Introducion
Lead is a common environmental pollutant used in many industries and enters the aquatic environment with effluents from mines, smelters, and chemical factories manufacturing pigments, paints, plastics, etc. [1, 2]. Lead toxicity has been reported in liver, muscles, kidneys, brain and gonads in common carp [3]. Moreover, the toxicity has been reported in this fish muscles and liver in Gorgan bay and Gomishan marsh, in Iran [4]. Also, lead has been detected in fish food in many situations [5]. Lead has caused pathological alterations in fish erythrocyte [6], cellular membranes [7], and often induces anemia in fish [8, 9]. This heavy metal has an extremely high affinity for erythrocytes [10, 11]. In fish and other aquatic animals, lead is responsible for direct erythrocytic injuries, basophilic stripping and inhibition of a key enzyme involved in hemoglobin formation, i.e. delta-aminolevulinic acid (ALA-D) [12]. Further, lymphocytosis and neutropenia have been reported in eel, Anguilla, at 0.3 mg/l Pb [13].
Similarly, Olanike et al. [8] observed a rise in lymphocyte percentage and a decline in neutrophils accompanied by a decrease in White Blood Cells (WBC) in African catfish (Clarias gariepinus) that were subjected to 25-250 mg/l of Pb for four days. Conversely, two other studies [14-16] have reported an increase in WBC in lead-exposed fish including common carps. Another study showed that leukocyte counts in Pb-exposed carps increased shortly followed by a decrease in WBC counts and lymphocytosis [16].
It is believed that Pb-induced tissue damage may activate the immune response in fish [13]. Salinity is one of the important factors in the aquatic ecosystem that affects fish growth and survival [17], and may cause a variety of physiological stress responses [18]. Alteration in water salinity also affects the Pb availability and subsequent toxicity by competing with metal ions for binding to sites on many biological molecules [19]. Ijeoma et al. [20] reported that in the fresh water at a salinity level of 2 ppt the Pb toxicity was higher than that observed for salinity at 12-18 ppt.
The bioavailability of Pb to aquatic organisms is dependent on the physical and chemical forms of this heavy metal [21, 22]. In this context, a decrease in the salinity level increases the toxicity associated with Pb bound to the assayed sediments. This increase permits a more readily transport of Pb through the plasma membranes and causes an increase in Metallothionein (MTs) [23]. Various physiological parameters have been used to investigate the sublethal toxicity of heavy metals in freshwater fish. One practical parameter is hematology, which provides a measure of the fish physiology relative to changes in the aquatic environment. Therefore, we conducted this study to examine the effects of varying salinity levels on lead toxicity in common carp, followed by evaluating the hematological parameters.
Materials and Methods
Fish and Treatment: The experiments were conducted in the fall of 2015 on healthy common carp (n=210), weighing 21±2 grams. The fish were obtained from the hatchery pond of the Inland Fisheries Institute in Ahvaz, Iran. They were acclimated to the laboratory setting for 2 weeks in an aerated flow-through tank, at 24±1° C, and dissolved oxygen saturation 70%-80%, supplied, at the density of 10 g/l. The Pb concentrations were set at 15, 30, 60 and 120 mg/l at each salinity level. Fish were fed daily with standard food (Faradaneh Co. Ahvaz, Iran) at the rate of 1% of the stock mass during the acclimation period.

Acute Toxicity Experiments at Varying Salinity Levels: All ethical considerations regarding the fish treatment were observed as set by the university guidelines. In addition we followed the Organization for Economic Cooperation and Development (OECD) Guideline No. 203 test conditions. The juvenile Common carp (n=210) were exposed to acute toxicity tests at varying salinity levels. The experimental Pb solutions were prepared, using the material Pb (C2H3O2)2; Kimia Gostar Pouyesh Co., Ahvaz, Iran). The concentrations of lead tested at varying salinity levels are shown in Table 1. The lead concentrations were selected according to a pilot study and 96 h LC50 value for common carp, reported as 15µg/l in water by the U.S. Environmental Protection Agency (USEPA) (Table 1). Ten fish were kept in each tank and the mortality rate was monitored daily for the initial four consecutive days. Thereafter, the 96 h LC50 was conducted, using probit regression analysis [24] (Table 2).

