Introduction
Malaria is one of the deadly infectious diseases globally and remains one of the oldest life-threatening parasitic diseases in Africa, despite the control programs [1]. Malaria affects the lives of almost all people but young children and pregnant women are at the highest risk where morbidity and mortality occur the most [2]. There are an estimated 100 million malaria cases with over 300,000 deaths per year in Nigeria. It is estimated that about 80% of rural populations still depend on herbal medicine and validations of these claims in treating malaria have yielded significant benefits [3].
The use of plants in traditional medicine in the treatment of various diseases is known since the beginning of human history [4]. Traditional folk medicine derived from plants has always guided researchers to search for novel drugs aimed at improving the health of humans and animals. The active ingredients extracted from these plants have led to the development of several lifesaving drugs, which are being used today in various communities globally [1].
Nauclea latifolia (N. latifolia), also known as Sarcocephalus latifolus (S. latifolus) is commonly called the African pincushion tree. It is used in traditional medicine in various regions of West Africa in the treatment of illnesses, such as malaria, pain and many infections [5]. The plant is known for its anti-inflammatory, anti-pyretic and anti-plasmodial properties, among others [6].
Terminalia glaucescens (T. glauscens) belongs to the Combretaceae family, believed to have anti-malarial activity but little systematic research has been conducted to explore its properties. The plant has been used traditionally to treat pain, dysentery, venereal diseases, coughs, dysmenorrhea, intestinal parasitosis, ulcers, leprosy and malaria [7]. The ethanolic extract has exhibited aldose inhibition plus anti-plasmodial and anti-cytotoxic effects [8]. In this study, the phytochemical and anti-plasmodial activities of T. glaucescens and N. latifolia were investigated in mice infected with Plasmodium berghei (P. berghei).
Materials and Methods
Collection and identification of plant materials: The stem bark of N. latifolia and T. glaucescens were collected with the help of local traditional medicine practitioners in Nso, North west region of Cameroon. Their scientific identification and authentication were established at the National Herbarium in Yaoundé, Cameroon (voucher specimen No.: 56386/HNC and 33322SRF/Cam). The plant samples were dried at room temperature and transferred to the laboratory in the Department of Biochemistry at Federal University of Technology, Minna, Nigeria, for further analyses.
Animals: Adult, Swiss albino mice, weighing 18-25 g, were obtained from the National Veterinary Research Institute (NVRI) in Vom, Plateau State, Nigeria. The animals had access to standard mice feed and water ad libitum. The mice were separated into male and female groups three weeks before starting the study.
Parasite: The parasite, chloroquine sensitive P. berghei (NK-65), was obtained from the National Institute of Pharmaceutical Research National Institute for Pharmaceutical Research and Development (NIPRD) Idu, Abuja, Nigeria. Parasitized erythrocytes were obtained from an infected donor mouse via cardiac puncture and were maintained by continuous reinfection in mice (serial passages).
Preparation of the plant materials: The plant materials were dried at room temperature and homogenized into a fine powder, using an electric blender. A 100 g sample of each plant material was extracted with 750 mL of 80% v/w methanol at 60o C for 2hr in a reflux extractor. The extract was filtered with a muslin cloth followed by Whatman paper filter No.1. The filtrate was evaporated to dryness using a water bath (45o C). The extracts were stored in tightly stoppered bottles and kept in a refrigerator at -4o C until used for further assays.
Quantitative phytochemical analyses: The plant extracts were subjected to quantitative phytochemical analyses, using standard procedures. Total phenols concentration was estimated using an established method [9]. The total alkaloids were measured according to the method of Harborne [10], saponins were determined as described by Oloyede [11] while tannins and flavonoids were assayed as described by Association of Official Analytical Chemists [12].
Acute toxicity of the extracts: The acute oral toxicity studies of the extracts were estimated in mice, using the modified Lorke’s method [13, 14]. The study was conducted in 2 phases with the extract concentrations at 10, 100 or 1000 mg/kg in phase 1, and 1600, 2900 and 5000 mg/kg in phase 2. The LD50 was calculated as the squared root of the product of the lowest lethal dose and the highest non-lethal dose. Specifically, this was the geometric mean of the consecutive dose for which 0 or 100% survival rates were recorded in the second phase. The median oral lethal dose was determined, using the following formula: LD50= Maximum survival dose x minimum lethal dose.
