Ethics code: Not applicable


1- Tashkent Institute of Chemical Technology, Department of Biotechnology, Tashkent, Uzbekistan. , hamzagiza6@gmail.com
2- Institute of Bioorganic Chemistry of the Academy of Sciences of the Republic of Uzbekistan.
3- Tashkent Institute of Chemical Technology, Department of Biotechnology, Tashkent, Uzbekistan.
Full-Text [PDF 494 kb]
(190 Downloads)
|
Abstract (HTML) (382 Views)
Full-Text: (5 Views)
Introduction
The growing interest in natural compounds with therapeutic potential has intensified research on flavonoid-rich food sources, such as Fagopyrum esculentum (buckwheat). As a pseudocereal, buckwheat is valued not only for its nutritional content but also for its abundant bioactive constituents, particularly flavonoids like rutin and quercetin [1]. Fagopyrum esculentum, commonly known as common buckwheat, is a plant with high biological value. Its composition includes proteins, polyphenolic compounds, flavonoids, and essential minerals, all of which contribute positively to human health. Notably, these cultivars were found to contain measurable amounts of the flavonoid rutin, which is recognized for its cardiovascular protective effects and its ability to scavenge free radicals. Mineral analysis revealed that buckwheat seeds are rich in copper (Cu), manganese (Mn), and magnesium (Mg) [2]. Buckwheat bioactive compounds and essential minerals play vital roles in maintaining human health, contributing to antioxidant defense, metabolic functions, and the prevention of age-related disorders [3]. Polyphenolic compounds, such as quercetin and rutin, abundantly found in Fagopyrum esculentum, are recognized for their potential to mitigate oxidative stress (OS)-related pathologies, including cancer, coronary artery disease, and atherosclerosis. Nevertheless, the biological effects of these compounds are dose-dependent, as excessive concentrations may exhibit pro-oxidant properties, potentially impairing cellular viability and growth [4]. Rutin and quercetin exhibit protective effects against doxorubicin-induced hepatotoxicity by reducing OS, enhancing antioxidant enzymes, such as superoxide dismutase (SOD), glutathione-S-transferase (GST), and glutathione peroxidase (GPx), downregulating tumor necrosis factor-alpha (TNF-α) and p53, and activating nuclear factor erythroid 2–related factor 2 (Nrf2). These findings highlight their potential as antitoxic agents [5]. Sirtuins (SIRT1–7) constitute a group of NAD⁺-dependent histone deacetylases that influence numerous cellular functions by modifying both histone and non-histone proteins. Emerging evidence highlights their involvement in OS regulation. Specifically, SIRT1, SIRT3, and SIRT5 contribute to cellular defense against reactive oxygen species (ROS), whereas SIRT2, SIRT6, and SIRT7 are involved in controlling gene expression and signaling pathways linked to oxidative damage [6]. Recent studies highlight the importance of sirtuins—NAD⁺-dependent deacetylases—as regulators of cellular homeostasis, aging, and detoxification. The SIRT2, the most studied member, is particularly involved in DNA repair, mitochondrial biogenesis, OS resistance, and metabolic regulation. Natural compounds capable of activating sirtuins are of growing interest as potential anti-aging and detoxification agents [7].
Endocrine-disrupting chemicals (EDCs) pose increasing health concerns due to their ability to exert direct or indirect hormonal effects, even at low estrogenic activity. Recent studies highlight endoplasmic reticulum (ER) stress as a key pathway mediating EDC-induced cellular dysfunction, including organ damage, apoptosis, and altered cell proliferation [8]. Phthalates, such as di(2-ethylhexyl) phthalate (DEHP) and di(n-butyl) phthalate (DBP), along with bisphenol A (BPA), are categorized as EDCs. These compounds possess estrogenic activity, exhibit anti-androgenic effects, and disrupt thyroid hormone (TH) homeostasis [9]. Dietary polyphenols, such as flavonoids, are abundant in plant-based foods and have gained attention for their antioxidant properties. Evidence from preclinical and clinical studies indicates that long-term consumption of polyphenol-rich diets may reduce the risk of OS-related diseases, including cardiovascular diseases (CVDs), neurodegenerative disorders, cancer, diabetes, and infections. Their protective effects are primarily attributed to their ability to neutralize ROS and modulate inflammation and cellular signaling pathways and exert their protective effects by inhibiting pro-inflammatory signaling pathways such as NF-κB, modulating genes involved in cell survival and proliferation, and activating deacetylase enzymes like sirtuin 1 (SIRT1), which are associated with cellular stress resistance and longevity [10,11].