Hematological Parameters: Based on the LC50 results of lead test at varying salinity levels, four Pb concentrations were selected for the evaluation of hematological parameters: 15, 30, 60 and 120 mg/l at identical salinities used for the LC50 test, i.e. 0, 0.1, 0.2 and 0.4 g/l of Pb added to pure water. The fish were kept in groups of 10 per tank. Control group was subjected to the same treatment except for the Pb exposure. Blood samples were collected from the caudal peduncle vein, using heparinized needles, and kept in heparinized eppendorf tubes after 96 h of Pb exposure [24].
The following hematological parameters were evaluated and compared among the fish groups: hematocrit (Ht), erythrocytes count (RBC: Red Blood Cells), Blood Hemoglobin concentration (Hb), Packed Cell Value (PCV), White Blood Cell (WBC), and the differential WBC counts.
Additionally, erythrocyte indices were calculated, including Mean Cell Volume (MCV), Mean Cell Hemoglobin (MCH), and Mean Corpuscular Hemoglobin Concentration (MCHC). The PCV was measured, using the microhematocrit method in heparinized capillaries after centrifugation at 11,000 rpm for 5 min. The RBC and WBC counts were determined, using a hemocytometer unit with the blood sample diluted 200 times with natheric solution.
The Hb level was measured in a spectrophotometer at 540 nm, using the cyanmet-hemoglobin method [24]. The derived parameters were calculated according to the following formulas (1, 2 & 3):
1. MCV = (Ht×10)/RBC
2. MCH = Hb/RBC
3. MCHC = (Hb×100)/Ht
Differential leukocyte counts were performed at 1000 magnification, using a Nikon Eclipse 300 light microscope on blood smears stained with Giemsa solution, and the results were presented in percentages. One hundred leukocytes were examined per blood smear [24].
Statistical Analyses: Statistical analyses were performed using SPSS V. 19 software and the data were presented as Mean±SD. The data were tested for normality (Kolmogorov–Smirnov test), and analyzed by one-way Analysis of Variance (ANOVA). The significant means were analyzed by Tukey’s test and a P<0.05 was considered to be statistically significant.
Results
Swimming at surface, erratic swimming (in vertical motions) and lethargy were the main behavioral changes observed in Pb-exposed fish throughout the experiment. These behavioral changes were mostly noted at high lead concentrations.
Acute Lead Toxicity vs. Salinity: All of the fish were kept in the tank at 60mg/l of lead concentration and salinity of 0 and 0.1 ppt (0 and 0.1 g/l). Another group of fish were kept at lead concentration 120mg/l and salinity of 0 and 0.1 ppt (0 and 0.1 g/l). LC50 showed that the increased in salinity led to an increase in toxicity (Table 2).