Evaluation of anti-plasmodial activity: Rane’s test was performed as reported by Iyiola et al. [15] with minor modifications. On the first-day, adult mice were inoculated intraperitoneally by injection of 0.2 mL of standard inoculum containing about 1.0×107 erythrocytes parasitized with P. berghei. These mice were randomly divided into 8 groups (A to H) of 3 animals each. The administration of the extracts and standard drug (chloroquine) was initiated three days’ post infection when parasitaemia had been established. The drugs and extracts were administered orally once a day at 24-hour intervals for five days (D0 to D4).
Mice in groups A, B and C were given of N. latifolia at graded doses of 100, 300 or 500 mg/kg, respectively. Groups D, E and F were given T. glaucescens extract at 100, 300 or 500 mg/kg, respectively. Group G mice received only 2 mL normal saline (negative control) while group H received 5 mg/kg chloroquine (positive control). Parasitaemia were counted on a daily basis according to the method described by Jigam et al. [16]. The body weights and packed cell volume (PCV) were monitored during the study period. The percent suppression of parasitaemia (Equation 1) and the mean survival days (Equation 2) of the mice were calculated for each test concentration, using the following equations:

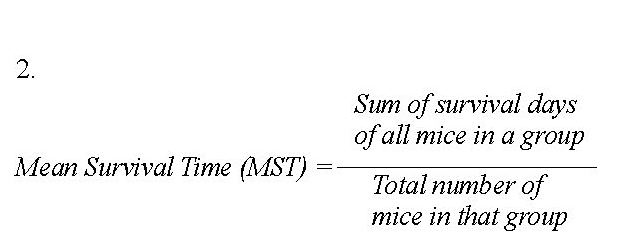
Results
Percent yield and phytochemical composition: Stem barks of N. latifolia and T. glaucescens had the extract yields of 17.32% and 18.71%, respectively. The quantitative phytochemical constituents of the crude extracts are presented in Table 1. The alkaloids were the most and while flavonoids were the least abundant secondary metabolites from both plants. Phenols and saponins were present at high concentration in T. glaucescens while flavonoids were found at high concentration in N. latifolia.
Acute oral toxicity profile of the extracts: The extracts of N. latifolia showed no signs of toxicity at any dose as shown in Table 2. The animals were calm and showed no strange reactions on the first phase of the experiment. However, as the dosage increased in the second phase, the animals were restless but the initial signs of weakness were reversed on the same day. All of the animals survived the experiments throughout the study. The LD50 was estimated to be greater than 5000 mg/kg.

The toxicity studies of T. glauscecens in the first phase did not cause any animal death (Table 3). The mice appeared normal, and no treatment-related signs of toxicity were observed during and after the treatments. In the second phase, as the dosage increased (1600 to 5000 mg/kg), the extract produced such physical signs as gasping for air, depression, and low food intake. Also, one death occurred after the administration of T. glauscecens at 2900 mg/kg followed by another death at 5000 mg/kg. Thus, the LD50 was determined to be 3808 mg/kg.
Anti-plasmodial activities of the extracts: The administration of the extracts was started 72 h post infection. On day one, the parasitaemia was determined before treatment in all the animals to be within 30%-37% and kept declining post treatment through day five. As shown in Figure 1 and 2, after five days of treatment, the parasitaemia was significantly lower (P<0.05) in the groups treated with the extract compared to the infected but untreated mice (negative control). The parasitaemia declined to below 15% in all groups treated with the extract or chloroquine (<2%; positive controls). The groups treated with 500 mg/kg, exhibited the lowest parasitaemia as compared with other doses, thus indicating a dose-dependent effect. However, parasitaemia was significantly lower (P<0.05) in the group treated with chloroquine compared to the highest dose of the extract (500 mg/kg) in all of the treated groups. The parasitaemia increased to 66.67% in the infected but untreated group (negative controls) after five days of being infected.

Effects of the extracts on parasitaemia suppression: The parasitaemia suppression and mean survival times of the mice treated with the extracts of N. latifolia and T. glaucescens are presented in Table 4. The highest suppression (66.79%) and mean survival days (27.67±1.45) was found in the groups treated with the N. latifolia extract at 500 mg/kg. The infected but untreated group survived only 9.33±0.88 days while chloroquine-treated groups lived for 31.33±0.88 days. For T. glaucescens, the highest suppression (65.37%) and mean survival days (30.33±0.33) was recorded for the groups treated with the extract at 500 mg/kg.
