







































BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijt.arakmu.ac.ir/article-1-1446-en.html
2- Renewable Energies and Environment Department, Faculty of New Sciences and Technologies, university of Tehran, Tehran, Iran
3- Department of Mechanical Engineering, Ayatollah Boroujerdi University ,
Introduction
Desulfurization of various fuel oils, such as gasoline, kerosene, jet fuels, and diesel, is valuable in industrial oil and transportation. Sulfur trioxide gas and sulfur dioxide are produced from the reaction of sulfur in the combustion process. The sulfur dioxide exposure to oxygen in the air can lead to sulfuric acid formation, and thus, acid rain or acid deposition. Sulfur oxides have a different impact on the environment and health. For instance, some respiratory ailments, such as lung infection, bronchitis, asthma, etc. Sulfur oxide compounds detriment catalytic converters in vehicles, which are responsible for volatile organic compounds, CO, and NO oxidation reactions [1,2]. One of the main practical actions to inhibit sulfur emission is to follow strict environmental regulations to reduce sulfur content to less than 10 ppm in China, the European :union:, Japan, and the United States. Furthermore, the sulfur content of transportation should be decreased to 10-15 ppm according to environmental regulations [3]. The technology of desulfurization is needed to eliminate sulfur content in fossil fuels, achieve clean fuel, and reduce environmental pollution. Desulfurization is a method to reduce sulfur content to an acceptable value. Desulfurization was classified as a pre-combustion and post-combustion process to remove sulfur before and after the fuel burning, respectively. The conventional method of desulfurization is Hydrodesulfurization (HDS); however, this method has some drawbacks. This method requires high consumption of energy due to operating at high pressure and high temperature (3 MPa to 6 MPa and 300°C to 440°C). Moreover, this technology requires a high amount of hydrogen, which leads to high expensive costs. Moreover, some components, such as BT, DBT, and thiophenes, have low reactivity in the HDS process. Therefore, studies have been conducted on the use of new technologies instead of HDS. One of the main technologies is the oxidative desulfurization process (ODS), which is promising due to its operation at ambient temperature and pressure. Moreover, this process has high potential in the removal of aromatic sulfur compounds that are not reactive in HDS [4-6]. The ODS process includes two steps: first, the conversion of sulfur compounds to sulfones, and second, the separation of sulfones and sulfoxides from fuel. Usually, liquid-liquid extraction is applied to the second step [7]. On the other hand, ultra-sound assisted oxidative desulfurization (UAOD) as an improved ODS method has been of interest in recent years. Ultrasound as an ODS assists the process in enhancing the component reactivity via cavitation by increasing the mass transfer process and fast oxidation reactions. This action leads to high affective desulfurization of fuel [8, 9]. Several studies investigated the UAOD process in different catalysts [10], phase-transfer agents (PTA) [11], oxidants [5], extractants, and adsorbents [12] and also investigations of the influential variables, such as temperature, pressure, ultrasonic [13] and economic analysis [14], have been considered. The amount of H2O2 as an oxidizer and the type of catalyst is essential in the UAOD process [15-17]. Khodaei et al. [13] investigated different operating parameters, including formic acid-to-sulfur molar ratio (nacid/ns), ultrasound power, oxidant-to-sulfur molar ratio (no/ns), temperature, and time in the UAOD process for nonhydrotreated kerosene. They used Response surface methodology based on Box Behnken design. The best result was obtained at 95.46% sulfur removal at the time of 10.5 min, nacid/ns = 107.8, no/ns = 15.02, and ultrasound power of 7.6 W/mL. In another work, Khodaei et al. [18] used the UAOD process