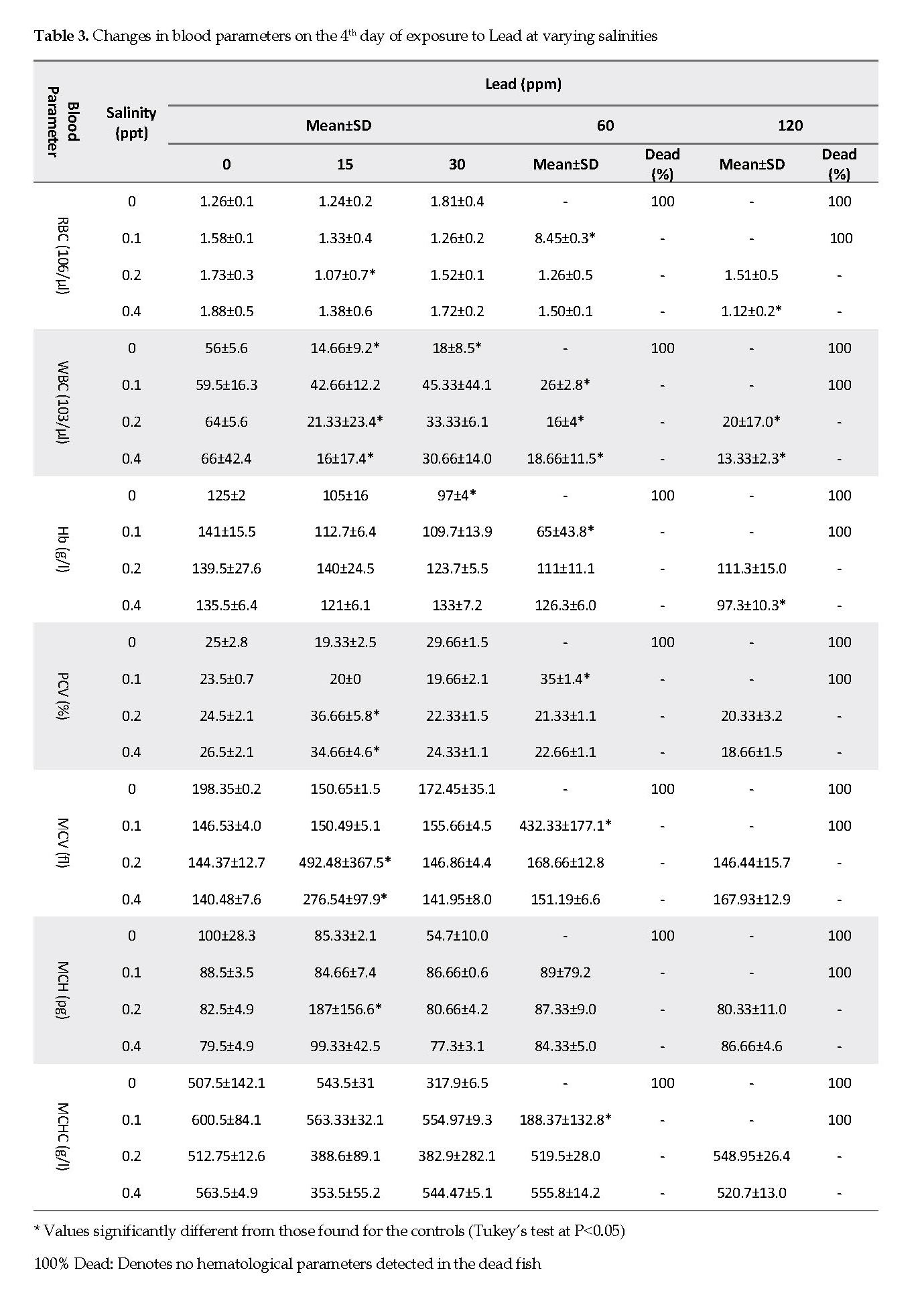
Hematological Parameters vs. Lead Exposure: Based on the data shown in Table 3, the RBC count did not show a significant change compared to that for the control group, with the exception of the fish kept at 60 ppm Pb and 0.1 ppt salinity. The WBC and Hb values decreased with the increasing lead concentrations. Further, most changes in PCV, MCV and MCHC values were seen at a salinity of 0.1, 0.2 or 0.4 ppt and Pb concentrations at 15 and 60 ppm.
The differential leukocyte count showed significant changes after exposure of the fish to certain Pb concentrations (Table 4). The leukocyte count in Pb-exposed fish gradually declined with the increasing lead concentration. The percentages of heterophiles were increased significantly at 0.4 ppt salinity, and 30, 60 and 120 ppm of Pb. Monocytes, basophils and eosinophils percentages were approximately less than 1%, except for those determined at 0.1 ppt salinity and 60 ppm Pb concentrations (Tables 3 & 4).
