Ethics code: IR.ARUMS.REC.1397.073


Farzaneh E, Bashardoust B, Bahreini A, Mostafazadeh B, Shadnia S, Mehrpour O et al . Results of Treatment with Peritoneal Dialysis in Patients with Aluminum Phosphide Poisoning. IJT 2025; 19 (4) :221-226
URL:
http://ijt.arakmu.ac.ir/article-1-1479-en.html
1- Department of internal medicine, school of medicine, Ardabil university of medical science, Ardabil, Iran.
2- School of Medicine, Ardabil University of Medical Science, Ardabil, Iran. , b_bashardoust@yahoo.com
3- Toxicological Research Center, Excellence Center & Department of Clinical Toxicology, Loghman Hakim Hospital, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
4- Michigan Poison & Drug Information Center, Wayne State University School of Medicine, Detroit, MI, USA.
5- Department of Pharmacology, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
Full-Text [PDF 426 kb]
(151 Downloads)
|
Abstract (HTML) (400 Views)
Full-Text: (6 Views)
Introduction
Aluminum phosphide, commonly called the "rice pill" in Iran, is a highly hazardous pesticide used to preserve rice and other grains in storage facilities and during transportation. Unfortunately, this pesticide is extensively employed in developing nations [1-3]. The toxicity of this pill is due to the production of phosphine gas in the presence of moisture in the air, water, or hydrochloric acid in the stomach. The main ways to contact this chemical are oral and respiratory routes [4]. Aluminum phosphide poisoning is a situation with high mortality due to the lack of a specific antidote [2, 5]. Poisonings usually manifest quickly, with most deaths being caused by cardiovascular problems. Deaths occurring after 24 hours are usually attributed to liver failure [6]. According to a meta-analysis, the mortality rate of aluminum phosphide poisoning is approximately 27%, with men having a higher mortality rate than women. Poisoning at a younger age is associated with better results. Severe hypotension and cardiac poisoning are among the most serious complications of this poisoning and are associated with a high rate of death [7].
There has been an increased report of self-poisoning with aluminum phosphide tablets from Iran in recent years [8]. Cardiogenic shock is the most important clinical feature, caused by direct myocardial toxicity of phosphine gas and severe metabolic acidosis [2, 9, 10]. Various interventions are advised to reduce the absorption of toxins and raise the blood pH to prevent or alleviate metabolic acidosis [11]. Correcting metabolic acidosis may improve the patient's overall condition [12].
Several extracorporeal treatments can help remove poisons from the body. Peritoneal dialysis (PD) is not considerably effective in treating acute poisoning because it can only achieve a clearance of <20 ml/min, which is much lower than the clearance achievable by hemodialysis (HD). However, PD is easier to perform in resource-limited areas and newborns [13]. The PD has been used in Iran as an alternative treatment along with other methods, but the percentage of its use has been much lower than HD. Considering the limitations of HD, such as being time-consuming and complications, such as a high prevalence of cardiovascular disease and high blood pressure, PD is more important [14]. In a study, Bashardoust et al. reported two successful oral aluminum phosphide treatment cases with PD. Moreover, PD may restore various body functions by improving acidosis and possibly aiding in eliminating these toxins [5].
Due to the recent popularity of agricultural poisons, such as rice tablets, there has been an increase in the number of people poisoned by these tablets in medical centers. Additionally, limited information has been reported on the effect of PD as a treatment method for rice tablet poisoning. Therefore, the present study aimed to investigate the results of patients' PD treatment.
Materials and Methods
The present research utilized a cross-sectional design to retrospectively analyze the medical records of patients diagnosed with aluminum phosphide poisoning and treated at Ardabil City Hospital in Iran between April 2015 and March 2017. The study focused exclusively on patients who had ingested rice tablets containing aluminum phosphide and subsequently underwent PD as part of their treatment regimen.
Inclusion and exclusion criteria
Patients were included in the study if they met the following criteria: confirmed ingestion of aluminum phosphide (rice tablets), treatment with PD, and comprehensive medical records detailing treatment before, during, and after the dialysis procedure. Patients were excluded if they received treatments known to affect metabolic parameters significantly before PD, such as magnesium sulfate and calcium gluconate, to isolate the effects of PD on the outcomes of aluminum phosphide poisoning.