The present study aimed to analyze the polyphenolic composition of roasted and green buckwheat (Fagopyrum esculentum) using high-performance liquid chromatography (HPLC). It also evaluated the detoxification potential of the major flavonoid rutin through its in silico interaction with SIRT1, emphasizing its role as a modulator of sirtuin-related protective pathways.
Materials and Methods
Plant materials
Two types of buckwheat (Fagopyrum esculentum Moench) seeds, green (unroasted) and roasted, were used in the present investigation. Both seed types were procured from a certified agricultural supplier in Uzbekistan, ensuring traceability and compliance with national quality standards. The green buckwheat seeds were collected in their raw, unprocessed form, while the roasted buckwheat samples underwent thermal processing typical for commercial grain preparation. Upon receipt, the seeds were visually inspected to confirm the absence of physical damage, foreign matter, or signs of microbial contamination.
Following inspection, each sample type was separately ground using a laboratory-grade stainless steel grinder to obtain a homogeneous fine powder. This step was essential to increase the surface area for efficient solvent penetration during the extraction process. The powdered materials were stored in airtight, light-resistant containers at 4°C to prevent oxidative degradation and preserve the integrity of bioactive compounds, particularly flavonoids and phenolic acids, prior to further experimental procedures.
Extraction and HPLC analysis of flavonoids
The identification and quantification of flavonoid compounds in the sample were carried out using HPLC. An accurately weighed portion of 5–10 g of the sample was transferred into a 300 mL flat-bottom flask. Subsequently, 50 mL of 70% ethanol was added. The mixture was equipped with a magnetic stirrer and a reflux condenser, and heated under reflux at 70–80 °C for 1 hour with continuous stirring. After this period, the mixture was allowed to stir at room temperature for an additional 2 hours. The resulting extract was allowed to settle and then filtered.
To maximize flavonoid recovery, the remaining solid residue was subjected to two additional extractions with 25 mL of 70% ethanol each. All filtrates were combined and transferred to a 100 mL volumetric flask, then brought to volume with 70% ethanol. The final solution was centrifuged at 6000–8000 rpm for 20–30 minutes. The supernatant obtained after centrifugation was used for chromatographic analysis.
According to the literature, phosphate and acetate buffer systems, along with acetonitrile, are commonly used as eluents for the separation of flavonoids and steroids by HPLC [12]. In this study, a phosphate buffer system combined with acetonitrile was employed as the mobile phase.
HPLC analysis
The HPLC conditions were as follows: Instrument: Agilent 1200 HPLC system (equipped with an autosampler); Column: Eclipse XDB-C18 (reverse-phase), 5 µm, 4.6 × 250 mm; Detector: Diode-array detector (DAD), wavelengths set at 254 nm and 272 nm for flavonoid identification. Mobile phase: Phosphate buffer: Acetonitrile (v/v) 0–5 min: 95:5, 6–12 min: 70:30, 12–13 min: 50:50, 13–15 min: 95:5; Flow rate: 0.8 mL/min; Column temperature: 30°C; Injection volume: 10 µL Initially, standard solutions of reference flavonoids were injected into the HPLC system to calibrate retention times and UV absorbance. Subsequently, the prepared sample solutions were analyzed under identical chromatographic conditions.
In silico docking
Molecular docking analysis was performed using CB-Dock2 to investigate the binding interactions of rutin and quercetin with the active site of the SIRT1 protein (PDB ID: 8QT1). The binding affinities of the ligands to the target protein were evaluated and compared based on their docking scores.
Results
The HPLC analysis revealed significant differences in flavonoid concentrations between roasted and green buckwheat samples (Figure 1).