kerosene by using the direct probe sonicator reactor. They investigated the effect of different operating parameters such as sonication, time, ultrasonic power, and pressure. The Box–Behnken design was employed by response surface methodology (RSM) to evaluate optimum conditions. The results showed sulfur removal 96%at pressure=0.03barg, power 390 W, and 22 min sonication time. Dana et al. [19] applied UAOD process for desulfurization of diesel using H2O2 and formic acid. They studied the effect of oxidant to sulfur molar ratio (5–35), formic acid to sulfur molar ratio (10–150), and residence time (2–24 min) for removal of sulfur by RSM. By considering the cost of chemical and electrical energy consumption in the continuous-flow oxidation stage, the optimum operating condition was obtained at 16 min of residence time, 54.47 of formic acid to sulfur molar ratio, and 8.24 of oxidant to sulfur molar ratio that lead to sulfur removal of 86.90%. Jima et al. investigated [20] heavy naphtha desulphurization by UAOD. They used from H2O2 as peroxide and acetic acid as catalyst. After the oxidation reaction, they used adsorption process by activated carbon as adsorbent. The results show Sulphur removal 89% under the normal condition. In this study desulfurization investigated by several parameters such as hydrogen peroxide, acetic acid, and activated carbon. The best oxidants content was 10 ml hydrogen peroxide100 ml of heavy naphtha. Also, the best sulfur removal was obtained 7.5 ml acid per 10 ml
oxidant. Carnaroglio et al. [21] applied UAOD
process with dibenzotiophene (DBT) and 4,6-dimethyldibenzothiophene (4,6-DMDBT) as fuel oil model. They are used from potassium superoxide, sodium persulfate, and Oxone®. Oxone® showed higher efficiency at 30 min sonication. The results showed a reduction of sulfur content to less than 10 ppm. Barilla et al. [22] synthesized an activated carbon-supported phosphotungstic acid catalyst for mixing assisted oxidative desulfurization (MAOD). They use a simulated diesel including 2.3 wt.% S of dibenzothiophene and benzothiophene in real fuel oil. The effect of different parameters such as mixing time, speed, and temperature was evaluated for oxidation reaction. The result showed the best variables at mixing time of 88.5 min, speed of 16,800 rpm, and temperature of 63.28◦C for oxidation of 62.37%.
Considering the importance of using the desulfurization process in removing sulfur from petroleum-based compounds, there is a need to manage the consumption of materials such as oxidizers and catalysts. Therefore, the aim of this study is to investigate the ultrasonic ability in sulfur removal from commercial naphtha with a sulfur content of 2000 ppm, so that the lowest oxidizer and catalyst can be used to achieve the best sulfur removal. In this regard, different catalysts, including homogeneous catalysts at various loadings, were used.
Materials and Methods
Materials
Phosphotungstic acid (H3PW12O40: 99.9 % purity), Ferrous ion (FeSO4), hydrogen peroxide (H2O2: 35 vol%) were purchased from Merck Inc. Acetic acid and acid formic 90 wt.% was bought from Merck Inc. N-methyl pyrrolidone were procured from Sigma-Aldrich. The naphtha was used as fuel from a commercially available fuel.
Experimental Method
The experiments were performed by preparing commercial naphtha as feed under ultrasonic irradiation in a jacket-type glass reactor (Figure 1). The feed specifications are shown in Table 1. The reactor was equipped with a circular water bath through the reactor jacket to maintain a constant temperature due to the nature of the exothermic reaction, along with a thermometer to display the temperature. The model used for the UAOD was UTD 400 from the Ultrasound Technology Development Company, Iran. All reactions were conducted at a frequency of 20 kHz. Initially, 50 ml of naphtha was poured into the reactor so that 1 cm of the probe remained immersed in the naphtha. The fuel was heated to 40 °C, and then the catalyst and oxidizer were added to the reactor drop by drop. The prepared samples are shown in Table 2.