Method setting
For PD, the catheter was inserted by a general surgeon parallel to the umbilicus on either the left or right side of the peritoneum, directed toward the right lower quadrant (RLQ) area. Due to the inadequacy of the acute catheter, the surgeon opted for the largest size orange Nelaton catheter, featuring additional side holes. A 1.5% glucose-based solution was administered, with the dwell time initially set to 1 hour. Once the arterial blood gas (ABG) was corrected, the dwell time was extended to 2 hours. If the acid-base balance remained stable for 6 hours, it was further adjusted to 6-hour and 1-hour intervals. Finally, after three days of monitoring pressure and ABG levels with no complications, the catheter was discontinued.
Additional routine treatments for patients include gastric lavage, activated charcoal, gluconate administration, magnesium sulfate, bicarbonate vials, and vitamins E and C. Intubation was performed when required.
Data extraction
Data were extracted using a standardized checklist developed for this study. The checklist included demographic information (age and gender), the amount of aluminum phosphide ingested, clinical symptoms upon arrival (including vital signs and gastrointestinal, respiratory, and neurological symptoms), laboratory test results (pH, PCO2, bicarbonate, open base, blood pressure, electrolytes including K+ and Na+), and the outcome for the patient (recovery or death). The comprehensive data collection effort was designed to fully understand the patient's condition and how they responded to treatment.
Statistical analysis
The data collected from the 27 patients included in this study were analyzed using the SPSS (version 21) software (IBM Corp., Armonk, NY, USA). Descriptive statistics, including mean ± standard deviation (SD) for continuous variables and frequencies (percentages) for categorical variables, were used to summarize the demographic and clinical characteristics of the patient cohort. The primary aim of the statistical analysis was to compare clinical and laboratory parameters at the time of admission and discharge, thereby assessing the effectiveness of PD in treating aluminum phosphide poisoning.
The normality of data distribution was assessed using the Shapiro-Wilk test for continuous variables. According to these results, paired t-tests (for normally distributed data) or Wilcoxon signed-rank tests (for data not normally distributed) were applied to identify significant changes in clinical and laboratory parameters from admission to discharge. The choice of test was guided by the distribution characteristics of each variable, ensuring the appropriateness of the statistical methods used.
A significance level of p<0.05 was predetermined for all statistical tests to identify statistically significant differences. Pearson's correlation coefficient was also calculated to explore the relationships between patients' outcomes (recovery or death) and key biochemical parameters (e.g., blood pH and bicarbonate levels).
Sample size justification
The study sample comprised all 27 patients meeting the inclusion criteria during the specified study period. This sample size was determined by the availability of cases fitting the study criteria, acknowledging that the relatively rare incidence of aluminum phosphide poisoning limits the potential for a larger cohort. The implications of this sample size for the study's power and generalizability are discussed in the limitations section.
Results
Among all the patients, 13 (48.13%) were 30 or younger, and the rest were over 30. Moreover, 13 people (48.13%) were female. The average age of patients was 35.1±16.9 years, with an age range from 16 to 73 years. Patients stayed in the hospital for an average of 3.3±2.04 days.
The distribution of clinical symptoms is presented in Figure 1. The most prevalent symptom was nausea and vomiting, observed in 18 cases (66.67%), followed by abdominal pain in 11 cases (40.74%). Additionally, six patients (22.2%) underwent intubation. Patients consumed an average of 1.5±1.00 tablets of aluminum phosphide.
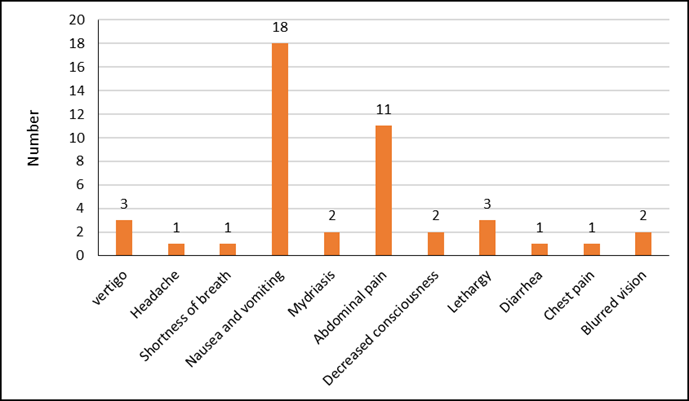
Figure 1. Distribution of clinical symptoms in patients with aluminum phosphide poisoning.