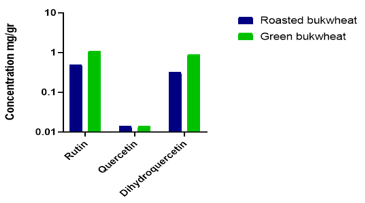
Figure 1. Comparison of flavonoid concentrations (mg/g) dihydroquercetin, rutin, and quercetin in roasted and green buckwheat samples as determined by HPLC analysis.
The content of dihydroquercetin was markedly higher in green buckwheat (0.9 mg/g) compared to roasted buckwheat (0.32 mg/g), indicating that thermal processing may lead to degradation of this compound. Similarly, the level of rutin was almost twice as high in green buckwheat (1.09 mg/g) as in roasted samples (0.5 mg/g), suggesting greater preservation of this flavonoid in unprocessed grains. In contrast, the concentration of quercetin remained unchanged (0.014 mg/g) in both forms, implying either thermal stability or naturally low abundance in buckwheat. These findings suggest that green buckwheat is a richer source of bioactive flavonoids, particularly rutin and dihydroquercetin, and may offer enhanced potential for use in functional foods aimed at antioxidant support and detoxification.
The molecular docking simulation revealed a stable interaction between rutin and the active site of the sirtuin protein. As visualized in the 3D structure (Figure 2), the rutin molecule is positioned within the binding pocket of the sirtuin enzyme, establishing multiple stabilizing interactions.
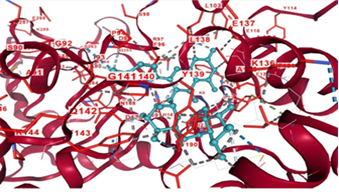
Figure 2. Molecular docking analysis of rutin with sirtuin.
(It has been reported that the data on binding affinity and the degree of interaction between the ligand and the target protein ).
Table 1. Predicted binding pockets of rutin on SIRT1 (PDB ID: 8QT1). C1 pocket showed the strongest affinity (–9.0 kcal/mol) and largest cavity volume, indicating the most favorable binding site
| Curpocket ID |
Vina Score |
Cavity volume (Å3) |
Center (x,y,z) |
Docking Size (x,y,z) |
| C1 |
-9.0 |
3567 |
-3,19,15 |
25,31,35 |
| C5 |
-8.0 |
408 |
6,34,14 |
25,25,25 |
| C4 |
-8.3 |
326 |
4,1,21 |
25,25,25 |
| C3 |
-8.5 |
160 |
16,20,12 |
25,25,25 |
| C2 |
-8.7 |
103 |
11,18,2 |
25,25,25 |
Several key amino acid residues within the sirtuin active site were found to participate in the binding. Notably, Tyr139 (Y139) is involved in π-π stacking interactions with the aromatic rings of rutin, enhancing the stability of the complex. Lys136 (K136) and Glu137 (E137) are likely to form hydrogen bonds or electrostatic interactions with rutin's hydroxyl and glycosidic functional groups. The Gly141–Gly143 segment contributes to the flexibility and accommodation of the ligand within the binding groove. Additional polar and charged residues such as Asp9 (D9), Asp17 (D17), and Asp190 (D190) also form hydrogen bonds (indicated by grey dashed lines), further stabilizing the ligand-protein complex. These interactions suggest that rutin fits tightly into the sirtuin active site, likely contributing to potential inhibitory or modulatory effects on enzymatic activity. The docking pose highlights rutin’s affinity for the sirtuin binding pocket through a combination of hydrogen bonding, π-π stacking, and hydrophobic contacts, which collectively support its role as a bioactive compound capable of modulating sirtuin function. Such binding behavior underpins rutin’s reported antioxidant and anti-aging properties at the molecular level.
Discussion
The present study highlights the differences in polyphenolic content, particularly rutin, between roasted and green buckwheat (Fagopyrum esculentum), and explores the molecular interaction of rutin with the sirtuin protein SIRT2. HPLC analysis confirmed that green buckwheat contains significantly higher levels of rutin (1.09 mg/g) compared to roasted buckwheat (0.5 mg/g), suggesting that thermal processing may degrade heat-sensitive polyphenols. This observation is consistent with previous findings [13-15] that highlight the vulnerability of flavonoids, such as rutin, to high temperatures during roasting or cooking processes.