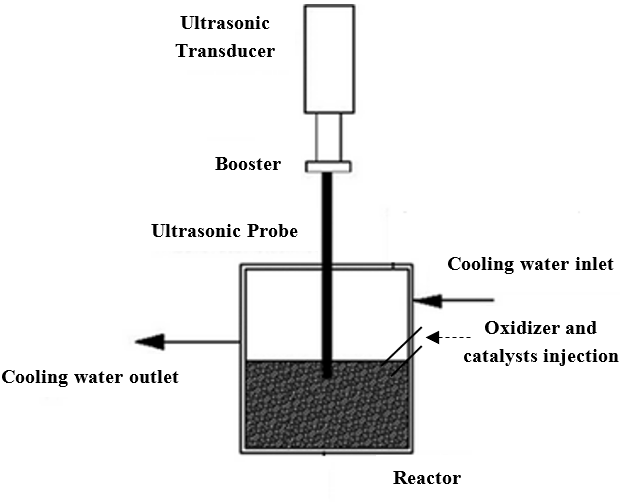
Figure 1. Schematic of the ultrasonic system used in this study.
Table 1. Used feed specification
|
Row |
Property |
Unit |
Test Method |
Specification |
Typical value |
|
1 |
Density@15 ˚C |
g/cm3 |
ASTM D1298 |
0.710 Max |
0.708 |
|
2 |
Total Sulfur |
ppm |
ASTM D4294 |
3000 Max |
2890 |
|
3 |
Mercaptan Content |
ppm |
ASTM D3227 |
2000 Max |
1760 |
|
4 |
R.V.P. |
PSI |
11 Max |
9 |
|
|
5 |
Benzene |
- |
- |
3 Max |
2.1 |
|
6 |
Total Aromatics |
- |
- |
14 Max |
12.1 |
|
7 |
N.Paraffins |
- |
- |
|
31.2 |
|
8 |
Paraffin |
Vol.% |
ASTM D6729 |
|
79.2 |
|
9 |
Olefins |
- |
- |
|
4.1 |
|
10 |
Naphthenes |
- |
- |
|
15.5 |
|
11 |
Chlorides |
ppm |
|
0.3 |
|
|
12 |
Mercury |
ppb |
AAS |
|
0.1 |
|
13 |
Total oxygenate |
ppm |
ASTM D6729 |
|
Max 50 |
|
IBP |
˚C |
ASTM D86 |
35 Min |
37 |
|
|
15 |
FBP |
˚C |
- |
200 Max |
181 |
|
16 |
Color |
- |
ASTM D158 |
20 Min |
25 |
Table 2. Prepared samples in this study.
|
Sample |
Oxidizer |
Catalyst |
|
1 |
H2O2 |
Formic acid |
|
2 |
H2O2 |
Acetic acid |
|
3 |
H2O2 |
FeSO4 |
|
4 |
H2O2 |
Phosphotangestic acid |
The ultrasonic power was adjusted to 150 W for all experiments. The residence time of the reactions was selected according to previous published studies. After the reaction time ended, the organic and aqueous phases were separated and washed with a non-polar solvent. In this section, 30 ml of the hydrocarbon phase was poured into a flask. Then, 30 ml of N-methyl pyrrolidone (NMP) was added as an extracting agent to eliminate the sulfur oxide compounds from the naphtha. The extraction process was performed on a stirrer for 30 min. After that, the mixture was poured into a separatory funnel to separate the hydrocarbon and aqueous phases. Additionally, the hydrocarbons were washed with water and separated again.
Characterizations
The EDXRF Sulfur Analyzer RX-360SH was used to measure the total sulfur content of the samples. The energy dispersive X-ray fluorescence technique was applied in this test according to the ASTM D4294. Moreover, fuel characterization such as density, mercaptan content, R.V.P., benzene, paraffins, chlorides, total oxygenate, IBP, FBP, and color were measured by ASTM D1298, ASTM D3227, ASTM D323, ASTM D6729, ASTM D5808, ASTM D86, ASTM D158.