There was a significant difference between the values of acidity (pH), partial pressure of carbon dioxide (PCO2), bicarbonate, base excess, blood pressure (BP), creatinine (Cr), sodium (Na), and potassium (K) at the time of starting the treatment and at the time of discharge. The pH, PCO2, bicarbonate, and base excess values increased at discharge; however, the other variables, including BP, Cr, Na, and K, decreased. Temperature and respiratory rate variables did not change significantly (Table 1). It is noteworthy that the mortality rate was 14.81% (four cases).
Pearson's correlation results showed a significant correlation between patients' outcomes and blood pH and bicarbonate levels (P=0.0001 and 0.022, respectively).
Table 1. Comparison of measurements of evaluated parameters at admission and discharge
| Parameters |
Admission (Mean±SD) |
Discharge (Mean±SD) |
P-value |
| pH |
7.35±0.21 |
7.45±0.12 |
0.04* |
| Partial pressure of carbon dioxide (PCO2) mmHg |
22.64±2.5 |
41.17±3.03 |
0.001*** |
| Bicarbonate (mmol/L) |
11.16±2.84 |
23.8±2.77 |
0.001*** |
| Base excess |
-14.82±2.72 |
1.38±2.86 |
0.001*** |
| Blood pressure (mmHg) |
14.9±0.18 |
13.8±0.15 |
0.02* |
| Creatinine (Cr) mg/dl |
1.11±0.39 |
1.01±0.37 |
0.001*** |
| Sodium (Na) mmol/L |
146.6±1.83 |
140.57±1.11 |
0.001*** |
| Potassium (K) mmol/L |
4.7±0.13 |
4.16±0.1 |
0.001*** |
| Temperature |
36.76±0.37 |
36.85±0.22 |
0.16 |
| Respiratory rate |
18.52±5.5 |
18.26±3.65 |
0.41 |
*P≤0.05 indicates statistical significance; ***P≤0.001 indicates high statistical significance.
Discussion
The present study represents one of the most comprehensive evaluations of PD as a primary treatment option for severe metabolic acidosis resulting from aluminum phosphide poisoning, offering new insights into its efficacy and mechanism of action in this context. It was observed that there was a significant difference between pH, PCO2, bicarbonate, base excess, blood pressure, and blood electrolytes (K+, Na+) at admission and after PD.
The timely detection and initiation of treatment are crucial in addressing the effects of phosphine gas, which can lead to organ failure and other serious complications [15]. The research conducted by Taramsary et al. and Pajoumand et al. concluded that in cases of poisoning with rice tablets, rapid emptying of stomach contents and gastric lavage with a potassium permanganate solution as an oxidant are recommended. In addition, intravenous administration of magnesium sulfate and calcium gluconate solution is considered an effective oxidant [3, 16]. Other studies obtained some promising results using high-dose insulin therapy, Extracorporeal Membrane Oxygenation (ECMO) with Trimetazidine and Magnesium, Intra-aortic Balloon Pump (IABP), and N-acetyl cysteine (NAC) [17-23].
In this recent research, following the findings of previous studies, all patients underwent rapid gastric emptying and lavage upon admission. Additionally, patients were administered activated charcoal, calcium gluconate, magnesium sulfate, and a vial of sodium bicarbonate.
A study by Mehrpour et al. previously demonstrated that among individuals poisoned and presenting with hyponatremia, hypokalemia, hyperkalemia, bicarbonate levels below 15, and bicarbonate levels between 15 and 20, the mortality rates were 66%, 87.5%, 100%, 74.19%, and 64%, respectively. These findings underscore the significance of electrolyte disorders in determining patient prognosis [24]. The results of the present research indicated a significant relationship between mortality and pH and bicarbonate.
Several theories suggest that PD effectively treats severe metabolic acidosis. Only Bashardoust et al. reported two cases successfully treated with PD. Following the PD procedure, there was an improvement in the PH, PCO2, bicarbonate level, and base excess [5]. The present study also indicated that PH, PCO2, bicarbonate level, and base excess increased significantly after PD, indicating the treatment's effectiveness. In addition, the levels of sodium, potassium, and creatinine decreased significantly. While previous research has pointed to the potential of PD in treating aluminum phosphide poisoning, the findings of the present investigation extend this knowledge by quantitatively assessing changes in key biochemical parameters, thereby providing a clearer picture of the treatment's physiological impact.
