Ethics code: PAAU/CHS/PRV/CHSREC/Vol-1/029


Emmanuel F T, Iliyasu M O, Omede A, Akor S E, Abubakar A, Alabi O J, et al . Impact of Oral Lacatomtom Consumption on Brain Antioxidant Enzyme Response and Oxidative Stress in Wistar Rats. IJT 2025; 19 (4) :213-220
URL:
http://ijt.arakmu.ac.ir/article-1-1501-en.html
1- Department of Medical Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, Prince Abubakar Audu University, Anyigba, Nigeria. , friday.et@ksu.edu.ng
2- Department of Anatomy, Faculty of Basic Medical Sciences, College of Health Sciences, Prince Abubakar Audu University, Anyigba. Nigeria.
3- Department of Medical Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, Prince Abubakar Audu University, Anyigba, Nigeria.
4- Department of Medical Laboratory Science, College of Health Sciences, Prince Abubakar Audu University, Anyigba, Nigeria.
5- College of Medicine, University of Nigeria, Enugu Campus, Nigeria.
6- Department of community medicine, college of health science Prince Abubakar Audu,University, Anyigba,Nigeria.
7- Abu Arish General Hospital, Saudi Arabia.
Full-Text [PDF 595 kb]
(173 Downloads)
|
Abstract (HTML) (447 Views)
Full-Text: (5 Views)
Introduction
The increasing global prevalence of psychoactive substance abuse represents a significant public health challenge, with a particularly pronounced impact on young populations [1]. In various regions, including Nigeria and other parts of Africa, there has been a notable rise in the use of novel psychoactive substances (NPS), frequently prepared as informal, homemade concoctions [1, 2]. Among these emerging substances, "Lacatomtom" (LTT), colloquially known as "gigabyte," has gained considerable attention. The LTT is typically formulated by dissolving tomtom candies in Lacasera beverage [2-4]. The motivations behind its consumption are multifaceted, encompassing curiosity, peer pressure, and the pursuit of more affordable alternatives to substances like codeine, which have faced import and sales bans [2-4].
The LTT is visually characterised by its dark-brown colouration and a fizzy appearance, which results from the rapid nucleation of carbon dioxide gas. It also possesses a distinctive minty aroma [2-4]. The escalating, informal use of LTT, coupled with a notable scarcity of scientific investigation into its precise chemical constituents and physiological effects, underscores its critical importance as an under-researched public health threat [1]. This lack of comprehensive understanding highlights the urgency for rigorous scientific inquiry into its toxicological profile.
The brain exhibits a unique vulnerability to oxidative stress, primarily due to its high metabolic rate and oxygen consumption, its rich composition of polyunsaturated fatty acids (which are highly susceptible to peroxidation), and its comparatively limited endogenous antioxidant defence mechanisms [5, 6, 1]. Oxidative stress arises from an imbalance between the generation of reactive oxygen species (ROS) and the biological system's capacity to neutralise these highly reactive molecules [5, 6, 1]. When ROS production overwhelms antioxidant defences, it can lead to significant cellular damage, including lipid peroxidation, protein oxidation, DNA damage, apoptosis, and ultimately, neurodegeneration [5, 6, 1].
The brain's defence against oxidative damage relies on a sophisticated network of antioxidant components. Key enzymatic antioxidants include superoxide dismutase (SOD), which catalyzes the dismutation of superoxide radicals to hydrogen peroxide; catalase (CAT), which converts hydrogen peroxide to water and oxygen; and glutathione peroxidase (GPX), which reduces hydrogen peroxide and organic hydroperoxides to less harmful alcohols [1]. Complementing these enzymes are non-enzymatic antioxidants, notably reduced glutathione (GSH), a crucial tripeptide involved in directly neutralizing free radicals and serving as a substrate for GPX [1]. Therefore, monitoring alterations in brain antioxidant enzyme activity and increases in lipid peroxidation, as indicated by malondialdehyde (MDA) levels, provides critical biomarkers for assessing neurotoxicity and potential neurological dysfunction induced by exogenous substances [1]. Despite the increasing prevalence of LTT abuse, comprehensive preclinical data regarding its effects, particularly on the delicate oxidative balance within the brain, remain notably limited [2, 3]. While previous investigations have explored LTT's broader toxicological profile, including its impact on liver, kidney, and reproductive functions, as well as its psychoactive potential, a direct and detailed assessment of its influence on brain antioxidant enzymes is essential for elucidating its neurotoxic mechanisms [2, 3, 7-9].
The present study aimed to evaluate the response of key brain antioxidant enzymes (SOD, CAT, GSH, GPX) and the extent of lipid peroxidation (MDA) following oral consumption of LTT and its individual components (Lacasera and Tomtom in water [TTW]) in Wistar rats. By dissecting the contributions of the individual components versus the complete LTT mixture, this investigation aimed to differentiate whether observed neurotoxicity is attributable to a specific constituent, a combined effect, or the synergistic action of the mixture itself. This approach will provide valuable insights into the oxidative stress profile induced by LTT within the central nervous system.
Materials and Methods
Chemicals and reagents
All chemicals and reagents used were of analytical grade and were obtained from reputable scientific chemical organizations.
Preparation of lacatomtom samples
The test samples were prepared to closely approximate the dosages commonly reported by LTT users [10]. The LTT solution, designated as Sample B, was prepared by dissolving three tomtom candies (Cadbury Nigerian Plc, Nigeria), weighing a total of 13.16 grams, in 350 mL of Lacasera drink (Lacasera Company Plc, Nigeria). This preparation yielded a concentration of 37 mg of tomtom per 1 mL of the Lacasera drink. Lacasera, used as Sample C, was administered as 1 mL of the commercial Lacasera drink. Tomtom in water, referred to as Sample D, was prepared by dissolving three tomtom candies (13.16 g) in 350 mL of distilled water. Distilled water, identified as Sample A, served as the control group.
Experimental animals and grouping
A total of 24 Male Wistar rats obtained from the College of Health Experimental Animal House, Prince Abubakar Audu University, Anyigba, Nigeria, were utilised for this study [3, 7-10]. The animals were divided into four distinct experimental groups to facilitate comparative analysis:
Group A: Administered with distilled water, serving as the control.
Group B: Administered with 1 mL of the prepared LTT solution per kilogram body weight of the rat.
Group C: Administered with 1 mL of Lacasera drink per kilogram body weight of the rat.
Group D: Administered with 1 mL of tomtom dissolved in water per kilogram body weight of the rat.
Ethical Consideration
The rats were maintained under standard laboratory conditions and fed rodent cubes. The study received approval from the College of Health Sciences Research and Ethics Committee, with the ethical number PAAU/CHS/PRV/CHSREC/Vol-1/029.
Oral administration protocol
All prepared samples were administered orally to the respective groups, with dosages adjusted per kilogram of the rat's body weight. The administration was conducted once daily for 30 days.
Collection of tissue sample
At the end of the 30 days experimental period, six rats were randomly selected and sacrificed from each of the rat groups and dissected following internationally accepted principles for laboratory use and care of European Community (EECdirective of 1986: 86/609/EEC) and the regulations of the local ethics committee of College of Health of Science, Prince Abubakar Audu University, Anyigba, Nigeria. Brain samples were carefully collected from the sacrificed rats and taken to the laboratory for further analysis.
Biochemical assays for brain antioxidant enzymes (SOD, CAT, GSH, GPX) and lipid peroxidation (MDA)
The specific methodologies were employed for the biochemical assays of SOD, CAT, GSH, GPX, and MDA. The MDA levels were measured using methods described by Ohkawa et al. (1979) [11]; SOD and GPx activities were determined using methods described by Ashibi et al. [9] using commercially available assay kits ( Cayman assay kit); CAT activity was assayed spectrophotometrically as described by Goth (1991) [12]; and GSH levels were estimated by the method described by Ashibi et al. [9] using assay kits (Calbiochem, USA).
Statistical analysis
One-way analysis of variance (ANOVA) was used to compare mean values across the treatment groups. Significant differences were determined using Tukey's post-hoc test, and all statistical significance was set at a p-value of < 0.05.
Results
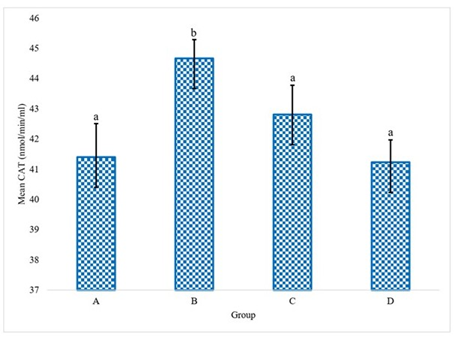
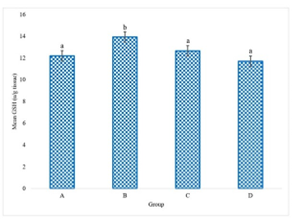
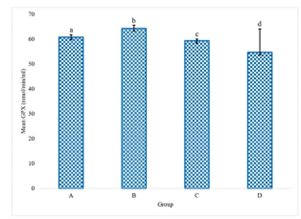
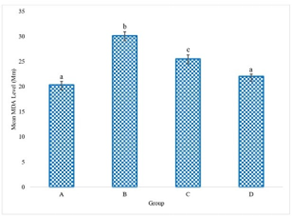
The experimental data delineating the brain antioxidant enzyme activities and lipid peroxidation levels in Wistar rats across the various treatment groups are presented in Figures 1 to 5. These figures provide direct quantification of the biochemical responses within the brain, enabling a direct comparison between the control group (distilled water), the LTT group, the Lacasera group, and the TTW group. A comparative analysis of the data reveals several important trends. In rats administered with LTT (Group B), there was a noticeable increase in the activities of SOD, as shown in Figure 1, CAT (Figure 2), and GPX (Figure 4), as well as in the levels of GSH (Figure 3), compared to the control group (Group A). Concurrently, MDA levels in Figure 5, a marker of lipid peroxidation, were significantly elevated (p<0.05) in Group B compared to Group A.
Figure 1 indicates SOD activity in the brains of rats. The graph shows a significant difference in SOD activity across the treatment groups (LTT, LC, and TTW) compared to the control group (water), while Figure 2 shows CAT activity in the brains of rats. This figure illustrates the varying levels of CAT activity across the groups, with significant differences observed.
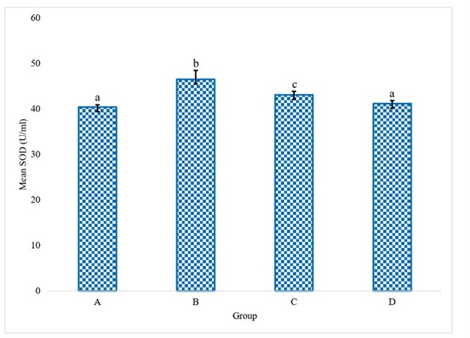
Figure 1. Superoxide dismutase (SOD) activity in the rats administered with water (A), LTT (B), LC(C) and TTW (D). Bars differ significantly at p < 0.05.
Figure 2. Catalase activity (CAT) in the rats administered with water (A), LTT (B), LC(C) and TTW (D). Bars differ significantly at p < 0.05.
Figure 3 indicates GSH levels in the brains of rats. This graph compares the GSH levels, highlighting the impact of each treatment on this key antioxidant, while Figure 4 demonstrates GPX activity in the brains of rats. The figure shows the GPX activity in each group, indicating the different levels of this antioxidant enzyme in response to the treatments.
Figure 3. Reduced Glutathione (GSH) level in the rats administered with water (A), LTT (B), LC(C) and TTW (D). Bars differ significantly at p < 0.05.
Figure 4. Glutathione peroxidase (GPX) activity in the rats administered with water (A), LTT (B), LC(C) and TTW (D). Bars differ significantly at p <0.05.
Figure 5 demonstrates MDA levels in the brain of Wistar rats. The graph shows that the LTT group exhibited the highest MDA levels, indicating a significant increase in lipid peroxidation and severe oxidative stress. (p=0.034).
Figure 5. Melondialdehyde level (MDA) in the rats administered with water (A), LTT (B), LC(C) and TTW (D). Bars differ significantly at p<0.05.
Discussion
This finding indicates that oral consumption of LTT (Group B) induces significant oxidative stress within the Wistar rat brain. The marked increase in lipid peroxidation (MDA) serves as a direct and critical indicator of elevated oxidative damage to neuronal membranes, consistent with the brain's inherent vulnerability due to its high oxygen consumption and lipid-rich composition [5, 6, 9]. The concomitant upregulation of SOD, CAT, GSH, and GPX activities suggests an active and robust compensatory mechanism by the brain's endogenous antioxidant defence system [9]. This response is a typical biological attempt to counteract the increased production of ROS and mitigate oxidative insult [5, 6, 9]. However, despite this compensatory upregulation, the persistent and significant increase in MDA levels indicates that these defence mechanisms are either overwhelmed or insufficient to completely prevent oxidative damage, implying a net pro-oxidant effect of LTT on the brain.
