Introduction
Globally, the annual incidence of carbon monoxide (CO) poisoning is estimated to be 137 per million, with a mortality rate of 4.6 per million [1]. Mortality from CO poisoning decreased by 53.5% over the two-decade period due to continued public health education and advances in treatment [2]. CO toxicity arises from tissue hypoxia and direct effects, such as enzyme inhibition (e.g., cytochrome C oxidase). Apoptosis and intracellular oxidative stress are key factors in the pathogenesis of neurotoxicity following CO exposure [1].
The clinical course of CO poisoning may be monophasic or biphasic. The acute manifestations may be either vague, mimicking non-specific viral illnesses, or moderate to severe, resulting in convulsions, cardiovascular collapse, and even death. In the biphasic course, individuals may develop delayed neuropsychiatric sequelae (DNS) [3, 4].
DNS is the most serious complication of CO poisoning. After the acute phase of the poisoning has been successfully treated with medical intervention, DNS may occur in some patients [5]. DNS is characterized by a series of neurological, cognitive, and psychiatric symptoms, including dementia, as well as pyramidal and extrapyramidal dysfunction [3].
Predicting DNS complications should be considered a preventive measure. It is incredibly challenging to identify patients with acute CO poisoning who are likely to develop DNS. The patient's initial presentation does not accurately predict the development of DNS. In addition, there is no screening tool to identify the high-risk group [6].
Creatine kinase (CK) is an intracellular muscle enzyme most often used to diagnose myopathies, including muscle injury. Its concentration in patients with CO poisoning is known to be elevated due to CO-induced ischemia, inflammatory reaction, and low consciousness level [7].
Brain-type CK (CK-BB) is released from the brain into the blood after experimental and clinical brain injury. The presence of a high level of CK-BB in the serum is associated with a poor neurological prognosis [8].
Neurogranin (Ng) is a protein involved in brain signaling. It is expressed at high levels in the brain. It can be detected in serum or cerebrospinal fluid in conditions that result in damage to brain tissue [9, 10]. CO poisoning causes neurological damage, particularly in the basal ganglia (especially globus pallidus and putamen), thalamus, hippocampus, and corpus callosum, where Ng is abundant [11].
Several studies have investigated tools to predict DNS in patients with CO poisoning; however, there are no standard screening tools or guidelines to predict the development of DNS accurately [7]. Determining accurate predictors for the prognosis of CO poisoning is highly necessary; therefore, this study aimed to investigate the prognostic value of initial serum Ng and CK-BB levels and their role as predictors of DNS in patients with acute CO poisoning.
Materials and Methods
Study design, date, and setting
This prospective cohort study was conducted on patients with acute CO poisoning admitted to the University Poison Control Center. The study was conducted from February 2022 to January 2023.
Ethical considerations
The study received approval from the Ethical Committee of the University Hospitals, Egypt (approval code: 35238/1/22). Written informed consent was obtained from each patient or their legal guardians in the case of unconscious patients. Data confidentiality was maintained by assigning a unique code number to each patient that was only known to the investigators.
Eligibility criteria
Patients of both genders with acute CO poisoning who were presented to the Poison Control Center within 24 hours of exposure were included in this study. Patients were diagnosed based on a history of acute CO exposure, clinical symptoms (e.g., headache, altered mental status, seizures, chest pain, and difficulty in breathing), and elevated COHb levels of > 3-4% in nonsmokers or > 10% in smokers, which confirmed the diagnosis during the initial visit to the Emergency Department.
The diagnosis of DNS was confirmed based on the recurrence of initial neurological or psychological symptoms after recovery periods or the development of new symptoms in patients included in the study.
Exclusion criteria included patients with insufficient clinical and toxicological data to confirm CO poisoning, combined exposure to CO and other intoxicants, and conditions that may lead to a preexisting rise in CK-BB and Ng levels. In addition, patients with a history of substance abuse due to its harmful effect on memory and cognitive function, those with neuropsychiatric disorders that could impact outcome assessment, cases with a history of chronic CO toxicity or prior exposure, those with previous treatment before admission to the Poison Control Center, and the participants who were lost to follow-up were excluded from the study [12].
Methods of the study
All patients were subjected to:
- History-taking, which includes sociodemographic data (age, gender, residence, education, and occupation), medical history (smoking status, medications, and drug allergies), and toxicological history (the source and duration of CO exposure, delay before medical arrival, and presenting complaints).