The primary pathology of aluminum phosphide poisoning involves cellular ischemia, leading to multiorgan failure, and persistent hypotension is a result of this process. Cellular ischemia, vasodilation, and the release of free radicals can accelerate this process and result in death. The PD may improve acidosis and potentially remove these toxins, restoring various organ functions [5, 25]. The significant correlations identified between patients' outcomes and blood pH and bicarbonate levels post-PD treatment underscore the importance of metabolic correction in managing aluminum phosphide poisoning, a connection that has not been thoroughly explored in earlier studies. The current work contributes to novel findings on the role of PD in mitigating the effects of severe metabolic acidosis induced by aluminum phosphide. It suggests that PD can be more than just a supportive treatment; it can be a lifesaving intervention in critical cases.
The present research fills a critical gap in the literature by presenting detailed outcomes of PD treatment in a larger cohort of aluminum phosphide poisoning cases than previously reported, offering robust evidence of its potential benefits and limitations. By demonstrating the effectiveness of PD in improving patient outcomes in a resource-limited setting, this study challenges existing paradigms and advocates for a broader consideration of PD in the standard treatment protocols for aluminum phosphide poisoning, especially where HD is not feasible.
The methodology employed constitutes its primary strength. By conducting a comprehensive analysis of patient data before and after PD treatment, the authors have established a replicable framework for future research. This framework is poised to validate and enhance the findings of the present study, potentially culminating in the formulation of standardized treatment guidelines.
Limitations and future research directions
While the present study presents new insights into treating aluminum phosphide poisoning with PD, several limitations must be addressed to enhance the robustness and applicability of findings to broader contexts.
1. Small Sample Size and Generalizability: The study was conducted with a relatively small population of 27 patients, limiting the statistical power and generalizability of the results. Future studies should aim to include larger sample sizes by possibly conducting multicenter research. This approach would not only increase the number of participants but also diversify the patient population, thereby enhancing the generalizability of the findings.
2. Retrospective Design: The study's retrospective nature restricts the control over variables and reliance on the accuracy and completeness of medical records. Prospective studies are recommended to allow for standardized data collection, more rigorous control of confounding variables, and the ability to adjust the study design and data collection methods in real time.
3. Lack of Control Group: The present study did not include a control group of patients treated without PD, making it difficult to isolate the effect of PD from other treatments or the natural progression of the poisoning. Future research should consider employing a comparative design, including control groups receiving alternative treatments, to ascertain PD's efficacy more definitively.
4. Incomplete Data: Excluding cases due to incomplete information highlights the challenge of relying on existing medical records. Implementing more stringent data collection and management protocols in clinical settings can mitigate this issue. Additionally, future studies could employ more rigorous inclusion criteria and methods to handle missing data, such as multiple imputation techniques, to maximize the use of available information.
5. Geographic and Demographic Limitations: The findings of the present study are based on a specific geographic region and demographic, which may not fully represent populations in other areas where aluminum phosphide poisoning is prevalent. Expanding future research to include a broader range of geographic locations and demographic groups would help assess PD's effectiveness across different settings.
6. Detailed Mechanistic Insights: While the study provides evidence of the efficacy of PD in treating severe metabolic acidosis caused by aluminum phosphide poisoning, it lacks a detailed exploration of the underlying mechanisms. Further research should aim to elucidate the physiological and molecular mechanisms by which PD exerts its beneficial effects, possibly by including more detailed biochemical analyses and exploring toxin removal rates.
Engaging in multicenter and international collaborations can enhance the robustness and generalizability of research on the effectiveness of PD in treating severe metabolic acidosis by enabling the collection of larger, more diverse datasets. Future research may prioritize prospective study designs to standardize data collection and manage confounding variables effectively, incorporating control groups to provide comparative insights into PD's efficacy. Additionally, adopting enhanced data collection protocols will improve data quality and completeness, while mechanistic studies on PD's role in treating specific conditions like aluminum phosphide poisoning could offer critical insights into optimizing treatment protocols.
Conclusions
The present work compellingly demonstrates that PD offers a viable and effective treatment for severe metabolic acidosis resulting from aluminum phosphide poisoning, emphasizing the integration of PD with conventional treatments as a promising management strategy for this critical condition. It highlights the importance of rapid and specific treatment interventions to correct severe metabolic disturbances caused by the poisoning. A comprehensive approach is recommended to improve patient outcomes and advance the treatment of aluminum phosphide poisoning, incorporating multidisciplinary strategies that merge the latest research findings, innovative treatment modalities, and patient-focused care. This approach should cover a spectrum of interventions, from immediate clinical actions, including swift medical response, thorough laboratory testing, and early treatment initiation, to more extensive preventive efforts, such as controlling the substance's distribution, public education to reduce exposure risks, introducing safer agricultural practices, and researching PD's potential for toxin removal. This integrated strategy aims to enhance patient care and prevent future poisoning incidents by addressing the immediate and broader challenges associated with aluminum phosphide poisoning.
