Savolainen H. Methanol and
formic acid toxicity: biochemical mechanisms. Pharmacol
Toxicol. 1991;69(3):157-163. [doi:
10.1111/j.1600-0773.1991.tb01290.x] [pmid: 1665561]
21.
Pietzke M, Meiser J, Vazquez A. Formate metabolism in health and disease. Mol Metab. 2020; 33:23-37. [doi: 10.1016/j.molmet.2019.05.012] [pmid: 31402327]
22.
Miller MA, Zachary JF. Mechanisms and morphology of cellular
injury, adaptation, and death. Pathologic Basis of Veterinary Disease.
Elsevier; 2017: 2-43.e19. [doi: 10.1016/B978-0-323-35775-3.00001-1]
23.
Ilyas S, Hutahaean S, Elimasni, Ar-roisyi DK. Effect of
turmeric rhizome extract (Curcuma longa L.) on liver histology of
preeclampsia rat (Rattus norvegicus L.). IOP Conf Ser Earth Environ Sci.
2019;305(1):012077. [doi:10.1088/1755-1315/305/1/012077]
24.
Silvia R, Wahyuni WT, Rohaeti E, Aisyah S, Anggraini Septaningsih
D, Hudatul
Karomah A, et al. LC-HRMS-based metabolomics approach reveals antioxidant
compounds from Centella asiatica leaves extracts.
Indonesian Jo Chem. 2024;24(6):1861. [doi:10.22146/ijc.90782]
25.
Crisman E, Duarte P, Dauden E, Cuadrado
A, Rodríguez-Franco MI, López MG, et al. KEAP1‐NRF2
protein–protein interaction inhibitors: Design, pharmacological properties and
therapeutic potential. Med Res Rev. 2023;43(1):237-287. [doi:10.1002/med.21925] [pmid: 36086898]
26.
Rasmi RR, Sakthivel KM, Guruvayoorappan
C. NF-κB inhibitors in treatment and prevention
of lung cancer. Biomed Pharmacother. 2020; 130:110569.
[doi:
10.1016/j.biopha.2020.110569] [pmid:32750649]
27.
Alsawaf S, Alnuaimi
F, Afzal S, Thomas RM, Chelakkot AL, Ramadan WS, et
al. Plant flavonoids on oxidative stress-mediated kidney inflammation. Biology
(Basel). 2022;11(12):1717. [doi:10.3390/biology11121717] [pmid; 36552226]
28.
Papi S, Ahmadizar F, Hasanvand
A. The role of nitric oxide in inflammation and oxidative stress. Immunopathologia Persa. 2019;5(1): e08. [doi:10.15171/ipp.2019.08]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |