Ethics code: IR.LUMS.REC.1401.089


Hasanvand A, Astaraki P, Shamsinia S, Nourmohammadi M, Hatami F, Birjandi M, et al . Hepatoprotective Effects of the Adenosine Monophosphate-Activated Protein Kinase Signaling Pathway in the Fluoxetine-Induced Hepatotoxic Model in Rats. IJT 2025; 19 (3) :182-188
URL:
http://ijt.arakmu.ac.ir/article-1-1468-en.html
1- Hepatitis Research Center, Department of Physiology and Pharmacology, School of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran.
2- Department of Internal Medicine, School of Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran.
3- Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran.
4- Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran
5- Department of Biostatistics, School of Health and Nutrition, Lorestan University of Medical Sciences, Khorramabad, Iran.
6- Department of Pathology, School of Medicine, Lorestan University of Medical Science, Khorramabad, Iran.
7- Department of English, School of Medicine, AJA University of Medical Sciences, Tehran, Iran.
8- Health and Environment Research Center, Ilam University of Medical Sciences, Ilam, Iran. , nrahimi@razi.tums.ac.ir
Full-Text [PDF 794 kb]
(162 Downloads)
|
Abstract (HTML) (378 Views)
Full-Text: (132 Views)
Introduction
Drug-induced liver injury (DILI) continues to account for a considerable proportion of acute liver failure cases [1]. DILIs present with a broad spectrum of clinical manifestations, ranging from asymptomatic abnormalities in liver tests to acute liver disease accompanied by symptoms, disability, prolonged jaundice, and even overt acute or subacute liver failure. The diagnosis of DILI can be difficult and is often delayed because other, more common causes of liver injury must first be ruled out [2]. The risk of DILI increases as a drug becomes more widely prescribed [3]. Evidence suggests that many antidepressants, even at therapeutic doses, may cause hepatotoxicity. Furthermore, DILI can present with hepatocellular, cholestatic, or mixed patterns of liver injury [4]. Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), is prescribed for the management of various psychiatric and behavioral disorders, including depression, obsessive–compulsive disorder, panic disorder, fibromyalgia, bulimia nervosa, premature ejaculation, and trichotillomania [5,7]. Fluoxetine is a fluorinated antidepressant with a long half-life, primarily metabolized in the liver and eliminated through the urine [8,9]. However, several studies have reported that fluoxetine use may cause hepatotoxicity and alter liver enzyme activity [10,12]. Long-term use of fluorinated drugs may lead to liver injury, recognized as one of their potential adverse effects. Studies have indicated that high doses of fluoxetine can elevate liver function biomarkers and cause liver damage through oxidative stress [13]. Fluoxetine metabolism may lead to the excessive production of free radicals, which can potentially cause liver damage. Additionally, inflammation associated with hepatic injury has been linked to elevated levels of reactive oxygen species (ROS), including hydrogen peroxide (H₂O₂), superoxide (O₂•−), and hydroxyl radicals (OH•), which can recruit phagocytes and exacerbate tissue damage [14]. Animal studies have demonstrated that fluoxetine can induce hepatic necrosis and ischemia at various doses, as well as cause steatosis, cholestasis, lobular and portal inflammation, and hepatomegaly [15,17]. Metformin, a frequently used treatment for type 2 diabetes [18], is a biguanide that inhibits complexes I and III of the mitochondrial electron transport chain during its metabolism. As a result, partial leakage in the electron transport chain increases cellular ROS production, leading to a slight reduction in ATP levels. In metformin’s metabolic pathway, this decrease in cellular ATP typically activates the adenosine monophosphate-activated protein kinase (AMPK), which subsequently phosphorylates and activates the transcription factor FOXO3a, playing a central role in regulating cell growth and death, glucose metabolism, ROS detoxification, and lifespan [19, 20]. Elevated cellular levels of antioxidant enzymes, such as catalase (CAT), help reduce oxidative stress by detoxifying ROS. This process ultimately facilitates the removal of superoxide via manganese superoxide dismutase (MnSOD) and the breakdown of hydrogen peroxide [21,22]. It has been hypothesized that fluoxetine may promote fat accumulation in hepatocytes and induce hepatotoxicity by suppressing AMPK activation and inhibiting its signaling pathway [23]. In contrast to fluoxetine, metformin activates AMPK, which suppresses SREBP1, reduces lipogenesis, and decreases fat accumulation, thereby exerting a protective effect on the liver. The mammalian system has developed numerous enzymatic and non-enzymatic pathways to counteract the adverse effects of drugs. Several meta-analyses have demonstrated that metformin can improve liver enzyme profiles [24,25]. Several studies have shown that metformin exhibits hepatoprotective effects through the AMPK signaling pathway [26,27]. The present study investigated the role of metformin-induced AMPK activation in preventing fluoxetine-induced hepatotoxicity in rats.
Materials and Methods
Animals
A total of 20 adult male Wistar rats, weighing 240–260 g, were obtained from the Animal Laboratory of Lorestan University of Medical Sciences, Iran. The animals were housed under standard conditions at 21–23°C, with a 12-hour light/dark cycle, and had free access to food and water. To acclimate to the laboratory environment, the rats were individually housed for one week prior to the start of the experiment, with unrestricted access to food and water. We have added a statement regarding ethical considerations. All experimental procedures were approved by the Ethics Committee of Lorestan University of Medical Sciences (IR.LUMS.REC.1401.089) and conducted in accordance with international guidelines for the care and use of laboratory animals.
Experimental Design
Metformin hydrochloride (Sigma-Aldrich, St. Louis, MO, USA), Dorsomorphin (Sigma-Aldrich, St. Louis, MO, USA), and Fluoxetine hydrochloride (Arya Pharmaceutical, Iran) were purchased and prepared for administration. Metformin and fluoxetine (Flux) solutions were dissolved in distilled water. Prior to the experiment, the rats were fasted for 20–24 hours. The animals were then randomly assigned to four groups, each consisting of five rats. Group I served as the control and received 1.0 ml of tap water once daily, while Group II received fluoxetine at a dose of 10 mg/kg/day for seven days [28]. Animals in Group III received fluoxetine at 10 mg/kg/day via intraperitoneal (IP) injection and metformin at 300 mg/kg/day via oral gavage. Group IV animals received fluoxetine (10 mg/kg/day, IP), metformin (300 mg/kg/day, gavage), and dorsomorphin at 10 mg/kg/day via IP injection. In Groups III and IV, the rats received metformin and/or dorsomorphin starting three days before hepatotoxicity induction and continuing for seven days afterward. The 300 mg/kg dose of metformin, recognized as a low dose, was selected for this study due to its ability to stimulate the AMPK signaling pathway [29] and confer liver protection [30, 31]. The animals were randomly assigned to experimental groups, and the allocation was performed using a simple randomization method to ensure unbiased distribution (Schematic Diagram 1).
Serum-Specific Marker of the Liver
Blood was obtained through cardiac puncture and centrifuged at 1,500 × g for 10 min at 4°C. To evaluate liver function, the activities of key serum enzymes—alanine transaminase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP)—were subsequently measured [32].
Histopathology Analysis
Following sacrifice, liver fragments were fixed in 10% formalin, dehydrated through an alcohol series, embedded in paraffin, and subsequently stained with hematoxylin and eosin (H&E) to assess tissue histology [33].
Enzyme-Linked Immunosorbent Assays (ELISA)
Glutathione peroxidase (GPx) and CAT activities were measured using a commercial kit with an automated analyzer. Liver tissues were first sectioned into small pieces, and 3 mL of 1.12 M KCl was added per gram of tissue. The samples were homogenized at −4°C, and the resulting suspension was centrifuged at 9,000 rpm for 21 min using a refrigerated centrifuge. The supernatant was then analyzed with the automated analyzer to determine GPx and CAT enzyme activities [34].
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 9 software. Central tendency and dispersion indices were computed. Data with normal distribution were analyzed using one-way analysis of variance (ANOVA), while non-normally distributed data were assessed using the Kruskal–Wallis test. Significant results were further evaluated with Tukey or Bonferroni post-hoc tests. A p-value less than 0.05 was considered statistically significant.
Results
Effects of AMPK on ALT, AST, and ALP
The results indicated that serum levels of ALT, AST, and ALP were significantly elevated on day 7 following fluoxetine administration in Group II, compared to the healthy control group (P < 0.001). Treatment with metformin at 300 mg/kg markedly reduced ALT, AST, and ALP levels compared to Group II. Conversely, co-administration of metformin and dorsomorphin resulted in increased levels of ALT, AST, and ALP (P < 0.001, Group IV vs. Group III; Figure 1).
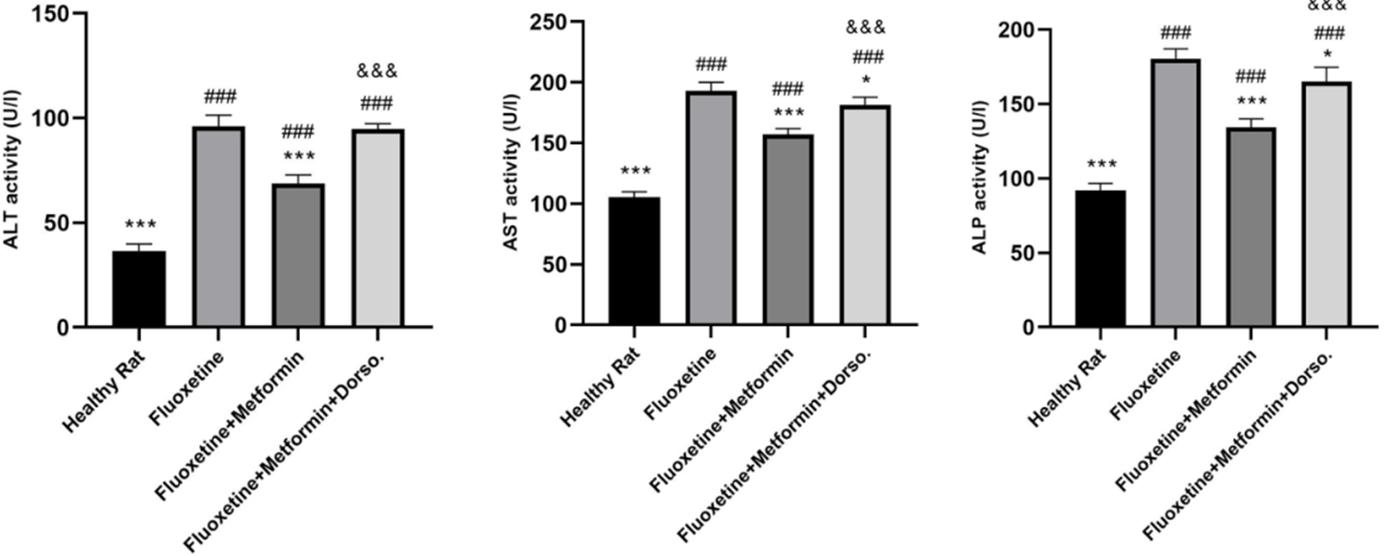
Figure 1. Comparison of the effect of drug administration on serum ALT, AST, and ALP activity. Group I: Healthy rats, Group II: Fluoxetine, Group III: Fluoxetine + Metformin, Group IV: Fluoxetine + Metformin + Dorsomorphin. * P <0.05 vs Group II, *** p< 0.001 vs Group II, ### p< 0.001 vs Group I, &&& p< 0.001 Group IV vs Group III.
Effects of AMPK on oxidative markers
Fluoxetine administration significantly increased GPx levels (P < 0.001, Figure 2) and decreased CAT levels (P < 0.001, Figure 3) compared to the healthy control group. In contrast, metformin treatment significantly elevated CAT levels (P < 0.01) and reduced GPx levels compared to Group II (P < 0.001). However, co-administration of dorsomorphin with metformin abolished the beneficial effects of metformin on these oxidative stress markers.
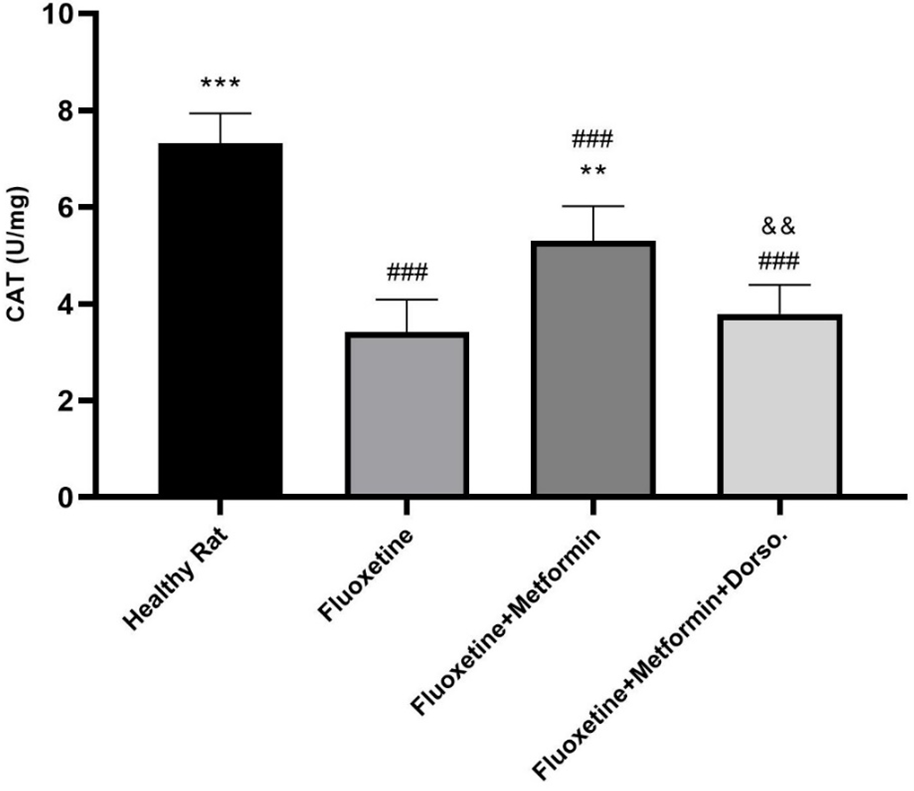
Figure 2. Comparison of the effect of drug administration on catalase (CAT) activity. Group I: Healthy rats, Group II: Fluoxetine, Group III: Fluoxetine + Metformin, Group IV: Fluoxetine + Metformin + Dorsomorphin. ** P <0.01 vs Group II, *** p< 0.001 vs Group II, ### p< 0.001 vs Group I, && p< 0.01 Group IV vs Group III.
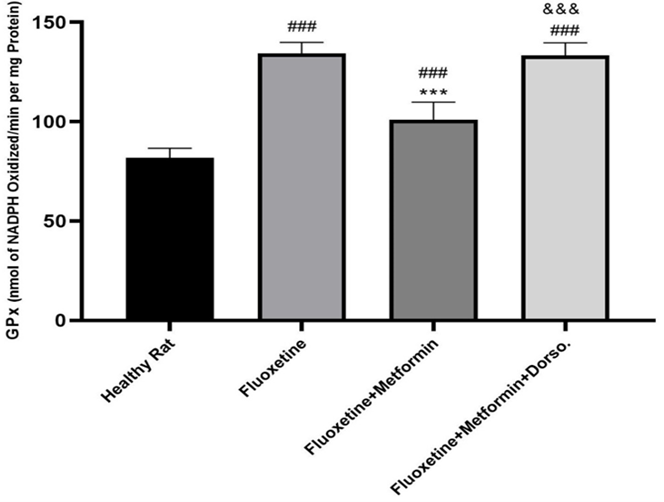
Figure 3. Comparison of the effect of drug administration on GPx activity. Group I: Healthy rats, Group II: Fluoxetine, Group III: Fluoxetine + Metformin, Group IV: Fluoxetine + Metformin + Dorsomorphin. *** p< 0.001 vs Group II, ### p< 0.001 vs Group I, &&& p< 0.001 Group IV vs Group III.
Effects of AMPK on Histopathology
Figure 4 illustrates the grading of necrosis, edema, and inflammatory cell infiltration in the liver. Histological examination of Group II revealed a marked increase in bile duct proliferation, hepatic inflammation, and hepatocellular alterations. In contrast, Group III, treated with metformin, exhibited a significant reduction in bile duct proliferation, hepatic inflammation, and hepatocellular changes. However, in Group IV, which received combined metformin and dorsomorphin treatment, dorsomorphin antagonized the hepatoprotective effects of metformin, resulting in a significant increase in bile duct proliferation, hepatic inflammation, and alterations in hepatocytes.

Figure 4. Effects of the AMPK signaling pathway on histopathological examination of Fluoxetine-induced hepatotoxicity. The yellow arrow indicates the location of inflammation, the blue arrow indicates the hepatocyte changes, and the green arrow indicates the proliferation of the bile ducts. A: Healthy rat (sham-operated), B: Fluoxetine (10 mg kg/day, IP), C: Fluoxetine (10 mg kg/day, IP) + Metformin (300 mg/kg, gavage), D: Fluoxetine (10 mg kg/day, IP) + Metformin (300 mg/kg, gavage) + Dorsomorphin (0.2 mg/ kg, IP).
Discussion
The results of this study demonstrated that treatment with metformin (300 mg/kg) for 10 days significantly improved serum markers of fluoxetine-induced hepatotoxicity, including ALT, AST, and ALP. Additionally, metformin mitigated oxidative stress and reduced histopathological liver damage. However, co-administration of dorsomorphin, an AMPK inhibitor, markedly diminished the hepatoprotective effects of metformin. These findings suggest that activation of the AMPK signaling pathway plays a critical role in protecting against fluoxetine-induced hepatotoxicity.
The findings of the present work demonstrated a significant increase in serum liver function markers (ALT, AST, and ALP) in fluoxetine-treated rats, in agreement with earlier reports [9, 28]. Metformin treatment significantly reduced serum levels of ALT, AST, and ALP compared to Group II. However, co-administration of dorsomorphin attenuated the beneficial effects of metformin, resulting in elevated levels of these enzymes. Several clinical studies have demonstrated that metformin can reduce elevations in liver enzymes [35,37], findings consistent with the present study, which suggests liver injury resulting from damage to cytoplasmic and mitochondrial membranes. Furthermore, ALP serves as a biomarker of hepatobiliary injury and cholestasis [28]. Elevations in ALT activity are generally more pronounced than those of AST in hepatic diseases, reflecting the cellular localization of this enzyme [38]. The increased activity of the cytoplasmic enzyme ALT in fluoxetine-treated animals indicates hepatic dysfunction, likely associated with drug-induced cellular membrane damage [17]. Considering that mitochondrial dysfunction is linked to the adverse effects of several drugs, the observed increase in AST activity—a mitochondria-associated enzyme—in fluoxetine-treated rats may reflect compromised mitochondrial membrane integrity in hepatocytes induced by fluoxetine [39]. Another mechanism by which fluoxetine induces liver injury involves the generation of free radicals and ROS [40, 41]. Elevated levels of these species can disrupt cellular signaling and homeostasis, ultimately leading to cell damage. Studies have demonstrated that the AMPK signaling pathway exhibits antioxidant activity, reducing the activity of enzymes related to oxidative stress and ultimately providing hepatoprotective effects [42,44]. Oxidative stress can inhibit ATP synthesis and production [45]; however, the AMPK signaling pathway regulates ATP production and consumption, thereby increasing ATP levels and promoting cellular homeostasis [46,47]. Moreover, AMPK, a serine/threonine kinase that functions as an energy sensor, is activated when the AMP/ATP ratio increases due to a decline in cellular energy level [20]. In mammals, AMPK is activated through phosphorylation of its catalytic α-subunit by either Ca²⁺/calmodulin-dependent protein kinase β (CaMKKβ) or liver kinase B1 (LKB1) [48]. Activation of AMPK inhibits anabolic pathways while promoting compensatory catabolic processes, including glucose uptake, glycolysis, and fatty acid oxidation [49]. Moreover, AMPK phosphorylation inhibits SREBP1, leading to reduced lipogenesis and lipid accumulation [50]. Fluoxetine is metabolized in the liver primarily via cytochrome P450, producing norfluoxetine and several other metabolites. Fluoxetine has been reported to affect hepatic lipid metabolism and may act as a hepatotoxic agent [8]. Furthermore, inhibition of AMPK activates SREBP1-mediated lipogenesis, resulting in the accumulation of chemically induced hepatic fat [51]. Studies have reported that fluoxetine’s effects on lipid metabolism are mediated by changes in the expression of genes involved in lipogenesis and lipolysis, which are regulated by AMPK signaling inhibition [23,52].
Conclusions
In conclusion, the present study highlights the pivotal role of the AMPK signaling pathway in maintaining liver health by exerting hepatoprotective and antioxidant effects. Modulation of AMPK activity appears to be a key mechanism through which metformin confers protection against DILI. These findings suggest that targeting AMPK could represent a promising therapeutic approach not only for fluoxetine-induced hepatotoxicity but potentially for other forms of chemically induced liver damage as well. Future studies are warranted to elucidate further the molecular pathways involved in AMPK-mediated hepatoprotection and to explore its broader therapeutic applications in preventing or mitigating hepatotoxicity from various pharmacological agents.
Ethical Considerations
The Ethics Committee approved all animal protocols for this study (IR.LUMS.REC.1401.089). The authors affirm that they have fully complied with all ethical standards of research, and confirm that there was no duplicate publication, data fabrication, or plagiarism in this study.
Conflict of Interests
The authors declared that there is no conflict of interest.
Funding
Financial support for this study was provided by Lorestan University of Medical Sciences, Iran.
Acknowledgement
The research team would like to express its gratitude to the distinguished Vice President of Research and Technology at Lorestan University of Medical Sciences, as well as to the staff at the Center for the Care and Maintenance of Laboratory Animals at the university.
Authors' Contributions
Project idea: Amin Hasanvand and Nader Rahimi. Data analyses: Amin Hasanvand and Mehdi Birjandi. Literature search: Amin Hasanvand, Peyman Astaraki, Shakiba Shamsinia. Laboratory experiments: Amin Hasanvand, Peyman Astaraki, Shakiba Shamsinia, Mohammadjavad Nourmohammadi, Fatemeh Hatami, Zahra Haghighatian. Manuscript writing: Amin Hasanvand and Nader Rahimi, Manuscript revision: Amin Hasanvand, Nader Rahimi, and Peyman Amanolahi Baharvand, Final approval: All authors.
References
- Hosack T, Damry D, Biswas S. Drug-induced liver injury: a comprehensive review. Therap Adv Gastroenterol. 2023;16:17562848231163410. [doi: 10.1177/17562848231163410] [pmid: 36968618]
- Gómez-Huelgas R, Sanz-Cánovas J, Cobos-Palacios L, López-Sampalo A, Pérez-Belmonte LM. Glucagon-like Peptide-1 receptor agonists and Sodium− Glucose cotransporter 2 inhibitors for cardiovascular and renal protection: A treatment approach far beyond their Glucose-Lowering effect. Eur J Intern Med. 2022;96:26-33. [doi: 10.1016/j.ejim.2021.11.008] [pmid: 34799233]
- Suzuki A, MinjunChen. Epidemiology and risk determinants of drug‐induced Liver Injury: Current knowledge and future research needs. Liver Int. 2025;45(4):16146. [doi: 10.1111/liv.16146] [pmid: 39494620]
- Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced Liver injury: a review for clinicians. Am J Psychiatry. 2014;171(4):404-15. [doi: 10.1176/appi.ajp.2013.13050709] [pmid: 24362450]
- Nguyen TMD, Klett D, Filliatreau L, Combarnous Y. Inhibition by fluoxetine of LH-stimulated cyclic AMP synthesis in tumor Leydig cells partly involves AMPK activation. Plos One. 2019;14(6):0217519. [doi: 10.1371/journal.pone.0217519] [pmid: 31163038]
- Crone C, Fochtmann LJ, Attia E, Boland R, Escobar J, Fornari V, et al. The American psychiatric association practice guideline for the treatment of patients with eating disorders. Am J Psychiatry. 2023;180(2):167-71. [doi: 10.1176/appi.ajp.23180001] [pmid: 36722117]
- Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, et al. Selective serotonin reuptake inhibitors and adverse effects: a narrative review. Neurology international. 2021;13(3):387-401. [doi: 10.3390/neurolint13030038] [pmid: 34449705]
- Zakaraya Z, Abu Assab M, Tamimi LN, Karameh N, Hailat M, Al-Omari L, et al. Pharmacokinetics and pharmacodynamics: a comprehensive analysis of the absorption, distribution, metabolism, and excretion of psychiatric drugs. Pharmaceuticals. 2024;17(3):280. [doi: 10.3390/ph17030280] [pmid: 38543065]
- Ganguly R, Kumar R, Pandey AK. Baicalin provides protection against fluoxetine-induced hepatotoxicity by modulation of oxidative stress and inflammation. World J Hepatol. 2022;14(4):729. [doi: 10.4254/wjh.v14.i4.729] [pmid: 35646277]
- Nahy AH, Ibrahim MA, Alhayder MN. Alleviation of the hepatotoxicity effects of Fluoxetine using acetylcysteine with the royal jelly in rats. Egypt Liver J. 2025;15(1):35. [Link]
- Ayyash A, Holloway AC. Fluoxetine-induced Hepatic Lipid accumulation is linked to elevated serotonin production. Can J Physiol Pharmacol. 2021;99(9):983-8. [doi: 10.1139/cjpp-2020-0721] [pmid: 33517848]
- Todorović Vukotić N, Đorđević J, Pejić S, Đorđević N, Pajović SB. Antidepressants-and antipsychotics-induced hepatotoxicity. Arch Toxicol. 2021;95(3):767-89. [doi: 10.1007/s00204-020-02963-4] [pmid: 33398419]
- Mohamed Kamel GA, Harahsheh E, Hussein S. Mechanisms underlying the hepatoprotective effect of Silymarin on Fluoxetine-induced liver injury in rats: the implication of peroxisome proliferator–activated Receptor-Gamma (PPAR-γ). Comp Clin Pathol. 2022;31(4):689-98. [Link]
- Gupta A, Kumar R, Ganguly R, Singh AK, Rana HK, Pandey AK. Antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica and its bioactive component ellagic acid against diclofenac induced oxidative stress and hepatotoxicity. Toxicol Rep. 2020;8:44-52. [doi: 10.1016/j.toxrep.2020.12.010] [pmid: 33391996]
- Yılmaz A, Elbey B, Yazgan ÜC, Dönder A, Arslan N, Arslan S, et al. Protective effects of Caffeic Acid Phenethyl Ester on fluoxetine-Induced hepatotoxicity: an experimental study. BioMed Res Int. 2016;2016. [doi: 10.1155/2016/1247191] [pmid: 27144157]
- Mohamed Kamel GA. Vinpocetine attenuates fluoxetine-induced liver damage in rats; Role of Nrf2 and PPAR-γ. Hum Exp Toxicol. 2021;40(12):509-18. [doi: 10.1177/09603271211051597] [pmid: 34669537]
- Zlatković J, Todorović N, Tomanović N, Bošković M, Djordjević S, Lazarević-Pašti T, et al. Chronic administration of Fluoxetine or Clozapine induces oxidative stress in rat liver: a histopathological study. Eur J Pharm Sci. 2014;59:20-30. [doi: 10.1016/j.ejps.2014.04.010] [pmid: 24768740]
- Wang C-P, Lorenzo C, Habib SL, Jo B, Espinoza SE. Differential effects of Metformin on age related comorbidities in older men with type 2 diabetes. J Diabetes Complications. 2017;31(4):679-86. [doi: 10.1016/j.jdiacomp.2017.01.013] [pmid: 28190681]
- Hartwig J, Loebel M, Steiner S, Bauer S, Karadeniz Z, Roeger C, et al. Metformin attenuates ROS via FOXO3 activation in immune cells. Front Immunol. 2021;12:581799. [doi: 10.3389/fimmu.2021.581799] [pmid: 33953705]
- Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48(7):245. [doi: 10.1038/emm.2016.81] [pmid: 27416781]
- Rasheed Z. Therapeutic potentials of catalase: mechanisms, applications, and future perspectives. Int J Health Sci. 2024;18(2):1-6. [pmid: 38455600]
- Grujicic J, Allen AR. Manganese superoxide dismutase: structure, function, and implications in human disease. Antioxidants. 2025;14(7):848. [doi: 10.3390/antiox14070848] [pmid: 40722952]
- Xiong J, Yang H, Wu L, Shang W, Shan E, Liu W, et al. Fluoxetine suppresses AMP-activated protein kinase signaling pathway to promote hepatic lipid accumulation in primary mouse hepatocytes. Int J Biochem Cell Biol. 2014;54:236-44. [doi: 10.1016/j.biocel.2014.07.019] [pmid: 25102273]
- Huang Y, Wang X, Yan C, Li C, Zhang L, Zhang L, et al. Effect of metformin on nonalcoholic fatty liver based on meta-analysis and network pharmacology. Med. 2022;101(43):31437. [doi: 10.1097/md.0000000000031437] [pmid: 36316840]
- Lian J, Fu J. Efficacy of various hypoglycemic agents in the treatment of patients with nonalcoholic liver disease with or without diabetes: a network meta-analysis. Front Endocrinol. 2021;12:649018. [doi: 10.3389/fendo.2021.649018] [pmid: 33841337]
- Yang X, Liu Y, Li M, Wu H, Wang Y, You Y, et al. Predictive and preventive significance of AMPK activation on hepatocarcinogenesis in patients with liver cirrhosis. Cell Death Dis. 2018;9(3):264. [doi: 10.1038/s41419-018-0308-4] [pmid: 29449537]
- Cai L, Hu K, Lin L, Ai Q, Ge P, Liu Y, et al. AMPK dependent protective effects of Metformin on tumor necrosis factor-induced apoptotic liver injury. Biochem Biophys Res Commun. 2015;465(3):381-6. [doi: 10.1016/j.bbrc.2015.08.009] [pmid: 26265045]
- Elgebaly HA, Mosa NM, Allach M, El-Massry KF, El-Ghorab AH, Al Hroob AM, et al. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2018;98:446-53. [doi: 10.1016/j.biopha.2017.12.101] [pmid: 29278855]
- Hauton D. Does long-term metformin treatment increase cardiac lipoprotein lipase? Metabolism. 2011;60(1):32-42. [doi: 10.1016/j.metabol.2009.12.015] [pmid: 20153488]
- Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest. 2012;92(7):1020-32.[doi: 10.1038/labinvest.2012.75] [pmid: 22525431]
- Westerkamp AC, Fujiyoshi M, Ottens PJ, Nijsten MWN, Touw DJ, de Meijer VE, et al. Metformin preconditioning improves hepatobiliary function and reduces injury in a rat model of normothermic machine perfusion and orthotopic transplantation. Transplant. 2020;104(9):271-80. [doi: 10.1097/tp.0000000000003216] [pmid: 32150043]
- Mohammadi A, Kazemi S, Hosseini M, Najafzadeh Varzi H, Feyzi F, Morakabati P, et al. Chrysin effect in prevention of Acetaminophen-Induced hepatotoxicity in rat. Chem Res Toxicol. 2019;32(11):2329-37. [doi: 10.1021/acs.chemrestox.9b00332] [pmid: 31625388]
- Aljobaily N, Viereckl MJ, Hydock DS, Aljobaily H, Wu TY, Busekrus R, et al. Creatine alleviates Doxorubicin-Induced liver damage by inhibiting liver fibrosis, inflammation, oxidative stress, and cellular senescence. Nutr. 2020;13(1):41. [doi: 10.3390/nu13010041] [pmid: 33374297]
- Saeedi Saravi SS, Hasanvand A, Shahkarami K, Dehpour AR. The protective potential of metformin against acetaminophen-induced hepatotoxicity in BALB/C mice. Pharm Biol. 2016;54(12):2830-7. [doi: 10.1080/13880209.2016.1185633] [pmid: 27252117]
- Resuli B, Demiraj V, Babameto A, Sema K, Malaj V. Metformin superior to lowfat diet for the treatment of patients with nonalcoholic fatty liver disease and/or steatohepatitis. Pol Arch Med Wewn. 2012;122(Suppl 1):68-71. [pmid: 23222203]
- Khoshbaten M, Beheshtirouy S, Shayanrad S, Gharekhani A, Rezaee H. Comparison of the efficacy of pioglitazone and metformin on ultrasound grade and liver enzymes level in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Drug Res. 2023;73(4):232-7. [doi: 10.1055/a-1997-0401] [pmid: 36791804]
- Shargorodsky M, Omelchenko E, Matas Z, Boaz M, Gavish D. Relation between augmentation index and adiponectin during one-year metformin treatment for nonalcoholic steatohepatosis: effects beyond glucose lowering? Cardiovasc Diabetol. 2012;11:61. [doi: 10.1186/1475-2840-11-61] [pmid: 22676459]
- Oh RC, Hustead TR, Ali SM, Pantsari MW. Mildly elevated liver transaminase levels: causes and evaluation. Am Fam Physician. 2017;96(11):709-15. [pmid: 29431403]
- Todorović Vukotić N, Đorđević J, Pejić S, Đorđević N, Pajović SB. Antidepressants-and antipsychotics-induced hepatotoxicity. Arch Toxicol. 2021;95(3):767-89. [doi: 10.1007/s00204-020-02963-4] [pmid: 33398419]
- Karimi-Khouzani O, Heidarian E, Amini SA. Anti-inflammatory and ameliorative effects of Gallic Acid on Fluoxetine-induced oxidative stress and liver damage in rats. Pharmacol Rep. 2017;69(4):830-5. [doi: 10.1016/j.pharep.2017.03.011] [pmid: 28599245]
- Beigi T, Safi A, Satvati M, Kalantari-Hesari A, Ahmadi R, Meshkibaf M-H. Protective role of ellagic acid and taurine against Fluoxetine induced hepatotoxic effects on biochemical and oxidative stress parameters, histopathological changes, and gene expressions of IL-1β, NF-κB, and TNF-α in male Wistar rats. Life Sci. 2022;304:120679. [doi: 10.1016/j.lfs.2022.120679] [pmid: 35662648]
- Kim YW, Lee SM, Shin SM, Hwang SJ, Brooks JS, Kang HE, et al. Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radical Biol Med. 2009;47(7):1082-92. [doi: 10.1016/j.freeradbiomed.2009.07.018] [pmid: 19616619]
- Lu Q, Shu Y, Wang L, Li G, Zhang S, Gu W, et al. The protective effect of Veronica ciliata Fisch. Extracts on relieving oxidative stress-induced liver injury via activating AMPK/p62/Nrf2 pathway. J Ethnopharmacol. 2021;270:113775. [doi: 10.1016/j.jep.2021.113775] [pmid: 33406386]
- Gao L, Chen X, Fu Z, Yin J, Wang Y, Sun W, et al. Kinsenoside alleviates alcoholic liver injury by reducing oxidative stress, inhibiting endoplasmic reticulum stress, and regulating AMPK-dependent autophagy. Front Pharmacol. 2022;12:747325. [doi: 10.3389/fphar.2021.747325] [pmid: 35115920]
- Ebanks B, Chakrabarti L. Mitochondrial ATP synthase is a target of oxidative stress in neurodegenerative diseases. Front Mol Biosci. 2022;9:854321. [doi: 10.3389/fmolb.2022.854321] [pmid: 35237666]
- Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66(6):789-800. [doi: 10.1016/j.molcel.2017.05.032] [pmid: 28622524]
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121-35. [doi: 10.1038/nrm.2017.95] [pmid: 28974774]
- Hasanvand A. The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: a new perspective for treatment and prevention of diseases. Inflammopharmacol. 2022;30(3):775-88. [doi: 10.1007/s10787-022-00980-6] [pmid: 35419709]
- Ha J, Guan KL, Kim J. AMPK and autophagy in glucose/glycogen metabolism. Mol Aspects Med. 2015;46:46-62. [doi: 10.1016/j.mam.2015.08.002] [pmid: 26297963]
- Quan HY, Kim SJ, Jo HK, Kim GW, Chung SH. Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK–mTOR–SREBP signaling pathway. Biochem Pharmacol. 2013;85(9):1330-40. [doi: 10.1016/j.bcp.2013.02.007] [pmid: 23435355]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224-36. [doi: 10.1016/j.cmet.2008.07.007] [pmid: 18762023]
- Feng XM, Xiong J, Qin H, Liu W, Chen RN, Shang W, et al. Fluoxetine induces Hepatic Lipid accumulation via both promotion of the SREBP 1c‐Related lipogenesis and reduction of lipolysis in primary mouse hepatocytes. CNS Neurosci Ther. 2012;18(12):974-80. [doi: 10.1111/cns.12014] [pmid: 23137031]
Type of Study:
Research |
Subject:
General










































 , Peyman Astaraki2
, Peyman Astaraki2 

 , Shakiba Shamsinia3
, Shakiba Shamsinia3 

 , Mohammadjavad Nourmohammadi4
, Mohammadjavad Nourmohammadi4 

 , Fatemeh Hatami3
, Fatemeh Hatami3 

 , Mehdi Birjandi5
, Mehdi Birjandi5 

 , Zahra Haghighatian6
, Zahra Haghighatian6 

 , Peyman Amanolahi Baharvand7
, Peyman Amanolahi Baharvand7 

 , Nader Rahimi *8
, Nader Rahimi *8 