Data Access and Responsibility
The data used or analyzed in this research is available upon request from the corresponding author.
Ethical Considerations
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Ethics Committee of Ardabil University of Medical Sciences, Iran (Code: IR.ARUMS.REC.1397.073). Although this study involved the analysis of existing patient records and posed minimal risk to participants, all patient information was anonymized, and confidentiality was maintained throughout the research process. We had no participants under the age of 16. Informed consent was obtained from all participants. For cases where patients had a severely reduced level of consciousness, informed consent was obtained from their legal guardian or an appropriate representative on their behalf.
Authors' Contributions
EF & BB designed and evaluated research. AM was responsible for gathering the data for the study. OM, BM & SS ensured the content’s scientific accuracy and tackled any grammatical and syntax issues. MR wrote the manuscript and updated the paper following the comments of the editors and the reviewers. All authors have read and approved the final version of the manuscript.
Acknowledgement
The authors express their gratitude to the management and staff of Ardabil City Hospital for their support and approval of the study protocol prior to its implementation.
Conflict of Interests
The authors declared thate there is no competing interests.
Funding
This research project received no financial support.
References
- Farzaneh E, Mostafazadeh B, Naslseraji F, Shafaiee Y, Ghobadi H, Amani F. Study Clinical symptoms and para-clinical findings in poisoning patient with aluminum phosphide in patients referred to Imam Khomeini Hospital in Ardabil (Northwest of Iran). Int J Med Toxicol Forensic Med. 2015;5(4):175-9. [DOI: 10.22037/ijmtfm.v5i4(Autumn).9285]
- Mostafazadeh B, Farzaneh E. A novel protocol for gastric lavage in patients with aluminum phosphide poisoning: a double-blind study. Acta Med Iran. 2012;50(8):530-4. [PMID: 23109024]
- Rahbar Taramsary M, Orangpoor R, Zarkami T, Palizkar M, Mousavian S. Survey patients poisoned with aluminum phosphide (rice tablet). J Guilan Univ Med Sci. 2006;14(56):42-7. [Link]
- Khodabandeh F, Kahani A, Soleimani G. The study of fatal complications of “rice tablet “poisoning. Iran J Forensic Med. 2014;20(2):27-36. [Link]
- Bashardoust B, Farzaneh E, Habibzadeh A, Sadeghi MSS. Successful treatment of severe metabolic acidosis due to acute aluminum phosphide poisoning with peritoneal dialysis: a report of 2 cases. Iran J Kidney Dis. 2017;11(2):165-7. [PMID: 28270650]
- Taheri SK, Afzali S, Khaled Naghshbandi M, Norouzi F, Mohammadi N. Report of two cases of accidental poisoning due to" rice tablet" misuse. Iran J Forensic Med. 2011;17(3):199-203. [Link]
- Abbaspour A, Nasri Nasrabadi Z, Ghorbani A, Marashi SM. Successful treatment of acute aluminum phosphide poisoning induced heart failure: a case report. Razi J Med Sci. 2013;20(107):78-83. [Link]
- Etemadi-Aleagha A, Akhgari M, Iravani FS. Aluminum phosphide poisoning-related deaths in Tehran, Iran, 2006 to 2013. Medicine. 2015;94(38):e1637. [DOI: 10.1097/MD.0000000000001637] [PMID: 26402837]
- Bumbrah GS, Krishan K, Kanchan T, Sharma M, Sodhi GS. Phosphide poisoning: a review of literature. Forensic Sci Int. 2012;214(1-3):1-6. [DOI: 10.1016/j.forsciint.2011.06.018]
- Mehrpour O, Farzaneh E, abdollahi M. Successful treatment of aluminum phosphide poisoning with digoxin: a case report and review of literature. Int J Pharmacol. 2011;7(7):761-4. [DOI:10.3923/ijp.2011.761.764]
- Jaiswal S, Verma R, Tewari N. Aluminum phosphide poisoning: Effect of correction of severe metabolic acidosis on patient outcome. Indian J Crit Care Med. 2009;13(1):21-4. [DOI: 10.4103/0972-5229.53111]
- Ashegh H, Rezaii J, Esfandiari K, Roueentan A, Abouzari M. Laparoscopic placement of peritoneal dialysis catheters in CAPD patients: complications and survival. Tehran Univ Med Sci J. 2008;66(3):186-90. [Link]
- Ghannoum M, Roberts DM. Management of poisonings and intoxications. Clin J Am Soc Nephrol. 2023: 18(9):1210-21. [DOI: 10.2215/CJN.0000000000000057]
- Abbaszadeh A, Javanbakhtian R, Salehee S, Motvaseliyan M. Comparative assessment of quality of life in hemodialysis and kidney transplant patients. JSSU. 2010;18(5):461-8. [Link]
- Manouchehri A, Ghareghani S, Shamaei S, Nilechi M, Bossaghzadeh F. A review on Aluminum phosphide (Rice Tablets) poisoning; from exposure to the applicable and new strategies of clinical management. Adv Life Sci. 2021;8(4):326-32. [DOI:10.62940/als.v8i4.1208]
- Pajoumand A, Jalali N, Abdollah M, Shadnia S. Survival following severe aluminium phosphide poisoning. J Pharm Pract Res. 2002;32(4):297-9. [DOI:10.1002/jppr2002324297]
- Anbalagan LC, Arora N, Pannu AK. Management of acute aluminum phosphide poisoning: has anything changed? Drug Metab Lett. 2021;14(2):106-16. [DOI: 10.2174/1872312814666210813115625] [PMID: 34818996]
- Sedaghattalab M. Treatment of critical aluminum phosphide (rice tablet) poisoning with high-dose insulin: a case report. J Med Case Rep. 2022;16(1):192. [DOI: 10.1186/s13256-022-03425-4]
- Rao CC, Himaaldev GJ. STEMI in young befogged by aluminum phosphide toxicity—role of ECMO as salvage therapy and trimetazidine and magnesium to suppress arrhythmias. Indian J Crit Care Med. 2020;24(8):727-30. [DOI: 10.5005/jp-journals-10071-23533] [PMID: 33024386]
- Mehrpour O, Asadi S, Yaghoubi MA, Azdaki N, Mahmoodabadi N, Javadmoosavi S. Cardiogenic shock due to aluminum phosphide poisoning treated with intra-aortic balloon pump: a report of two cases. Cardiovasc Toxicol. 2019;19(5):474-81. [DOI: 10.1007/s12012-019-09513-0] [PMID: 30949845]
- Bagherian F, Kalani N, Rahmanian F, Abiri S, Hatami N, Foroughian M, et al. Aluminum phosphide poisoning mortality rate in Iran; a systematic review and meta-analysis. Arch Acad Emerg Med. 2021;9(1):e66. [DOI: 10.22037/aaem.v9i1.1396] [PMID: 34870232]
- Mehrpour O, Amouzeshi A, Dadpour B, Oghabian Z, Zamani N, Amini S, et al. Successful treatment of cardiogenic shock with an intraaortic balloon pump following aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2014;65(1):121-6. [DOI: 10.2478/10004-1254-65-2014-2393] [PMID: 24445229]
- Taghaddosinejad F, Farzaneh E, Ghazanfari-Nasrabad M, Eizadi-Mood N, Hajihosseini M, Mehrpour O. The effect of N-acetyl cysteine (NAC) on aluminum phosphide poisoning inducing cardiovascular toxicity: a case–control study. Springerplus. 2016;5:1948. [DOI: 10.1186/s40064-016-3630-2]
- Mehrpour O, Shadnia S, Soltaninezhad K, Yaghmaei A. Evaluation of electrolytes and blood glucose level in aluminum phosphide poisoning. Iran J Forensic Med. 2009;15(1):49-53. [Link]
- Hosseini SF, Forouzesh M, Maleknia M, Valiyari S, Maniati M, Samimi A. The molecular mechanism of aluminum phosphide poisoning in cardiovascular disease: Pathophysiology and diagnostic approach. Cardiovasc Toxicol. 2020;20:454-61. [DOI: 10.1007/s12012-020-09592-4] [PMID: 32712815]
Type of Study:
Research |
Subject:
General










































 , Bahman Bashardoust *2
, Bahman Bashardoust *2 

 , Amin Bahreini3
, Amin Bahreini3 

 , Babak Mostafazadeh3
, Babak Mostafazadeh3 

 , Shahin Shadnia3
, Shahin Shadnia3 

 , Omid Mehrpour4
, Omid Mehrpour4 

 , Maral Ramezani5
, Maral Ramezani5 