The administration of Lacasera alone (Group C) resulted in slight increases in SOD, CAT, and GSH levels when compared to the control group. However, GPX levels decreased marginally. The MDA levels in Group C were elevated compared to Group A, though the increase was less pronounced than that observed in Group B. Lacasera, a primary component of LTT, contains aspartame [3].Scientific investigations have demonstrated that aspartame can significantly increase brain MDA levels and nitric oxide, while simultaneously decreasing GSH concentrations [13]. These biochemical alterations are associated with impaired memory performance [13]. The elevated MDA levels in Group C strongly suggest that aspartame is a primary driver of the observed brain oxidative stress induced by Lacasera.
Rats receiving tomtom dissolved in water (Group D) exhibited slight increases in SOD and CAT activities compared to the control. Conversely, GSH and GPX levels were marginally decreased. The MDA levels in Group D were slightly elevated compared to Group A, but to a lesser extent than in Groups B and C. Tomtom candies contain menthol [3]. Studies have linked menthol to increased intracellular calcium influx and the generation of mitochondrial ROS at the cellular level, indicating its potential to induce oxidative stress [14]. While the TTW group exhibited a milder oxidative stress profile compared to the full LTT mixture or Lacasera alone, menthol's established capacity to induce ROS and its known neurological effects suggest it contributes to the overall neurotoxic burden of LTT, possibly by enhancing the effects of other psychoactive compounds.
The findings indicate that oral consumption of LTT (Group B) appears to induce the most pronounced oxidative stress within the Wistar rat brain, as evidenced by the highest MDA levels and strongest compensatory antioxidant response. This indicates a significant cellular battle. MDA levels are a marker of lipid peroxidation, which is a direct measure of oxidative damage. The body's natural defense against this damage is its antioxidant system, which includes enzymes like SOD, CAT, and GPX. When a toxic substance like LTT induces a high level of oxidative stress (evidenced by high MDA), the cells respond by dramatically increasing the activity of these antioxidant enzymes in an attempt to neutralize the damaging ROS. The fact that this compensatory response is at its "strongest" suggests that the cells are under immense stress and are fighting a vigorous defensive battle. However, the persistence of high MDA levels indicates that the antioxidant defense is being overwhelmed, and damage is still occurring despite the body's best efforts.
Furthermore, the individual components of LTT demonstrate differential effects on brain oxidative stress markers, suggesting a complex interplay within the full mixture. Lacasera (Group C) contributes substantially to the observed oxidative stress, as indicated by its elevated MDA levels. In contrast, TTW (Group D) exhibits a milder and more varied impact on antioxidant enzymes, with some even showing a decrease. This comparison suggests that Lacasera components are significant contributors to oxidative stress, whereas Tomtom alone might be less potent, or its effects may be modulated by other components when part of the complete LTT mixture. The combination of Lacasera and Tomtom in LTT (Group B) results in the most pronounced oxidative stress, accompanied by the strongest compensatory antioxidant response. This observation indicates that the psychoactive mixture, as consumed, poses a greater acute oxidative challenge to the brain than its individual components. This heightened effect could be attributed to synergistic interactions between the compounds or higher effective concentrations of active compounds when combined.