Molecular docking simulations provided mechanistic insights into the interaction between rutin and the sirtuin protein. Blind docking identified five potential binding pockets (C1–C5) on SIRT1, with docking scores ranging from –8.7 to –9.0 kcal/mol. Among these, pocket C1 indicated the strongest binding affinity (–9.0 kcal/mol) and the largest cavity volume, highlighting it as the most favorable site for ligand accommodation. Detailed analysis of the C1 binding site revealed that multiple amino acid residues in chain A (VAL83, GLY84, ALA85, GLY86, ILE87, THR89, SER90, GLY92, ILE93, PRO94, ASP95, PHE96, ARG97, SER98, PRO99, SER100, THR101, GLY102, LEU103, TYR104, ASP105, GLU116, GLU120, GLU137, LEU138, PRO140, GLN167, ASN168, PHE235, GLY236, GLY261, THR262, SER263, LEU264, GLN265, VAL266, PHE269, ASN286, LYS287, GLU288, GLY322, GLU323, CYS324) and in chain B (GLY8, GLY9) collectively formed a well-defined binding pocket. These residues established a network of hydrogen bonds, hydrophobic interactions, and van der Waals forces, which stabilized the rutin–protein complex. The observed π–π stacking interaction between TYR104 and rutin’s aromatic ring further enhanced the stability of the complex, while electrostatic and hydrogen bonding contributions from GLU137 and LYS287 strengthened ligand anchoring. Such interactions indicate that rutin is not only capable of fitting tightly into the sirtuin cavity but may also modulate its conformational dynamics.
These findings are consistent with earlier docking studies on plant-derived flavonoids, which demonstrated that rutin exhibits strong binding affinity to NAD⁺-dependent deacetylases, such as SIRT1 and SIRT2 [16]. By occupying the C1 pocket, rutin may influence the enzymatic activity of sirtuins, thereby regulating pathways involved in OS response, mitochondrial homeostasis, and longevity. This finding is in line with experimental evidence suggesting that rutin activates sirtuin-mediated antioxidant defense, including the upregulation of SOD, CAT, and GPx enzymes [17]. Taken together, the docking results reinforce the biological plausibility of rutin as a natural modulator of sirtuin function. This mechanism supports its reported antioxidant, anti-aging, and detoxification properties, underscoring the importance of buckwheat as a dietary source of polyphenols with pharmacological potential.
This interaction highlights rutin’s capacity to activate sirtuin-dependent cellular protection. The findings align with the growing body of evidence suggesting that natural flavonoids can positively regulate sirtuin signaling pathways, contributing to reduced OS, inflammation, and enhanced mitochondrial activity.
This result aligns with recent literature suggesting that dietary polyphenols, particularly flavonoids, can activate sirtuins and contribute to the reduction of OS and inflammation [14]. Moreover, the ability of rutin to modulate detoxification-related pathways through sirtuin signaling could offer therapeutic implications for mitigating damage caused by environmental toxins and OS.
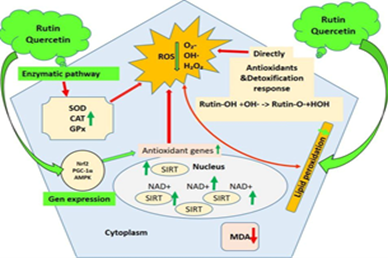
Figure 3. Proposed mechanism of rutin and quercetin action against OS. The flavonoids enhance antioxidant enzyme activities (SOD, catalase [CAT], GPx) and activate antioxidant-related genes via the SIRT1 pathway. This leads to reduced ROS, lipid peroxidation (MDA), and inflammation, supporting detoxification and cellular protection mechanisms
Molecular docking
Furthermore, the proposed mechanistic model (Figure 3) illustrates how rutin and quercetin exert their antioxidant and detoxifying effects at the molecular level. Both flavonoids appear to function through dual mechanisms: (i) direct radical scavenging of ROS, including superoxide (O₂⁻), hydroxyl radical (•OH), and hydrogen peroxide (H₂O₂) [15], and (ii) indirect activation of enzymatic pathways involving antioxidant enzymes, such as SOD, CAT, and GPx [16]. Notably, rutin may modulate SIRT expression and related gene pathways, enhancing cellular defense via NAD⁺-dependent signaling [17]. The net effect includes reduced lipid peroxidation (as indicated by lower MDA levels) and the maintenance of redox homeostasis.