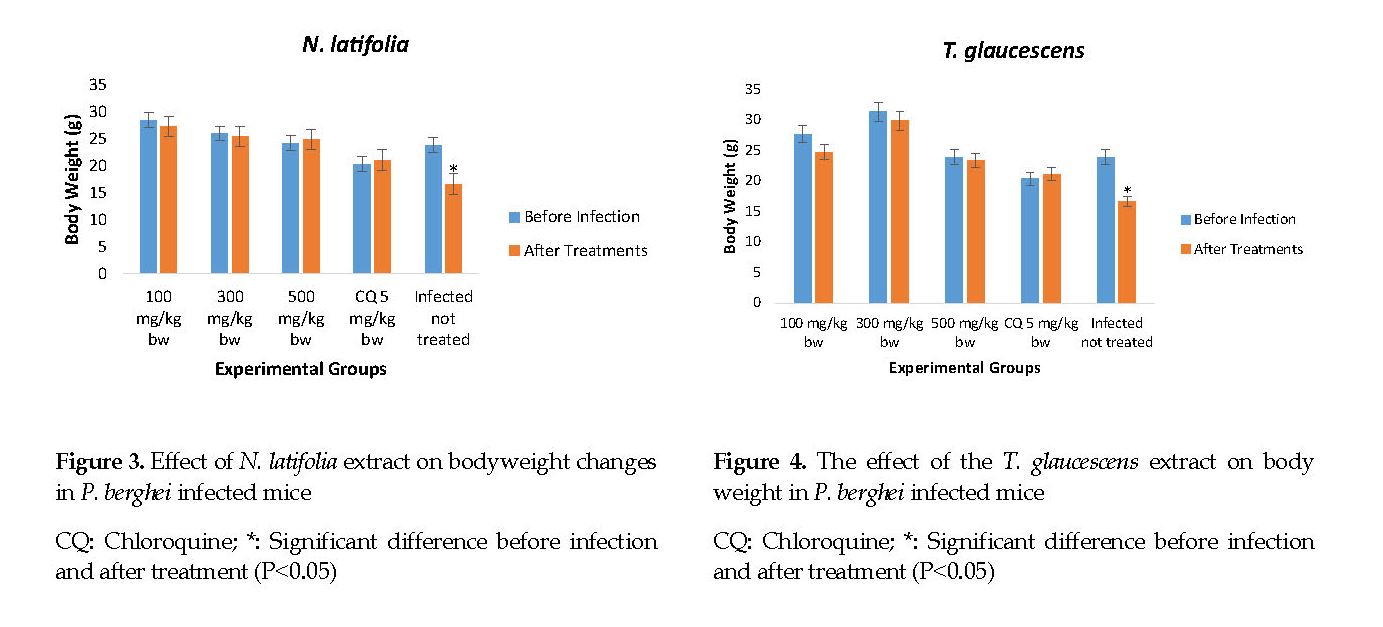
Effects of the extracts on changes in body weight: There was no significant difference (P>0.05) in the animals’ body weights before and after treatment (Figure 3). The mice treated with N. latifolia and T. glaucescens exhibited a significant increase (P<0.05) in weight compared to the infected but untreated group (negative controls). The chloroquine group (positive controls) showed a significant increase (P<0.05) in weight compared to those treated with the extracts. There was a significant loss (P<0.05) in the body weight of mice treated with 100 mg/kg of T. glaucescens compared to other treated groups (Figure 4).

Effect of the extracts on packed cell volume: The results indicated that the PCV for all of the treatments were not significantly different before the infection and after the treatment (Figures 5 & 6). The mean PCV recorded 5 days after treatment with the extracts showed an insignificant decrease in all of the treatment groups. However, The PCV of the infected but untreated group showed a significant decrease (P<0.05) in PCV compared to those in the treated groups.
Discussion
The preliminary phytochemical constituents detected were phenols, alkaloids, saponins, tannins and flavonoids (Table 1). Phytochemical constituents have been frequently reported for their therapeutic benefits in herbal medicines. The secondary metabolites, such as alkaloids have shown significant anti-malarial activities by blocking the protein synthesis in Plasmodium falciparum [17]. Saponins, flavonoids and tannins have been suggested to act as primary anti-oxidants or free radical scavengers, inhibiting the oxidative damages caused by malaria [18].