The chemical complexity of LTT is substantial, with Gas Chromatography-Mass Spectrometry (GC-MS) analysis identifying 47 distinct compounds [1]. Key constituents derived from both Tomtom candies and Lacasera beverage include menthol, various sugars, glucose syrup, aspartame, sodium benzoate, caffeine, and propionylcodeine, which has been identified as a top-docked psychoactive compound [15, 1]. The observed effects on brain oxidative stress markers can be attributed to the individual and combined actions of these components. The presence of aspartame in Lacasera (Group C), which contributed to elevated MDA levels, strongly suggests that aspartame is a primary driver of the observed brain oxidative stress. Its direct impact on brain neurotransmitter systems and oxidative markers provides a plausible and significant mechanism for the observed effects of LTT on brain oxidative balance and its documented influence on spatial memory [8]. While the TTW group (Group D) exhibited a milder oxidative stress profile compared to the full LTT mixture or Lacasera alone, menthol's established capacity to induce ROS and its known neurological effects (vertigo, dizziness, ataxia) suggest that it contributes to the overall neurotoxic burden of LTT. This contribution could arise from direct cellular oxidative mechanisms or potentially by enhancing the effects of other psychoactive compounds present in the mixture [14]. Lacasera is also believed to contain caffeine [3]. Caffeine's role in oxidative stress is complex and appears to be dose- and context-dependent, exhibiting both neuroprotective and neurotoxic properties [16,17]. The ambiguous nature of caffeine's effect makes its precise contribution to LTT's neurotoxicity challenging to isolate. However, its presence in Lacasera, which led to increased MDA levels in Group C, suggests that within the context of the LTT mixture, caffeine's pro-oxidant effects might be more dominant, or it may interact synergistically with other components to exacerbate overall oxidative stress. Sodium benzoate, a common preservative found in Lacasera,[3] has been indicated to induce oxidative stress and DNA damage at high concentrations [18]. It has also been implicated in reproductive toxicity, potentially through mechanisms involving oxidative stress [7]. However, some research indicates that sodium benzoate can exert positive cognitive effects by modulating N-methyl-D-aspartate (NMDA) receptors [19]. This contradictory profile suggests a complex, potentially dose-dependent, or context-specific role for sodium benzoate within LTT overall neurotoxicology. While its contribution to the observed brain oxidative stress is plausible, its precise mechanism and net effect within the LTT concoction warrant further dedicated investigation. Propionylcodeine has been identified as a top-docked compound within LTT [15], demonstrating the potential to permeate the blood-brain barrier and inhibit neuro-enzymes such as human monoamine oxidase (hMOA) and catechol O-methyltransferase (hCOMT) [15], which are crucial for dopamine and serotonin metabolism [1]. These properties suggest its psychoactive nature, consistent with its classification as an opiate derivative [1]. Opioids are known to induce respiratory depression, which can lead to brain hypoxia and subsequently contribute to oxidative stress [20]. While general oxidative stress is broadly linked to neurodegeneration [5, 6]. It is noteworthy that activation of delta opioid receptors (DOR) by opioids has been reported to decrease oxidative injury in neurons [21]. This presents an intricate role for propionylcodeine in LTT-induced brain oxidative stress. Its psychoactive effects might indirectly lead to hypoxia and oxidative stress, yet its direct interaction with specific opioid receptors could simultaneously confer neuroprotective effects against oxidative injury. This dual potential necessitates further investigation into the specific opioid receptor subtypes activated by propionylcodeine within the LTT mixture and their downstream consequences on brain redox balance.
LTT is recognised as a psychoactive mixture, with in silico studies indicating that its top-docked compounds, including propionylcodeine, (4-Methoxymethoxy-hex-5-ynylidene)-cyclohexane, and 3-(hydroxyphenylmethyl)-3,4-dimethyl-1-phenylpentan-2-one, possess the ability to permeate the blood-brain barrier [15, 1]. These compounds are also implicated in inhibiting neuro-enzymes like MAO and hCOMT, which play pivotal roles in the metabolism of neurotransmitters such as dopamine and serotonin [1]. Furthermore, LTT consumption has been demonstrated to significantly impair spatial memory in Wistar rats, suggesting a direct impact on central nervous system function, potentially mediated through the opioidergic system [8]. Research on other psychoactive substances, such as cannabis, provides a clear precedent, demonstrating a direct link between their consumption, alterations in brain neurotransmitter levels (e.g., dopamine, serotonin, norepinephrine, and acetylcholinesterase), and the induction of oxidative stress, evidenced by reduced levels of SOD, GSH, CAT, and GPx, alongside increased MDA [9].
The observed brain oxidative stress induced by LTT is therefore not merely a generalised toxic effect but is likely intricately linked to its psychoactive properties and the resulting neurological impairments. Oxidative stress is well-established to disrupt neurotransmission, impair neuronal function, and contribute to cognitive deficits [5, 6]. The LTT-induced oxidative stress could be a direct consequence of the psychoactive compounds' actions on neuronal metabolism, leading to increased ROS production, or an indirect effect resulting from altered neurotransmitter dynamics and subsequent metabolic perturbations. This issue establishes a comprehensive understanding where oxidative stress is not merely a side effect but potentially a fundamental mechanism underlying LTT's profound neurological impact.