Results & Discussion
According to the experimental methods, hydrogen peroxide converts to free radicals in the reaction between catalyst and oxidizer. The reactions in the absence and presence of the PTC are shown in Figure 2 (a) and Figure 2 (b), respectively. This reaction includes two steps. First, peroxyformic acid, an unstable compound, is produced as a result of the reaction between hydrogen peroxide and formic acid/acetic acid in the aqueous phase. Second, peroxyformic acid is transferred to the organic phase, where sulfur-containing compounds such as dibenzothiophene are converted to sulfones and sulfoxides. Moreover, the reaction mechanism of phosphotangestic acid with S-containing hydrocarbons is shown in Figure 2.
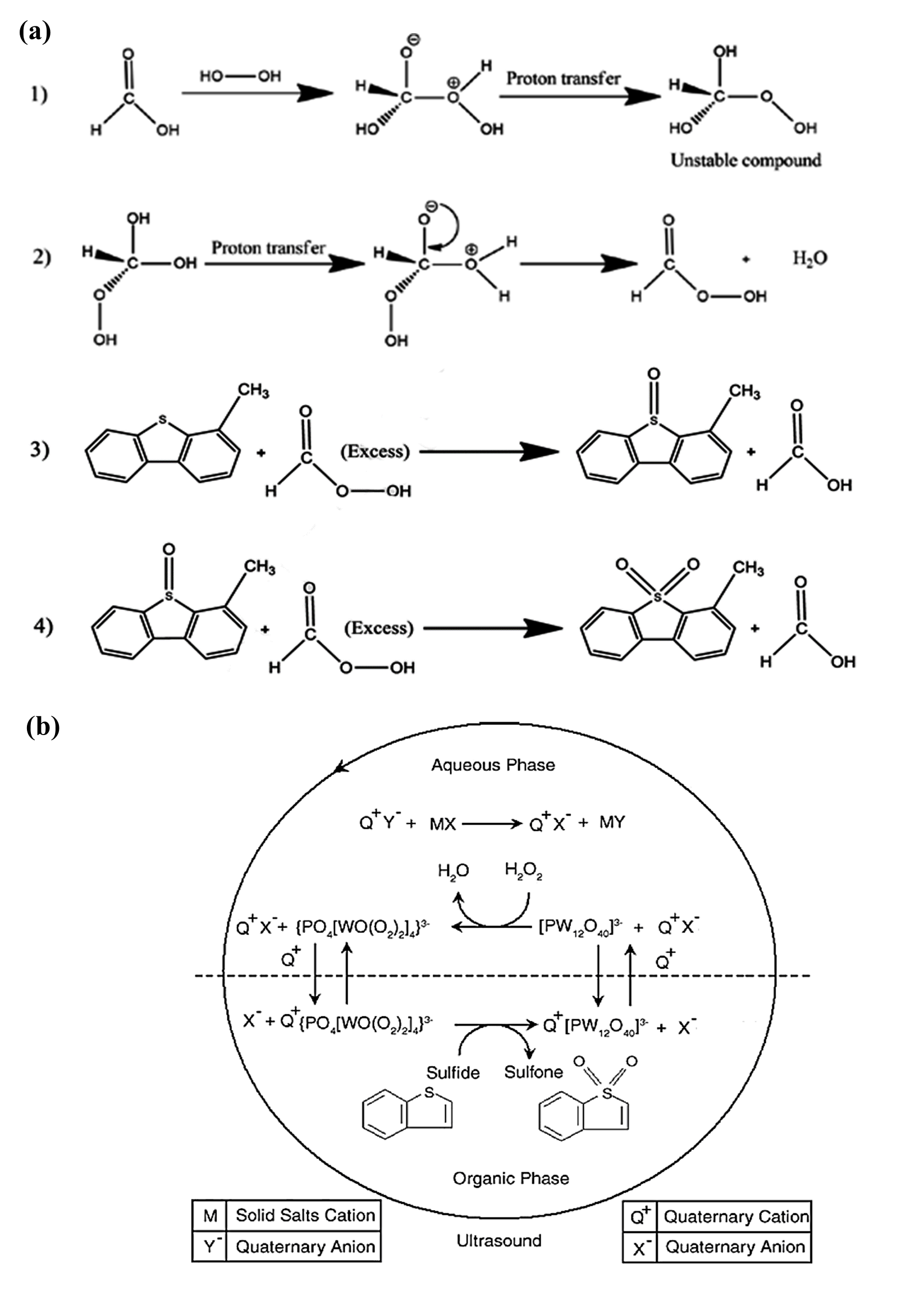
Figure 2. Mechanism reactions between (a) formic acid/acetic acid, (b) phosphotungstic acid and hydrocarbons.