Discussion
The present data showed that exposure of the fish to both salinity and lead caused variations in the hematological parameters. Johansson-Sjobeck and Larsson reported that reductions in RBC counts and Hb values accompanied by increased hematopoietic rates in rainbow trout after exposure to lead [25]. Moreover, hematological changes following exposure to lead have also been documented in other fish species. Examples of these are anemia in Salvelinus fontinalis [26], Colisa fasciatus [27], and Salmo gairdneri [25], and a significant elevation in the RBC count in Salmo gairdneri [28] and Oreochromis aureus [29]. Additional examples are RBC fragility and hemorrhage leading to hemolysis, damage to the hematopoietic tissues, and impairment in hemoglobin concentration [14]. Witeska et al. [16] showed that a short-term exposure of common carp to Pb resulted in moderate and transient alterations in WBCs, causing a temporary immune disturbance in the fish.
Also Małgorzata et al. [24] evaluated the effect of a short-term (3 h) exposure to 10mg/l of Pb (96 h; LC50) on the hematological parameters in common carp. In that study, the RBC parameters fluctuated over the experimental period with increasing frequency in morphological anomalies. Their observations suggest that lead disturbed the immune response in carp. In our study, a short-term exposure of common carp to Pb resulted in moderate and transient alterations in WBCs, causing a temporary immune disturbance.
This study demonstrated that the RBC counts decreased in all experimental groups except for the group that was treated at 0.1 ppt salinity and 100 ppm lead concentrations, in which the RBC level increased significantly (Table 3). In the latter group, the PCV was 35%, which might be due to the fish being in shortage of oxygen present in the water with low salinity and high lead concentrations. Similarly, the Hb value decreased with increasing lead concentration (Table 3). In addition, hemolysis was observed at high lead concentrations and low salinity levels. The most well-known effect of lead is the inhibition of δ-aminolevulinic acid dehydratase activity (ALA-D), an enzyme that catalyzes the formation of porphobilinogen from aminolevulinic acid. The disturbed hemoglobin synthesis due to the effect of lead on ALA-D might have also resulted in anemia in the fish [13].
Shah and Altindag reported that exposures to lethal concentrations of Hg and Cd resulted in a significant WBC reduction, while exposure to Pb caused a slight increase only. These findings suggest that the order of toxicity of these metals on the WBC of the fish are Hg > Cd > Pb [14]. In this study, we documented a significant decrease for WBCs as well (Table 3). We did not observe similar findings at varying salinity levels, which did not affect the hematological parameters in our study. Further, the LC50 decreased with increasing salinity concentration. The rate of packed cell value did not change significantly compared to that in the control group, except in group 3. The MCV, MCH and MCHC parameters did not vary significantly compared to that for the control group (Table 3).

In another study [16], transient leukopenia and neutropenia were observed and the reduced phagocytes’ activity was similar to those found in the current study (Table 4). Phagocytosis in fish is the most important and potent immune mechanism [30]. A previous study reported a suppressor effect for high Pb levels (5.0-100.0 mg/l) on the activity of carp phagocytes in vitro [16]. However, Witeska and Wakulska [30] observed no significant effects for Pb on the fish at 1-10 mg/l. Similarly to our findings, Pb at 15-120 ppm caused a gradual decrease in leukocyte count while enhancing heterophile and phagocytes’ activities. Lastly, we did not observe significant changes in the basophils, eosinophils and monocytes activities.
Conclusions
There is evidence that both salinity and lead concentrations cause pathologic alterations to the hematological parameters in common carp. RBC and Hb levels were decreased with increases in lead concentration. Also, hemolysis was observed in the fish at high lead and low salinity concentrations. Our findings demonstrated that salinity did not affect the hematological parameters; however, the LC50 decreased with increasing salinity. Other studies also suggest the necessity for using various fish species in order to evaluate the dose-response versus Pb toxicity. This will help elucidate the sensitivity of different fish species to Pb toxicity.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research was part of an investigation on the infectious diseases study of aquarium fishes of Ahvaz. This study is financially supported by research deputy of Shahid Chamran University of Ahvaz, Ahvaz, Iran.
Author's contributions
Conceptualization and methodology: Zahra Tulaby Dezfuly, Mojtaba Amir Aramoon and Mojtaba Alishahi; Formal analysis, investigation: Mostafa Halimi; Writing-original draft preparation and writing-review & editing: Roya Rahnama.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgements
The authors acknowledge the support of the office of Research Deputy at Shahid Chamran University, Ahvaz, Iran.