Beyond its direct impact on brain oxidative balance, LTT has demonstrated a concerning profile of systemic toxicity across multiple organ systems, further underscoring the severe public health risks associated with its consumption. LTT has been shown to significantly elevate plasma levels of liver enzymes, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP). This elevation, coupled with a reduction in the liver-to-body weight ratio, indicates substantial liver damage [3]. These hepatotoxic effects are attributed to the combined influence of LTT's constituents, such as menthol, aspartame, sodium benzoate, and caffeine [3]. Evidence suggests that LTT consumption can lead to increased plasma creatinine levels, indicative of kidney damage. This nephrotoxic effect is potentially linked to the tomtom component of the mixture [1, 3]. Prolonged intake of LTT has been associated with elevated plasma levels of total cholesterol and low-density lipoprotein (LDL)-cholesterol. Such alterations in lipid profiles pose a significant risk for the development of cardiovascular disorders [1, 3]. LTT has been found to negatively impact male reproductive function, characterized by an increase in sluggish sperm cells and a decrease in serum testosterone levels [7]. Sodium benzoate, a component of Lacasera, is implicated in contributing to these reproductive effects, potentially through mechanisms involving oxidative stress [7].
The multi-organ toxicity profile of LTT, encompassing damage to the liver, kidneys, and reproductive system, alongside its demonstrated general cytotoxicity, reinforces the profound public health hazard posed by its widespread abuse [1, 3]. Systemic inflammation and organ damage resulting from LTT consumption can indirectly exacerbate brain oxidative stress. This occurs by impairing the body's overall detoxification pathways and by releasing pro-inflammatory mediators that can cross the blood-brain barrier, thereby contributing to neuroinflammation and further oxidative insult within the central nervous system. This comprehensive toxicological picture highlights the interconnectedness of LTT's detrimental effects across various physiological systems.
Conclusions
Oral LTT consumption significantly induces brain oxidative stress in Wistar rats, marked by elevated lipid peroxidation (MDA) and a compensatory increase in antioxidant enzymes (SOD, CAT, GSH, GPX). Individual components, especially Lacasera, contribute to this oxidative burden, with aspartame being a strong candidate for driving brain oxidative stress and memory impairment. The complete LTT mixture shows more pronounced neurotoxic effects than its individual parts, suggesting synergistic actions. This brain oxidative stress is linked to LTT's psychoactive properties and its documented multi-organ toxicity, highlighting its serious public health threat. Further research is crucial to understand specific molecular mechanisms and develop mitigation strategies.
Data Access and Responsibility
The authors confirm that this article contains original work and accept full responsibility for its content.
Ethical Considerations
The rats were maintained under standard laboratory conditions and fed rodent cubes. The study received approval from the College of Health Sciences Research and Ethics Committee, with the ethical number PAAU/CHS/PRV/CHSREC/Vol-1/029.
Authors' Contributions
All authors contributed to the conception, design, drafting, and submission of the manuscript.
Acknowledgement
The authors extend their gratitude to Mr. Akogwu Victor, Mr. Iremide Saliu, and Mr. Joseph Daniel for their assistance during the experimental phase of this research.
Conflict of Interests
The authors declared that they have no competing interests to disclose.
Funding
The research was funded by the authors.
References
- Peacock A, Bruno R, Gisev N, Degenhardt L, Hall W, Sedefov R, et al. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. Lancet. 2019;394(10209):1668-84. [DOI:10.1016/S0140-6736(19)32231-7]
- Mustapha AK. Codeine: Addicts now use lacasera, tomtom to form “gigabyte” solution-NDLEA. People’s Daily Newspaper, 2018. [Link]