- Clinical assessment upon admission to the hospital involved vital signs, Glasgow Coma Scale evaluation, and examination of the cardiovascular, respiratory, genitourinary systems, and skin. In the current study, severity classification was based on the Olson and Smollin classification [13] and Tomaszewski. [14]. The clinical presentation and COHb level upon admission determined this classification. Mild cases exhibited dizziness and headache with COHb level <20%. Moderate cases presented confusion and syncope with COHb levels between 21% and 40%. Severe cases involved coma and COHb level >40%.
- Neuropsychiatric follow-up: Patients were given follow-up cards with their follow-up dates scheduled for 1 and 3 months after discharge. Both follow-up visits consisted of a comprehensive neurological examination and cognitive assessment. The Folstein Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) were utilized in the cognitive evaluation. The DNS manifestations included lethargy, emotional instability, personality changes, learning difficulties, psychosis, depression, Parkinsonism, apraxia, gait disorder, urinary incontinence, amnestic syndromes, dementia, and cognitive impairment [15].
Folstein Mini-Mental State Examination (MMSE)
The MMSE has a maximum score of 30 points, with five different domains of cognition analyzed: orientation (up to 10 points), memory (up to 6 points), attention and calculation (up to 5 points), language (up to 8 points), and design copying (up to 1 point). The maximum score on the MMSE is 30; a score of 24 or higher indicates normal cognition. Scores below 24 can suggest cognitive impairment: mild (19–23 points), moderate (10–18 points), and severe (≤9 points) [16].
Montreal Cognitive Assessment (MoCA)
The MoCA is a widely used and concise screening tool for detecting mild cognitive impairment by healthcare professionals. MoCA score ranges are commonly used to assess severity, with scores of 18–25 suggesting mild cognitive impairment, 10–17 indicating moderate impairment, and scores below 10 reflecting severe impairment [17].
- Laboratory investigations for each patient in this study. A 3 mL arterial blood sample and a 7 mL venous blood sample were withdrawn. The samples were taken immediately after admission and before the administration of any medications under completely aseptic conditions. Laboratory investigations included a Complete blood count, arterial blood gases, serum sodium (Na), potassium (K) levels, blood glucose, renal functions, alanine aminotransferase, aspartate aminotransferase, CK, CK-BB, and Ng assay. Measurement of CK-BB (U/L) was done by separating the isozymes of CK into well-resolved bands using electrophoresis. “Neurogranin was measured using ELISA kits (Manufacturer: [NEW TEST COMPANY], Germany, Catalog No: [DLR-NRGN-Hu-001). All procedures were performed according to the manufacturer’s instructions.
- Treatment that followed the established Poison Control Center protocol.
- Assessment of the outcome focused on the development of DNS. Non-complicated patients are those who were discharged from the hospital without any complications and did not develop any neuropsychological sequelae during the follow-up period. On the contrary, DNS-complicated patients refer to patients who had complete recovery of consciousness after an acute CO poisoning event; however, they then developed neuropsychological symptoms within days or months.
Sample size
The Sample size was calculated using Stephen Thompson's equation, with the following parameters: average population (60), error proportion (0.05), probability (50%), and a 95% confidence level (1.96). The sample size consisted of 40 patients.
Statistical analysis
Data were analyzed using IBM SPSS software package (version 20.0) (Armonk, NY: IBM Corp). Qualitative data were described using numbers and percentages. The Shapiro-Wilk test was used to verify the normality of the distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation, median, and interquartile range.
Chi-square test, Fisher’s Exact, or Monte Carlo correction for Chi-square were used for categorical variables. The Student t-test was used for normally distributed quantitative variables, and the Mann-Whitney test was used for abnormally distributed quantitative variables.
The receiver operating characteristic (ROC) curve was generated from the data to determine the optimal cut-off point, sensitivity, and specificity. The area under the curve (AUC) indicates the diagnostic performance of the test. It was graded as follows: 0.90-1=excellent; 0.80-0.90=good; 0.70-0.80=fair; and 0.60- 0.70=poor. Pairwise comparisons of the AUCs of the studied parameters were made. A P-value <0.05 indicated significance in interpreting the results of statistical tests.
Results
During the study period, 55 patients with acute CO poisoning were admitted to the Poison Control Center. They were screened for eligibility. Only 40 patients were ultimately enrolled in the study. The remaining 15 were excluded for various reasons: met exclusion criteria (n=6), undetermined storage errors (n=4), died during hospital stay (n=2), and refused follow-up (n=3).
Table 1 summarizes the characteristics of the studied patients with acute CO poisoning. Their ages ranged from 14 to 58 years. Men outnumbered women, with a median exposure duration of 1 hour. The source of exposure was a gas water heater in 55% of cases. Clinical manifestations and COHb levels indicated that 42.5% of cases had mild poisoning, while moderate grades were found in 45%, and severe grades in 12.5% of cases. Serum Ng levels ranged from 5.03 ng/dL to 26.82 ng/dL, and serum CK-BB levels ranged from 6.4 U/L to 4580 U/L. Elevated serum CK levels were found in 57.5% of patients. Delayed neuropsychiatric symptoms developed in 57.7% of patients within the first three months post-exposure (DNS complicated group). In contrast, 42.5% of patients didn’t develop DNS (non-complicated group). The frequency of detected symptoms within DNS complicated patients was memory loss (53.2%), concentration deficit (34.8%), and vegetative state (4.3%) (data is not tabulated).
Table 1. Baseline characteristics of patients with acute carbon monoxide poisoning
|
N=40 |
| Age (years) |
27.33±10.36 |
| Gender |
Male |
21(52.5%) |
| Female |
19(47.5%) |
| Delay (hours) |
3.0(2.0–4.0) |
| Duration of exposure (hours) |
1.0(0.50–2.0) |
| Source of CO |
Domestic butane |
2(5.0%) |
| Fire |
5(12.5%) |
| Gas water heater |
22(55.0%) |
| Charcoal burns in closed |
11(27.5%) |
| COHb level (%) |
24.50(17.5–35.0) |
| Severity of acute CO poisoning |
Mild (15-20) |
17(42.5%) |
| Moderate (21-40) |
18(45.0%) |
| Severe (41-59) |
5(12.5%) |
| Ng (ng/dl) |
12.82±4.80 |
| CKBB (U/L) |
22.20(15.50–42.10) |
| CK |
Normal |
23(57.5%) |
| Elevated |
17(42.5%) |
| DNS |
Yes |
23(57.5%) |
| No |
17(42.5%) |
Data is presented as mean ± SD or frequency (%) or median (IQR). CO: carbon monoxide, Ng: Neurogranin, COHb: carboxyhemoglobin, CK: creatine kinase; CK-BB: creatine kinase brain type, DNS: delayed neuropsychiatric sequelae.
According to the appearance of DNS after co-poisoning, acute CO-poisoned patients were divided into DNS-complicated and non-complicated patients.
The study found significant differences between the two patient groups in terms of age, gender, occupation, and special habits. On the contrary, no significant differences were found in other sociodemographic data. The two groups were comparable in terms of source of exposure and delay time; however, the DNS-complicated group had a significantly longer median duration of CO exposure. Upon admission, there were no significant differences in symptoms of CO poisoning, except for syncope, as shown in Table 2.
Table 2. Comparison between the DNS complicated group and the non-complicated group regarding sociodemographic data, source of CO, delay time, duration of exposure, and presenting symptoms
|
DNS
(n = 23) |
Non-DNS
(n = 17) |
Test (χ2) |
P |
| Age (years) |
31.17±11.23 |
22.12±6.16 |
t=3.260* |
0.002* |
| Gender |
Male |
19(82.6%) |
2(11.8%) |
19.673* |
<0.001* |
| Female |
4(17.4%) |
15(88.2%) |
| Marital Status |
Single |
10(43.5%) |
9(52.9%) |
0.351 |
0.554 |
| Married |
13(56.5%) |
8(47.1%) |
| Residence |
Rural |
17(73.9%) |
10(58.8%) |
1.015 |
0.314 |
| Urban |
6(26.1%) |
7(41.2%) |
|
|
| Education |
Read and write+ 1ry, perp |
7(30.4%) |
4(23.5%) |
1.154 |
0.562 |
| Secondary school |
7(30.4%) |
8(47.1%) |
| University and high institutes |
9(39.1%) |
5(29.4%) |
| Occupation |
Manual worker |
9(39.1%) |
0(0.0%) |
13.116* |
MCP=
0.004* |
| Housewife |
3(13.0%) |
7(41.2%) |
| Students |
5(21.7%) |
8(47.1%) |
| Employed |
6(26.1%) |
2(11.8%) |
| Special habit |
Smoking |
8(34.8%) |
0(0.0%) |
7.391* |
FEP=
0.013* |
| No |
15(65.2%) |
17(100.0%) |
| Source of CO |
Domestic butane |
2(8.7%) |
0(0.0%) |
3.452 |
MCP=0.374 |
| Fire |
4(17.4%) |
1(5.9%) |
| Gas water heater |
10(43.5%) |
12(70.6%) |
| Charcoal burn in closed |
%) 7(30.4 |
4(23.5%) |
| Delay (hours) |
3.50(2.0– 6.25) |
2.0(2.0–3.0) |
U=143.0 |
MCP=0.156 |
| Duration of exposure (hours) |
1.0(1.0–3.25) |
0.50(0.50–0.50) |
U=94.50* |
MCP=0.005* |
| Symptoms |
Syncope |
21(91.3%) |
10(58.8%) |
5.914* |
FEP=0.023* |
| Dizziness |
22(95.7%) |
13(76.5%) |
3.288 |
FEP=0.144 |
| Vomiting |
15(65.2%) |
9(52.9%) |
0.614 |
0.433 |
| Headache |
9(39.1%) |
10(58.8%) |
1.520 |
0.218 |
| Seizures |
5(21.7%) |
0(0.0%) |
4.224 |
FEP=0.061 |
| Urination/defecation |
%) 5(21.7 |
1(5.9%) |
6.625 |
MCP=
0.052 |
| Cyanosis |
4(17.4%) |
0(0.0%) |
| Compartments |
1(4.3%) |
0(0.0%) |
Data are presented as mean ± SD or frequency (%) or median (IQR). *Significant p value <0.05. DNS: Delayed neurological sequalae, CO: Carbon monoxide, X2: Chi square test; MC: Monte Carlo; FE: Fisher Exact, U: Mann Whitney test.
The clinical examination revealed significant statistical differences in diastolic blood pressure (DBP), GCS, and respiratory system evaluation between the two groups (P≤0.05). Patients were categorized based on the degree of impairment in their consciousness. There was a significant difference between the two groups regarding impairment in consciousness levels (P≤0.05). The laboratory investigations revealed that patients with DNS complications had significantly lower median HCO3 levels, mean PaCO2 levels, and SO2 compared to non-complicated patients. However, all laboratory tests, including Hb levels, blood cell counts, liver enzymes, and kidney function markers, were within normal values (data are not tabulated). The study found that non-invasive COHb, CK, CK-BB, and serum Ng levels were significantly higher in patients with DNS complications, compared to those without complications (P<0.05), as shown in Table 3.
Table 3. Comparison between the DNS complicated group and the non-complicated group as regards vital signs, consciousness level assessment, chest examination, ABG, level of COHb, levels of CK, CK-BB, and serum Ng level at admission
|
DNS
(n = 23) |
Non-DNS
(n = 17) |
Test |
P |
| Vital signs |
SBP (mmHg) |
107.8±17.83 |
117.6±10.91 |
t=2.007 |
0.052 |
| DBP (mmHg) |
69.35±14.17 |
79.41±10.88 |
t=2.442* |
0.019* |
| HR (beat /minute) |
95.83±24.42 |
89.18±14.64 |
t=1.071 |
0.291 |
| RR (cycle/minute) |
18.0(18.0–20.0) |
18.0(18.0–20.0) |
U=187.0 |
0.829 |
| Temperature (o C) |
36.94±0.24 |
37.0±0.0 |
t=1.153 |
0.261 |
| GCS |
14.0(8.50–14.0) |
15.0(14. 0–15.0) |
U=91.50* |
0.004* |
| Level of consciousness |
Minor (≥13) |
16(60.8%) |
5(94.1%) |
χ2=9.490* |
MCP= 0.018* |
| Moderate (9-12) |
3(13.0%) |
1(5.9%) |
| Severe (≤8) |
6(26.1%) |
0(0.0%) |
| Chest |
Normal |
17(73.9%) |
17(100.0%) |
χ2=5.217* |
FEP=
0.030* |
| Bilateral crepitation |
6(26.1%) |
0(0.0%) |
| ABG |
pH |
7.41±0.07 |
7.42±0.05 |
t=0.907 |
0.370 |
| PaCO2 (mmHg) |
30.08±7.20 |
37.88±8.58 |
t=3.124 |
0.003* |
| HCO3 (mEq/L) |
18.0(17.35–22.65) |
22.0(21.0–24.0) |
U=108.0* |
0.016* |
| PaO2 (mmHg) |
88.0(81.0–90.0) |
88.0(82.0–89.0) |
U=180.0 |
0.685 |
| SO2(%) |
92.91±6.18 |
96.94±3.07 |
t=2.707* |
0.011* |
| COHb (%) |
34.0(28.5–38.5) |
15.0(10.0–20.0) |
U=5.50* |
<0.001* |
| CK (U/L) |
Normal |
6(26.1%) |
17(100.0%) |
χ2=21.853* |
<0.001* |
| Elevated |
17(73.9%) |
0(0.0%) |
| CKBB (U/L) |
40.80(32.0–84.30) |
14.0(12.30–17.50) |
U=9.500* |
<0.001* |
| Ng (ng/mL) |
15.86±3.87 |
8.71±2.11 |
t=7.488* |
<0.001* |
Data are presented as mean ± SD or frequency (%) or median (IQR). *Significant p value <0.05. DNS: Delayed neuropsychiatric sequalae, COHb: Carboxyhemoglobin, ABG: Arterial blood gases, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, HR: Heart rate, RR: Respiratory rate, GCS: Glasgow coma scale, PCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen; HCO3: bicarbonate; pH: potential of hydrogen, SO2: Oxygen saturation, CK: Creatine kinase, CKBB: Creatine kinase brain type, Ng: Neurogranin level.
The severity of acute CO poisoning at presentation was significantly higher in DNS-complicated patients, compared to non-complicated ones, and the number of HBO sessions was higher in DNS-complicated patients. During follow-up, significant differences were found in neurological examination, median MMSE, and median MoCA test parameters between the two groups (Table 4).
Table 4. Comparison between the DNS complicated group and the non-complicated group regarding the severity of acute CO poisoning, number of HBO sessions, and follow-up visits at one and three months
|
DNS
(n = 23) |
Non-DNS
(n = 17) |
Test |
P |
| Severity of acute CO poisoning |
Mild |
1(4.3%) |
16(94.1%) |
χ2
34.469* |
<0.001* |
| Moderate |
17(73.9%) |
1(5.9%) |
| Severe |
5(21.7%) |
0(0.0%) |
| HBO therapy |
18(78.3%) |
12(70.6%) |
0.307 |
FEP=
0.717 |
| Number of HBO sessions |
3 (2.0 – 5.0) |
1.0 (1.0 – 1.50) |
U=46.50* |
0.008* |
| Follow-up visit at one month |
| Neurological examination |
Normal |
16(69.6%) |
17(100.0%) |
c2=6.271* |
0.014* |
| Abnormal |
7(30.4%) |
0(0.0%) |
| MMSE test |
22.0 (19.0 – 23.0) |
28.0 (26.0 – 28.0) |
U=7.500* |
<0.001* |
| MoCA test |
20.0 (17.50 – 22.0) |
26.0 (26.0 – 27.0) |
U=17.000* |
<0.001* |
| Follow-up visit at three months |
| Neurological examination |
Normal |
17(73.9%) |
17(100.0%) |
χ2 =5.217* |
0.030* |
| Abnormal |
6(26.1%) |
0(0.0%) |
| MMSE test |
25.0 (20.0 – 26.0) |
29.0 (28.0 – 29.0) |
U=23.500* |
<0.001* |
| MoCA test |
23.0 (19.50 – 25.0) |
28.0 (27.0 – 28.0) |
U=19.500* |
<0.001* |
Data are presented as mean ± SD or frequency (%) or median (IQR). *Significant p value <0.05. CO: Carbon monoxide, HBO: Hyperbaric oxygen, MMSE: Mini-Mental Examination, MoCA: Montreal Cognitive Assessment score.
Pearson correlation analysis revealed a strong positive correlation between Ng and memory loss (r = 0.78, P<0.001), while only weak to moderate correlations were observed between Ng and other DNS symptoms. CK-BB showed weak, mostly non-significant correlations with the studied symptoms (Table 6).
Table 5. Performance of COHb, serum Ng, serum CK-BB levels, and GCS for prediction of development of DNS in patients with acute carbon monoxide poisoning
|
COHb |
CK-BB |
Ng |
GCS |
| AUC |
0.986 |
0.976 |
0.962 |
0.766 |
| P |
<0.001* |
<0.001* |
<0.001* |
0.004* |
| 95% CI |
0.959 – 1.0 |
0.937 – 1.0 |
0.902 – 1.0 |
0.620 – 0.912 |
| Cut-off |
>20 |
>18.9 |
>10.42 |
≤14 |
| Sensitivity |
95.65 |
91.30 |
95.65 |
78.26 |
| Specificity |
94.12 |
88.24 |
88.24 |
64.71 |
| PPV |
95.7 |
91.30 |
91.7 |
75.0 |
| NPV |
94.1 |
88.24 |
93.8 |
68.7 |
| P value from pairwise comparison of AUCs |
| COHb |
------ |
0.624 |
0.388 |
0.002* |
| CKBB |
0.624 |
------ |
0.673 |
0.003* |
| Ng |
0.388 |
0.673 |
------ |
0.003* |
| GCS |
0.002* |
0.003* |
0.003* |
------ |
AUC: area under the curve, CI: confidence interval, PPV: positive predictive value, NPV: negative predictive value. * Significant P value<0.05. COHb: carboxyhemoglobin, Ng: Neurogranin, CK-BB: creatine kinase brain type, DNS: delayed neuropsychiatric sequelae, GCS: Glasgow coma scale.