References
Dojlido JR. [Chemia wód powierzchniowych (Chemistry of surface waters) (Polish)]. Warszawa: Wyd. Ekonomia i Środowisko; 1995.
Singh VK, Mishra KP, Rani R. Immunomodulation by lead. Immunol Res. 2003; 28(2):151-65. [DOI:10.1385/IR:28:2:151]
Ebrahimi M, Taherianfard M. [Concentration of four heavy metals (cadmium, lead, mercury, and arsenic) in organs of two cyprinid fish (Cyprinus carpio and Capoeta sp.) from the Kor River (Iran) (Persian)]. Envir Monitor Assess. 2010; 168(1-4):575-85.
Rademacher DJ, Steinpreis RE, Weber DN. Short-term exposure to dietary Pb and/or DMSA affects dopamine and dopamine metabolite levels in the medulla, optic tectum, and cerebellum of rainbow trout (Oncorhynchus mykiss). Pharmacol Biochem Behav. 2001; 70(2-3):199-207. [DOI:10.1016/S0091-3057(01)00597-4]
Olivera Ribeiro CA, Filipak Neto F, Mela M, Costa JA, Pelletier E. Hematological findings in Neotropical fish Hoplias malabarciusexposed to subchronic and dietary doses of methyl mercury, inorganic lead, and tributyltin chloride. Envir Res. 2006; 101(1):74-80. [DOI:10.1016/j.envres.2005.11.005] [PMID]
Hofer R, Weyrer S, Kock G, Pittracher H. Heavy metal intoxication of arctic charr (Salvelinus alpinus) in a remote acid Alpine Lake. Lugano: Lugano, 1992.
Tewari H, Gill TS, Pant J. Impact of chronic lead poisoning on the hematological and biochemical profiles of a fish, Barbus conchonius (Ham). Bulletin Envir Contam Toxicol. 1987; 38(5):748-52. [DOI:10.1007/BF01616696] [PMID]
Adeyemo OK, Ajani F, Adedeji OB, Ajiboye OO. Acute toxicity and blood profile of adult Clarias gariepinus exposed to lead nitrate. Int J Hematol. 2008; 4(2):1-8. [DOI:10.5580/10e7]
Nakagawa H, Nakagawa K, Sato T. Evaluation of erythrocyte 5-aminolevulinic acid dehydratase activity in the blood of carp Cyprinus carpio as an indicator in fish with water lead pollution. Fisheries Sci. 1995; 61(1):91-5. [DOI:10.2331/fishsci.61.91]
Alves LC, Wood CM. The chronic effects of dietary lead in freshwater juvenile rainbow trout (Oncorhynchus mykiss) fed elevated calcium diets. Aquat Toxicol. 2006; 78(3):217-32. [DOI:10.1016/j.aquatox.2006.03.005] [PMID]
Somero GN, Chow TJ, Yancey PH, Snyder CB. Lead accumulation rates in tissues of the estuarine teleost fish, Gillichthys mirabilis: Salinity and temperature effects. Arch Envir Contam Toxicol. 1977; 6(1):337-48. [DOI:10.1007/BF02097775] [PMID]
Santos MA, Hall A. Influence of inorganic lead on the biochemical blood composition of eel, Anguilla anguilla L. Ecotoxicol Envir. 1990; 20(1):7-9. [DOI:10.1016/0147-6513(90)90040-C]
Shah SL, Altindağ A. Alterations in the immunological parameters of Tench (Tinca tinca L. 1758) after acute and chronic exposure to lethal and sublethal treatments with mercury, cadmium and lead. Turkish J Veter Animal Sci. 2005; 29(5):1163-8.