- Emmanuel FT, Akor SE, Momoh S, Owemidu IO, Akor SE. Investigation of the hepatotoxicity of lacatomtom drink in albino rat. Niger J Biochem Mol Biol. 2022;37(1):26-31. [Link]
- Kuntzleman TS, Davenport LS, Cothran VI, Kuntzleman JT, Campbell DJ. New demonstrations and new insights on the mechanism of the Candy-Cola soda geyser. J Chem Educ. 2017;94(5): 569-76. [DOI:10.1021/acs.jchemed.6b00862]
- El-Shennawy L, Kamel MAE, Khalaf AHY, Yousef MI. Dose-dependent reproductive toxicity of sodium benzoate in male rats: Inflammation, oxidative stress and apoptosis. Reprod Toxicol. 2020:98:92-8. [DOI: 10.1016/j.reprotox.2020.08.014]
- Al-Ani BT, Al-Saadi RR, Wassef HF. Toxic effects of sodium benzoate on the rat testes. Indian J Public Heal Res Dev. 2019;10(10):2505. [DOI:10.5958/0976-5506.2019.03239.X]
- Anyanwu CF, Georgewill UO, Onuama VI, Phil-Ashiri NU. Potential reproductive toxicity of lacatomtom drink in male wistar rats. Trop J Nat Prod Res. 2024;8(6):7542-6. [DOI:10.26538/tjnpr/v8i6.33]
- Olumorin OI, Titus EF, Mohammed N, Emmanuel JO. Effect of lacatomtom drink on spatial memory in male wistar rat. Trop J Phytochem Pharm Sci. 2025;4(3):123-5. [DOI:10.26538/tjpps/v4i3.3]
- Ashidi JS, Emeya IE, Owagboriaye FO, Feyisola RT, Lawal OI, Okechukwu OC. Neurological behaviour of albino rats treated separately and in combination with cannabis sativa L. and cannabis indica L. Scientia Africana, 2021;20(2):37-50. [DOI:10.4314/sa.v20i2.4]
- Emmanuel FT, Gyebi GA, Oka SA, Omada AA, Junaidu Y, Gideon A, et al. In vivo analysis of neuromodulatory and biochemical effects of lacatomtom drinks on wistar rat brain. Trop J Drug Res. 2025;2(5):117-29. [DOI:10.26538/tjdr/v2i5.1]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351-8. [DOI: 10.1016/0003-2697(79)90738-3] [PMID: 36810]
- Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196(2-3):143-51. [DOI:10.1016/0009-8981(91)90067-M] [PMID: 2029780]
- Aguocha CM, Nwefoh E. Prevalence and correlates of substance use among undergraduates in a developing country. Afr Health Sci. 2021;21(2):875-3. [DOI: 10.4314/ahs.v21i2.49]
- Yusuf FA. Factors influencing substance abuse among undergraduate students in Osun State, Nigeria. Afr Res Rev. 2011;4(4):69233. [DOI:10.4314/afrrev.v4i4.69233]
- Emmanuel FT, Gyebi GA, Gyebi GA, Samuel AE, Emeje Pl. Ayeni G, et al. Chemical profiling, in-silico investigation and in vivo toxicity assessment of lacatomtom (a psycoactive mixture) on selected indices in albino wistar rat. Curr Biol chem. 2024:18(4):215-37. [DOI:10.2174/0122127968319253241024050426]
- Lapsley DK, Edgerton J. Separation-individuation, adult attachment style, and college adjustment. J Couns Dev. 2002;80(4):484-92. [DOI:10.1002/j.1556-6678.2002.tb00215.x]
- Adesida SA, Quadri MO, Adedeji AM. Use of psychoactive substances among students in a Nigerian University: An imperative for intervention programs. Sci Am. 2022:16:e01139. [DOI:10.1016/j.sciaf.2022.e01139]
- Oluwafunmilayo OV, John OO, Olabode ON, Blessing AO, Manirambona E, Vicerra P, et al. Prevalence and pattern of psychoactive substance use among government secondary school students in central Nigeria. PAMJ-One Health. 2022:8:17. [DOI:10.11604/pamj-oh.2022.8.17.35856]
- Onaolapo OJ, Olofinnade AT, Ojo FO, Adeleye O, Falade J, Onaolapo AY. Substance use and substance use disorders in Africa: An epidemiological approach to the review of existing literature. World J Psychiatry. 2022;12(10):1268-86. [DOI: 10.5498/wjp.v12.i10.1268]
- Alexandridou A, Mouskeftara T, Raikos N, Gika HG. GC-MS analysis of underivatised new psychoactive substances in whole blood and urine. J Chromatogr B Anal Technol Biomed Life Sci. 2020:1156:122308. [DOI:10.1016/j.jchromb.2020.122308]
- Yu W, MacKerell AD Jr. Computer-aided drug design methods. Methods Mol Biol. 2017;1520:85-106. [DOI: 10.1007/978-1-4939-6634-9_5] [PMID: 27873247]
Type of Study:
Research |
Subject:
General










































 , Musa Omoyine Iliyasu2
, Musa Omoyine Iliyasu2 

 , Ameh Omede3
, Ameh Omede3 

 , Shedrack Egbunu Akor4
, Shedrack Egbunu Akor4 

 , Al-Hassan Abubakar5
, Al-Hassan Abubakar5 

 , Oladele Joshua Alabi6
, Oladele Joshua Alabi6 

 , Mathew Akpa7
, Mathew Akpa7 

 , Alexander Lawrence4
, Alexander Lawrence4