Table 6. Correlation between Biomarkers (CKBB & Ng) and DNS Symptoms in patients with acute carbon monoxide poisoning.
| Symptom / Biomarker |
Ng (r, p) |
CK-BB (r, p) |
| Headache |
0.22 (p>0.05) |
-0.11 (p>0.05) |
| Insomnia |
0.30 (p>0.05) |
0.15 (p>0.05) |
| Memory loss |
0.78 (p<0.001) * |
0.28 (p>0.05) |
| Concentration deficit |
0.45 (p>0.05) |
0.20 (p>0.05) |
Ng: Neurogranin, CK-BB: creatine kinase brain type, DNS: delayed neuropsychiatric sequelae, *Significant p value <0.05, r: Pearson correlation coefficient
ROC curves were conducted to assess the accuracy of the studied markers (CK-BB & Ng) and for GCS and COHb in predicting DNS in patients with acute CO poisoning. Initial CK-BB levels had a sensitivity of 91.3% and a specificity of 88.24%, while initial Ng levels had a sensitivity of 95.6% and a specificity of 88.24%. COHb showed a sensitivity of 95.60% and a specificity of 94.12%, while GCS had a sensitivity of 78.26% and a specificity of 64.71%. Carboxy haemoglobin had the best area under the curve (AUC), followed by CKBB, Ng, and GCS. Pairwise comparisons revealed no significant differences between all predictors, except for GCS, which showed a substantial difference with all other parameters (Table 5 and Figure 1).
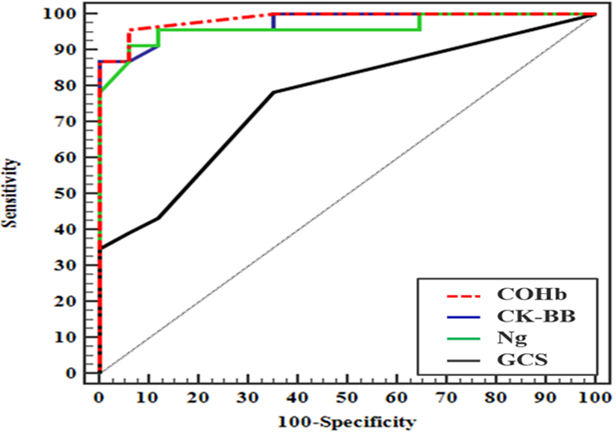
Figure 1. ROC curve for prediction of development of delayed neurological sequalae in patients with acute carbon monoxide poisoning using neurogranin level, creatine kinase brain type, Glasgow coma scale, and carboxyhemoglobin
Discussion
Preventing DNS is a crucial goal in treating CO poisoning; however, predicting risk factors is challenging, with limited clinical utility [18]. This study aimed to assess the prognostic value of initial serum Ng and CK levels in predicting DNS in patients with acute CO poisoning.
All authors contributed to the conception and design of the study. Material preparation, data collection, and analysis were performed by [M. M. E.], [B. H. F.] and [A. A. W.]. The first draft of the manuscript was written by [A. A. E.] and [M. S. E.]. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All figures of the manuscript were original.
References
- Zhang Y, Lu Q, Jia J, Xiang D, Xi Y. Multicenter retrospective analysis of the risk factors for delayed neurological sequelae after acute carbon monoxide poisoning. Am J Emerg Med. 2021;46:165-9. [doi: 10.1016/j.ajem.2020.06.090] [pmid: 33069546]
- Moberg ME, Hamilton EB, Zeng SM. Global, regional, and national mortality due to unintentional carbon monoxide poisoning, 2000–2021: results from the global burden of disease study 2021. Lancet Public Health. 2023;8(11):839-49. [doi: 10.1016/s2468-2667(23)00185-8] [pmid: 37813118]
- Huang YQ, Peng ZR, Huang FL, Yang AL. Mechanism of delayed encephalopathy after acute carbon monoxide poisoning. Neural Regen Res. 2020;15(12):2286-95. [doi: 10.4103/1673-5374.284995] [pmid: 32594050]
- Kassa BD, Yigzaw AA, Kassie YG, Kedimu MW, Mekuanint YF, Moges N. Delayed neuropsychiatric sequelae due to long-term effects of carbon monoxide poisoning in Ethiopia: A case report. Toxicol Rep. 2023;11:36-9. [doi: 10.1016/j.toxrep.2023.06.009] [pmid: 37448591]
- Zhang JJ, Bi WK, Cheng YM, Yue AC, Song HP, Zhou XD, et al. Early predictors of brain injury in patients with acute Carbon Monoxide poisoning and the neuroprotection of mild hypothermia. Am J Emerg Med. 2022;61:18-28. [doi: 10.1016/j.ajem.2022.08.016] [pmid: 36029667]