The effect of temperature on the ultrasound oxidation reaction in the range of 20 to 90 °C has been studied in various published studies. The maximum boiling point of the materials used is 90 °C; in other words, the materials are volatile. Therefore, there are two limitations to temperature increase: the decomposition of hydrogen peroxide at high temperatures and the evaporation of naphtha by light components. In this study, three temperatures were considered, as shown in Figure 3. As shown in Figure 3, the sulfur content increases at higher temperatures, which can be explained by the removal of peroxy intermediate due to its temperature sensitivity.
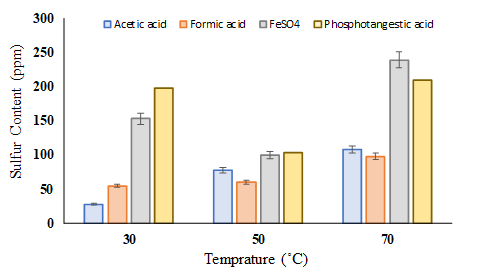
Figure 3. Temperature effect on the sulfur content at a time of 15 min.
Moreover, the effect of reaction time for sulfur removal is shown in Figure 4. The experiments were done during the 15 min. According to Figure 4, the increasing reaction time enhanced sulfur removal. However, the time increase improved sulfur removal, but the decline of operation cost is needed to identify the optimum time. H2O2, as the most common oxidant in the UAOD process, is a friendly environmental material. However, the increasing H2O2 enhances sulfur removal, but economic consideration should be noticed. One of the main topics in the ODS process is the recovery of chemical residues. Therefore, minimization of chemicals is essential.
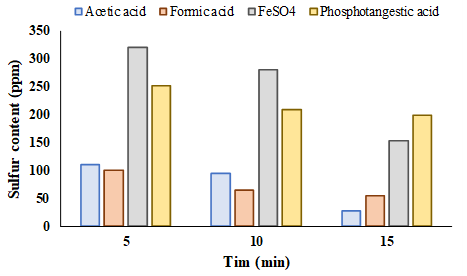
Figure 4. Reaction time effect for sulfur removal at T=30°C
Oxidative desulfurization technology using an ultrasonic reactor is one of the technologies that is effective and compatible with the environment for desulfurization. In recent years, the use of the desulfurization method of fuel materials such as diesel, kerosene, and gasoline has been comprehensively investigated. However, less attention is paid to the desulfurization of petroleum feedstocks. The reason can be due to the sulfur compounds of naphtha, where there are less refractory. Therefore, in large refineries where the naphtha reforming unit supplies hydrogen, it will be more cost-effective to remove refractory sulfur compounds from naphtha with HDS. However, for small refineries, isolated facilities, and those without access to hydrogen production units, alternative methods of oxidative desulfurization using an ultrasonic reactor are more suitable for petroleum. There are some commercial technologies for this purpose, such as presented techniques by Pure Path, International Ultrasonic Technologies Inc., and Hielscher Ultrasonics. However, this technology was first introduced by Professor Yen from the University of Southern California. SulphCo is one of the reputable companies with technical knowledge. This technology is registered under the names SulphCo™, Sonocracking™, Sonocracker™, Sonic Cracking™, Sonic Cracker™, and Sonocracked Crude. Since 1999, this company has developed the oxidative desulfurization process using an ultrasonic reactor and has been successful in this field. To date, the company has installed several 15,000 bbl/day modular Sonocracking units. According to this point, there is a significant need for desulfurization of crude oil and other petroleum derivatives such as gasoline, diesel, kerosene, and heating oil. Therefore, it needs modern technologies that are safe and require lower capital investment.