Ates B, Orun I, Talas ZS, Durmaz G, Yilmaz I. Effects of sodium selenite on some biochemical and hematological parameters of rainbow trout (Oncorhynchus mykiss Walbaum, 1792) exposed to Pb and Cu. Fish Physiol Biochem. 2008; 34(1):53-9. [DOI:10.1007/s10695-007-9146-5] [PMID]
Witeska M. Stress in fish-hematological and immunological effects of heavy metals. Elect J Ichthyol. 2005; 1(1):35-41.
Lin XY, Liu WB, Ye JC. Effects of salinity on survival activity index and growth of larvae and juvenile of Nibea diacanthus. J Oceanography in Taiwan Strait. 2005; 24(3):351.
Choi CY, An KW, An MI. Molecular characterization and mRNA expression of glutathione peroxidase and glutathione S-transferase during osmotic stress in olive flounder (Paralichthys olivaceus). Mol Integrat Physiol. 2008; 149(3):330-7. [DOI:10.1016/j.cbpa.2008.01.013] [PMID]
Heath AG. Water pollution and fish physiology. Florida: CRC Press; 1987.
Osuala FI, Bawa-Allah KA. Mortality assessment of Oreochromis niloticus fingerlings in varying salinity and influence of salinity changes on acute toxicity of lead. Afr J Envir Sci Technol. 2014; 8(11):664-9.
Luoma SN. Bioavailability of trace metals in aquatic organisms: A review. Sci Total Envir. 1983; 28(1-3):1-22. [DOI:10.1016/S0048-9697(83)80004-7]
Shah SL, Altindag A. Hematological parameters of tench (Tinca tinca L.) after acute and chronic exposure to lethal and sublethal mercury treatments. Bulletin Envir Contam Toxicol. 2004; 73(5):911-8. [DOI:10.1007/s00128-004-0513-y] [PMID]
Riba I, Garcia-Luque E, Blasco J, DelValls TA. Bioavailability of heavy metals bound to estuarine sediments as a function of pH and salinity values. Chemical Speciation & Bioavailability. 2003; 1;15(4):101-14. [DOI:10.3184/095422903782775163]
Witeska M, Kondera E, Szymanska M. Hematological changes in common carp (Cyprinus carpio L.) after short-term lead (Pb) exposure. Polish J Envir Stud. 2010; 19(4):825-31.
Johansson-Sjöbeck ML, Larsson Å. Effects of inorganic lead on delta-aminolevulinic acid dehydratase activity and hematological variables in the rainbow trout, Salmo gairdnerii. Arch Envir Contamin Toxicol. 1979; 8(4):419-31. [DOI:10.1007/BF01056348] [PMID]
Holcombe GW, Benoit DA, Leonard EN. Long-term effects of lead exposure on three generations of brook trout (Salvelinus fontinalis). J Fish Board Canada. 1976; 33(8):1731-41. [DOI:10.1139/f76-220]
Seddiqui AK, Mishra S. Blood dyscrasia in a teleost, Colisa fasciatus after acute exposure to sub lethal concentrations of lead. J Fish Biol. 1979; 14(2):199-204. [DOI:10.1111/j.1095-8649.1979.tb03511.x]
Hodson PV, Blunt BR, Spry DJ. Chronic toxicity of water-borne and dietary lead to rainbow trout (Salmo gairdneri) in Lake Ontario water. Water Res. 1978; 12(10):869-78. [DOI:10.1016/0043-1354(78)90039-8]
Allien P. Effects of acute exposure to cadmium (II) chloride and lead (II) chloride on the haematological profile of Oreochromis aureus (Steindachner). Compar Pharmacol. 1993; 105(2):213-7. [DOI:10.1016/0742-8413(93)90197-S]
Haux C, Larsson Å. Influence of inorganic lead on the biochemical blood composition in the rainbow trout, Salmo gairdneri. Ecotoxicol Envir Safe. 1982; 6(1):28-34. [DOI:10.1016/0147-6513(82)90077-X]
Witeska M, Wakulska M. The effects of heavy metals on common carp white blood cells in vitro. Alter Lab Animal. 2007; 35(1):87-92. [DOI:10.1177/026119290703500123] [PMID]








