- Hafez AS, El-Sarnagawy GN. S-100β in predicting the need of hyperbaric oxygen in CO-induced delayed neurological sequels. Hum Exp Toxicol. 2020;39(5):614-23. [doi: 10.1177/0960327119897104] [pmid: 31885284]
- Lee H, Kang H, Ko BS, Oh J, Lim TH, Cho Y. Initial creatine kinase level as predictor for delayed neuropsychiatric sequelae associated with acute carbon monoxide poisoning. Am J Emerg Med. 2021;43:195-9. [doi: 10.1016/j.ajem.2020.02.054 ] [pmid: 32165069]
- Mansueto P, Seidita A, Scozzari F, Rivaldo G, D'Alcamo AL, Adragna F, et al. CPK increase as an occult marker of cerebrovascular disease: a case report. Acta Medica Mediterr. 2012;28:83-8. [Link]
- Sarkis GA, Lees-Gayed N, Banoub J, Abbatielo SE, Robertson C, Haskins WE, et al. Generation and release of neurogranin, vimentin, and MBP proteolytic peptides, following traumatic brain injury. Mol Neurobiol. 2022;59(2):731-47. [ doi: 10.1007/s12035-021-02600-w ] [pmid: 34762230]
- Liu W, Lin H, He X, Chen L, Dai Y, Jia W, et al. Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimer's disease and mild cognitive impairment. Transl Psychiatry. 2020;10(1):125. [doi: 10.1038/s41398-020-0801-2] [pmid: 32350238]
- Yeşilyurt Ö, Cömertpay E, Vural S, Eroğlu O, Badem ND, Çankaya İ, et al . The diagnostic value of neurogranin in patients with carbon monoxide poisoning: Can it show early neurological damage? Am J Emerg Med. 2021;50:191-5. [doi: 10.1016/j.ajem.2021.07.052] [pmid: 34388687]
- Cadet JL, Bisagno V. Neuropsychological consequences of chronic drug use: relevance to treatment approaches. J Front Psychiatry. 2016;6:189. [ doi: 10.3389/fpsyt.2015.00189] [pmid: 26834649]
- Olson K, Smollin C. Carbon Monoxide poisoning (acute). BMJ Clin Evid. 2008;2008:2103. [pmid: 19445736]
- Tomaszewski C. Goldfrank’s toxicologic emergencies. CO poisoning. 2011;20:200-8.
- Cha YS, Kim H, Do HH, Kim HI, Kim OH, Cha KC, et al. Serum neuron-specific enolase as an early predictor of delayed neuropsychiatric sequelae in patients with acute Carbon Monoxide poisoning. Hum Exp Toxicol. 2018;37(3):240-6. [doi: 10.1177/0960327117698544] [pmid: 28349731]
- Gluhm S, Goldstein J, Loc K, Colt A, Liew CV, Corey-Bloom J. Cognitive performance on the mini-mental state examination and the montreal cognitive assessment across the healthy adult lifespan. Cogn Behav Neurol. 2013;26(1):1-5. [doi: 10.1097/wnn.0b013e31828b7d26] [pmid: 23538566]
- Julayanont P, Brousseau M, Chertkow H, Phillips N, Nasreddine ZS. Montreal cognitive assessment memory index score (MoCA-MIS) as a predictor of conversion from mild cognitive impairment to Alzheimer's disease. J Am Geriatr Soc. 2014;62(4):679-84. [doi: 10.1111/jgs.12742] [pmid: 24635004]
- Gao X, Wei W, Yang G-D. Clinical factors for delayed Neuropsychiatric sequelae from acute Carbon Monoxide poisoning: a retrospective study. Front Med. 2024;11:200-30. [doi: 10.3389/fmed.2024.1333197] [pmid: 38371510]
- Helal NE, Ashmawy MM, Saad KM, Draz EE. Role of Pentraxin 3, Ischemia-modified Albumin, and Myeloperoxidase in Predicting Acute Carbon Monoxide Poisoning Outcomes. Nat Sci. 2019;17(8):54-63. [doi: 10.7537/marsnsj170819.08]
- Shahin M, Allam A, Elkholy R, Lashin H. Hematological parameters as early predictors of delayed neurological sequelae in acute Carbon Monoxide poisoning. Ain-Shams J Forensic Med Clin Toxicol. 2020;35(2):61-72. [ doi: 10.21608/ajfm.2020.104446]
- Caballero-Bermejo AF, Ruiz-Antoran B, Ramio-Lluch C, Dueñas-Ruiz A, Pineda Torcuato Á, Homar-Amengual C, et al. Clinical features and predictors of delayed neurological syndrome in Carbon Monoxide poisoning: the AMICO study. Emergencias. 2024;36(2):116-22. [ doi: 10.55633/s3me/024.2023] [pmid: 38597618]