Conclusions
Ultrasonic desulfurization of commercial naphtha was performed by different catalysts such as acetic acid, formic acid, FeSO4, and phosphotangestic acid and hydrogen peroxide as oxidant. The results showed the high capacity of this process for commercial naphtha desulfurization. Furthermore, the best results were observed to acetic acid and formic acid and sulfur content reduce from 2890 ppm to 28 and 55 ppm, respectively. Studies factors were time, temperature and the type of catalyst with the aim of application in industrial scale.
Conflict of Interests
The authors declare no conflict of interest.
Funding
This research received no external funding.
Acknowledgement
The authors would like to acknowledge kind supports from Dr. Ali Afshar Ebrahimi and Iran Polymer and Petrochemical Institute.
Compliance with Ethical Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Authors' Contributions
All authors contributed in writing, conception, experimental measurement, and resources.
References
1. Barilla GRH, Chen CAW, Valencia MZM, Dugos N, Sy Choi AE. Oxidative desulfurization utilizing activated carbon supported phosphotungstic acid in the frame of ultrasonication. Chemical Engineering Communications. 2023;210(7):1154-1164. [doi:10.1080/00986445.2022.2059357]
2. Song C. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catalysis Today. 2003; 86(1-4): 211-263. [doi:10.1016/S0920-5861(03)00412-7]
3. Houda S, Lancelot C, Blanchard P, Poinel L, Lamonier C. Oxidative desulfurization of heavy oils with high sulfur content: A review. Catalysts. 2018;8(9):344. [doi:10.3390/catal8090344]
4. Rahimi M, Shahhosseini S, Movahedirad S. Continuous-flow ultrasound assisted oxidative desulfurization (UAOD) process: an efficient diesel treatment by injection of the aqueous phase. Ultrasonics Sonochemistry. 2017;39:611-622. [doi:10.1016/j.ultsonch.2017.05.033]
5. Wu Z, Ondruschka B. Ultrasound-assisted oxidative desulfurization of liquid fuels and its industrial application. Ultrasonics Sonochemistry. 2010;17(6):1027-1032. [doi: 10.1016/j.ultsonch.2009.11.005] [pmid: 20022546]
6. Aslam Abdullah M, Nagamalleswara R, Singh A. Novel methods of deep desulfurization : A review. Petroleum & Coal. 2019;61(4):647. [Link]
7. Sinhmar PS, Tiple A, Gogate PR. Combined extractive and oxidative desulfurization approach based on ultrasound and ultraviolet irradiation with additives for obtaining clean fuel. Environmental Technology & Innovation. 2021;22:101487. [doi:10.1016/j.eti.2021.101487]
8. Mohammed MM, Alalwan H, Alminshid AH, Ali S, Fakhir Mohammed M. Desulfurization of heavy naphtha by oxidation-adsorption process using iron-promoted activated carbon and Cu+2-promoted zeolite 13X. Catalysis Communications. 2022;169: 106473. [doi:10.1016/j.catcom.2022.106473]
9. Tugrul Albayrak A, Tavman A. Sono-oxidative desulfurization of fuels using heterogeneous and homogeneous catalysts: A comprehensive review. Ultrasonics Sonochemistry. 2022;83:105845. [doi: 10.1016/j.ultsonch.2021.105845] [pmid: 35151195]
10. Akbari A, Omidkhah M, Darian JT. Investigation of process variables and intensification effects of ultrasound applied in oxidative desulfurization of model diesel over MoO3/Al2O3 catalyst. Ultrasonics Sonochemistry. 2014: 21(2): 692-705. [doi: 10.1016/j.ultsonch.2013.10.004] [pmid: 24409467]