Chelating
Polyphenols are known to exhibit significant chelating properties due to the presence of multiple hydroxyl (-OH) groups in their flavonoid structures [18]. These phenolic groups can coordinate with heavy metal ions (e.g., Pb²⁺, Cd²⁺, or Hg²⁺), forming stable rutin–metal complexes. This interaction neutralizes the metal’s reactivity, reduces its bioavailability, and facilitates its excretion from the body [19]. The resulting neutral or anionic complex is less toxic and is more readily eliminated through renal or biliary pathways. This chelating mechanism contributes to rutin’s protective effects against heavy metal-induced OS and organ damage. OS and chronic inflammation are major factors in many diseases, and numerous studies confirm that flavonoids exert protective antioxidant and anti-inflammatory effects [20].
Conclusions
The present work demonstrates that green buckwheat (Fagopyrum esculentum) contains significantly higher levels of the bioactive flavonoid rutin compared to roasted buckwheat, as confirmed by HPLC analysis. Molecular docking results further support rutin’s strong binding affinity to the active site of the SIRT2 protein, suggesting its potential as a natural modulator of sirtuin-regulated detoxification and antioxidant pathways. These findings indicate that rutin, particularly from green buckwheat, may contribute to cellular protection against OS by activating antioxidant gene expression and reducing lipid peroxidation. Therefore, green buckwheat may serve as a promising dietary source of functional polyphenols for health promotion and disease prevention. Further in vitro and in vivo studies are needed to validate these protective effects and elucidate the precise mechanisms involved.
Data Access and Responsibility
The corresponding author had full access to all the data in this study and takes full responsibility for the integrity and accuracy of the data analysis.
Ethical Considerations
This study did not involve human participants or animal experiments. All experimental procedures were conducted in accordance with institutional and international ethical standards.
Authors' Contributions
The author confirms that they were solely responsible for the conception, design, data collection, analysis, and writing of this manuscript.
Acknowledgement
The authors gratefully acknowledge the Institute of Bioorganic Chemistry named after Academician A. S. Sodiqov, Academy of Sciences of the Republic of Uzbekistan, for providing research facilities and scientific support during the study.
Conflict of Interests
The authors declare that there is no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Sofi SA, Ahmed N, Farooq A, Rafiq S, Zargar SM, Kamran F, et al. Nutritional and bioactive characteristics of buckwheat, and its potential for developing gluten‐free products: An updated overview,” Food Sci Nutr. 2022;11(5):2256–76. [DOI: 10.1002/fsn3.3166]
- Podolska G, Gujska E, Klepacka J, Aleksandrowicz E. Bioactive compounds in different buckwheat species. Plants. 2021;10(5):961. [DOI:10.3390/plants10050961]
- Kurćubić VS, Stajić S, Jakovljevic V, Živković V, Stanišić N, Maskovic P, et al. Contemporary speculations and insightful thoughts on buckwheat—a functional pseudocereal as a smart biologically active supplement. Foods. 2024;13(16):2491. [DOI:10.3390/foods13162491]
- Alía M, Mateos R, Ramos S, Lecumberri E, Bravo L, Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur J Nutr. 2006;45(1):19-28. [DOI: 10.1007/s00394-005-0558-7] [PMID: 15782287]