- Alharthy N, Alanazi A, Almoqaytib A, Alharbi B, Alshaibani R, Albuniyan J, et al. Demographics and clinical characteristics of carbon monoxide poisoning for patients attending in the emergency department at a tertiary hospital in Riyadh, Saudi Arabia. Int J Emerg Med. 2024;17(1):25. [doi: 10.1186/s12245-024-00600-w] [pmid: 38408885]
- Gaballah SZ, Elkhishen IA-R, Hashim NA, Abdel Hamid OI. Predictors of delayed neurological sequelae after acute Carbon Monoxide poisoning at Zagazig University Hospitals. ZJFM. 2020;18(2):105-21. [doi: 10.21608/zjfm.2020.41674.1063]
- Mubarak MA E-GD, Abd El A, Wagih F, Maklad AI. Assessment of sTWEAK protein, neutrophil-lymphocyte ratio, blood lactate and carboxyhemoglobin as predictors of delayed neurological sequelae after acute Carbon Monoxide poisoning. Am J Emerg Med. 2024;6(1):1-9. [doi: 10.33545/27074447.2024.v6.i1a.68]
- Ghanem MA, El Shanawany SE, Ashry M, Abdelgaleel A, Gad NE, Kholeif W. Neutrophil-lymphocyte ratio and ischemia-modified albumin in predicting carbon monoxide-delayed neurological sequelae. Asia Pac J. 2022;11(3):112-20. [ Link ]
- Pepe G, Castelli M, Nazerian P, Vanni S, Del Panta M, Gambassi F, et al. Delayed neuropsychological sequelae after Carbon Monoxide poisoning: Predictive risk factors in the emergency department. A retrospective study. Scand J Trauma Resusc Emerg Med. 2011;19:16. [ doi: 10.1186/1757-7241-19-16] [pmid: 21414211]
- Ashry S, Khater AS, Wahdan M, Eweda SA. The possible role of partial pressure of Carbon Dioxide level changes in the outcome of acute Carbon Monoxide poisoning. ZJFM. 2023;21(2):131-47. [ doi: 10.21608/zjfm.2023.216580.1151]
- Yang J, Korley FK, Dai M, Everett AD. Serum neurogranin measurement as a biomarker of acute traumatic brain injury. Clin Biochem. 2015;48(13-14):843-8. [doi: 10.1016/j.clinbiochem.2015.05.015] [pmid: 26025774]
- Canturk IB, Kalkan A, Es AK, Bozan O, Unver SS, Senturk M. Serum neurogranin measurement as a biomarker of central nervous system infections: A preliminary study. Keio J Med. 2022;71(3):62-7. [ doi: 10.2302/kjm.2021-0019-oa] [pmid: 35718469]
- Kitamoto T, Tsuda M, Kato M, Saito F, Kamijo Y, Kinoshita T. Risk factors for the delayed onset of Neuropsychologic sequelae following Carbon Monoxide poisoning. Acute Med Surg. 2016;3(4):315-19. [doi: 10.1002/ams2.197] [pmid: 28163920]
- Alkholy UM, Abdalmonem N, Zaki A, Ali YF, Mohamed SA, Abdelsalam NI. Early predictors of brain damage in full-term newborns with hypoxic ischemic encephalopathy. Neuropsychiatr Dis Treat 2017;13:2133-9. [doi: 10.2147/ndt.s144225] [pmid: 28860770]
- Ning K, Zhou YY, Zhang N, Sun XJ, Liu WW, Han CH. Neurocognitive sequelae after Carbon Monoxide poisoning and hyperbaric oxygen therapy. Med Gas Res. 2020;10(1):30-6. [ doi: 10.4103/2045-9912.279981] [pmid: 32189667]
- Wu K, Liu M, He L, Tan Y. Abnormal degree centrality in delayed encephalopathy after carbon monoxide poisoning: a resting-state fMRI study. Neuroradiol. 2020;62(5):609-16. [doi: 10.1007/s00234-020-02369-0] [pmid: 31955235]
- Çevik S, Özgenç MM, Güneyk A, Evran Ş, Akkaya E, Çalış F.NRGN, S100B and GFAP levels are significantly ncreased in patients with structural lesions resulting from mild traumatic brain injuries. Clin Neurol Neurosurg. 2019;183:105380. [doi: 10.1016/j.clineuro.2019.105380] [pmid: 31234132]









