11. Jiang B, Yang H, Zhang L, Zhang R, Sun Y, Huang Y. Efficient oxidative desulfurization of diesel fuel using amide-based ionic liquids. Chemical Engineering Journal. 2016;283: 89-96. [doi:10.1016/j.cej.2015.07.070]
12. Ma X, Zhou A, Song C. A novel method for oxidative desulfurization of liquid hydrocarbon fuels based on catalytic oxidation using molecular oxygen coupled with selective adsorption. Catalysis Today. 2007;123(1-4): 276-284. [doi:10.1016/j.cattod.2007.02.036]
13. Khodaei B, Sobati MA, Shahhosseini S. Optimization of ultrasound-assisted oxidative desulfurization of high sulfur kerosene using response surface methodology (RSM). Clean Technologies and Environmental Policy. 2016;18(8):2677-2689. [doi:10.1007/s10098-016-1186-z]
14. Anderson, K., et al., Economic analysis of ultrasound-assisted oxidative desulfurization. Energy Sources, Part B: Economics, Planning, and Policy. 2017;12(4):305-311. [doi:10.1080/15567249.2014.917131]
15. Margeta D, Grčić I, Papić S, Sertić-Bionda K, Foglar L. Impact of ultrasound application on oxidative desulphurization of diesel fuel and on treatment of resulting wastewater. Environmental Technology. 2016; 37(3): 293-299. [doi:10.1080/09593330.2015.1068870] [pmid: 26166709]
16. Jalali MR, Sobati MA. Intensification of oxidative desulfurization of gas oil by ultrasound irradiation: Optimization using Box–Behnken design (BBD). Applied Thermal Engineering. 2017; 111: 1158-1170. [doi: 10.1016/j.applthermaleng.2016.10.015]
17. Ebrahimi SL, Khosravi-Nikou M, Hashemabadi SH. An experimental study on the operating parameters of ultrasound-assisted oxidative desulfurization. Iranian Journal of Oil and Gas Science and Technology. 2019; 8(3): 1-17. [doi:10.22050/ijogst.2019.171123.1492]
18. Khodaei B, Rahimi M, Sabati MA, Shahhosseini S, Jalali M. Effect of operating pressure on the performance of ultrasound-assisted oxidative desulfurization (UAOD) using a horn type sonicator: experimental investigation and CFD simulation. Chemical Engineering and Processing-Process Intensification. 2018;132:75-88. [doi:10.1016/j.cep.2018.08.006]
19. Dana M, Sabati MA, Shahhosseini S, Ansari A. Optimization of a continuous ultrasound assisted oxidative desulfurization (UAOD) process of diesel using response surface methodology (RSM) considering operating cost. Chinese Journal of Chemical Engineering, 2020;28(5):1384-1396. [doi:10.1016/j.cjche.2019.12.007]
20. Jima BB, Majeed NS. Oxidation desulphurization of heavy naphtha improved by ultrasound waves. Iraqi Journal of Chemical and Petroleum Engineering. 2020;21(1):9-14. [doi:10.31699/IJCPE.2020.1.2]
21. Carnaroglio D, Mantegna S, Moreira EM, Vicente de Castro A, Flores EMM, et al. Ultrasound-assisted oxidative desulfurization/denitrification of liquid fuels with solid oxidants. Energy & fuels. 2014;28(3):1854-1859. [doi:10.1021/ef402431e]
22. Barilla GRH, Chen CAW, Valencia MZM, Dugos NP, Sy Choi AE. Mixing assisted oxidative desulfurization using a synthesized catalyst of the activated carbon supported phosphotungstic acid: A process optimization study. South African Journal of Chemical Engineering. 2022;42:61-71.[doi:10.1016/j.sajce.2022.06.012]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