- Ahmed OM, Elkomy MH, Fahim HI, Ashour MB, Naguib IA, Alghamdi B, et al. Rutin and quercetin counter doxorubicin-induced liver toxicity in Wistar rats via their modulatory effects on inflammation, oxidative stress, apoptosis, and Nrf2. Oxid Med Cell. Longev. 2022;2022(3):2710607 [DOI:10.1155/2022/2710607]
- Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N. The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal. 2018;28(8):643-661. [DOI: 10.1089/ars.2017.7290] [PMID: 28891317]
- Sauve AA, Youn DY. Sirtuins: NAD(+)-dependent deacetylase mechanism and regulation. Curr Opin Chem Biol. 2012;16(5-6):535-43. [DOI: 10.1016/j.cbpa.2012.10.003] [PMID: 23102634]
- Kim K, Kwon JS, Ahn C, Jeung EB. Endocrine-disrupting chemicals and their adverse effects on the endoplasmic reticulum. Int J Mol Sci. 2022;23(3):1581. [DOI: 10.3390/ijms23031581] [PMID: 35163501]
- Baralić K, Buha Djordjevic A, Živančević K, Antonijević E, Anđelković M, Javorac D, et al. Toxic effects of the mixture of phthalates and bisphenol A-subacute oral toxicity study in Wistar rats. Int J Environ Res Public Health. 2020;17(3):746. [DOI: 10.3390/ijerph17030746] [PMID: 31979393]
- Rudrapal M, Khairnar SJ, Khan J, Dukhyil AB, Ansari MA, Alomary MN, et al. Dietary polyphenols and their role in oxidative stress-induced human diseases: insights into protective effects, antioxidant potentials and mechanism(s) of action. Front Pharmacol. 2022;13:806470. [DOI: 10.3389/fphar.2022.806470] [PMID: 35237163]
- Sarubbo F, Esteban S, Miralles A, Moranta D. Effects of resveratrol and other polyphenols on sirt1: relevance to brain function during aging. Curr Neuropharmacol. 2018;16(2):126-136. [DOI: 10.2174/1570159X15666170703113212]
- Staniak M, Wójciak M, Sowa I, Tyszczuk-Rotko K, Strzemski M, Dresler S, et al. Silica-based monolithic columns as a tool in HPLC-An overview of application in analysis of active compounds in biological samples. Molecules. 2020;25(14):3149. [DOI: 10.3390/molecules25143149] [PMID: 32660127]
- Nguyen HC, Hoang HTT, Miyamoto A, Nguyen TD, Nguyen HTT. Effects of roasting on antibacterial and antioxidant properties of sophora japonica buds—the involvements of rutin and quercetin constituents. Plants. 2024;13(23):3337. [DOI:10.3390/plants13233337]
- Zahra M, Abrahamse H, George BP. Flavonoids: antioxidant powerhouses and their role in nanomedicine. Antioxidants (Basel). 2024;13(8):922. [DOI: 10.3390/antiox13080922] [PMID: 39199168]
- Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. 2023;97(10):2499-2574. [DOI: 10.1007/s00204-023-03562-9] [PMID: 37597078]
- Chang WT, Huang SC, Cheng HL, Chen SC, Hsu CL. Rutin and gallic acid regulates mitochondrial functions via the SIRT1 pathway in C2C12 myotubes. Antioxidants (Basel). 2021;10(2):286. [DOI: 10.3390/antiox10020286] [PMID: 33668647]
- Gusti AMT, Qusti SY, Alshammari EM, Toraih EA, Fawzy MS. Antioxidants-related superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione-S-transferase (GST), and nitric oxide synthase (NOS) gene variants analysis in an obese population: a preliminary case-control study. Antioxidants (Basel). 2021;10(4):595. [DOI: 10.3390/antiox10040595] [PMID: 33924357]
- Jomova K, Alomar SY, Valko R, Liska J, Nepovimova E, Kuca K, et al. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem Biol Interact. 2025;413:111489. [DOI: 10.1016/j.cbi.2025.111489]
- Tamás MJ, Sharma SK, Ibstedt S, Jacobson T, Christen P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules. 2014;4(1):252-67. [DOI: 10.3390/biom4010252] [PMID: 24970215]
- Jomova K, Alomar SY, Valko R, Liska J, Nepovimova E, Kuca K, et al. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chemico-Biological Interactions. 2025;413:111489. [DOI: 10.1016/j.cbi.2025.111489]
Type of Study:
Research |
Subject:
General











