These secondary metabolites could have elicited the observed anti-plasmodial activity either singly or synergistically with each other, through the inhibition of protein synthesis and preventing the invasion of new red blood cells by the plasmodium parasites. The concept of phytochemical constituents contributing to anti-malarial activity is consistent with the results reported by Jigam et al. [16]. These authors investigated the anti-plasmodial, analgesic and anti-inflammatory effects of the extract of Guira senegalensi (Combretaceae) leaves in mice infected with P. berghei. Ettebong et al. have also reported the association of the phytochemicals with anti-malarial activity of Eleucine indica [19].
Safety margin of the extracts: The LD50 estimated to be greater than 5000 mg/kg for N. latifolia is consistent with the findings of Ogugua et al. who also reported the potential protective effects of the methanolic extract of N. latifolia on the liver and kidneys in experimental animals [20]. On the other hand, the LD50 of 3808 mg/kg for T. glaucescens is not consistent with those found by Konan et al. who suggested the toxicity of the aqueous T. glaucescens extract being greater than 5000 mg/kg [21]. This discrepancy could be attributed to the differences in the polarity of the extraction solvents. However, the highest dose (500 mg/kg) of the extracts, used to treat the infected mice with anti-malarial activity, was far lower than 1600 mg/kg, indicative of the extract safety. Any orally administered test substance with lethal dose less than 1000 mg/kg or higher than three times the minimum effective dose can be considered nontoxic [22].
Effect on physical activity: There was a general increase in the physical activities of the mice treated with the extracts compared with that in the parasitized but untreated mice. The results showed that the physical activities of the extracts-treated mice were better than that observed in the untreated animals. This could be due to the ameliorating effect of the plant extracts on malaria infection as confirmed by the reduction in parasite multiplication of the treated rats. The anti-malaria activity of T. laucescens, and N. latifolia, as demonstrated in this study, justifies the traditional use of these plants for the treatment of malaria in Nso, Cameroon.
Dose and time dependence: The extracts also showed that their anti-plasmodial activity in mice infected with P. berghei was exposure duration-dependent and required significantly higher amounts of the extract compared with the standard drug (chloroquine), as evidenced by the percentage of inhibition of the parasite and improving the survival time. The extracts may be considered as active anti-malarial agents, based on their ability to produce a minimum inhibition of parasitaemia (30%) and offering a greater percent of survival time, compared to the results noted in the infected but untreated mice [23].
Moreover, the extracts administered at a daily dose of 500 mg/kg for five days resulted in >50% reduction of the parasitaemia. This performance is likely to be improved if the extracts are purified and the active molecules identified. The observed activity suggests that the extracts may suppress the parasitic condition to lower levels in the long-run and its application in traditional medicine may be further justified. Our findings are also supported by previous studies that discovered anti-plasmodial potentials in the methanol extracts of Eucalyptus camadulensis and N. latifolia leaves in laboratory animals [24-26].
Although there are ample research published on some members of Combreteceae plant family for their anti-plasmodial and anti-protozoal activities [27]; however, studies on T. glaucescens are very scarce. To the best of our knowledge, the present study is the first in vivo research on the anti-plasmodial activity of the methanol extracts of T. glaucescens.
Weight loss: The weight loss is a notable manifestation in mice infected with P. berghei. This is likely to be due to the parasite depressant action on the appetite and other disturbed metabolic functions [28]. The body weight can be used to evaluate the anti-malarial activity of the mice treated with the above extracts [29]. The extracts showed a dose-dependent, preventive effect on body weight loss as compared to that observed in the untreated animals. However, the weight loss could be attributed to anti-nutritive factors that may be present in the crude extracts [30].
For instance, tannins inhibit growth by decreasing the digestion coefficient of most nutrients and the coagulation of proteins. The increase in body weight at higher doses of the crude extract may be due to the fact that higher level of parasitaemia significantly lowers the initial body weight so the significant change might be due to the regain of initial weight loss by the curative effects of higher doses of the extracts. The implication is that lower doses lack enough concentration of the active ingredients. Similar suggestions have been made by Jigam et al. who studied the Guiera senegalensis leaf extracts [16].
Packed Cell Volume: Erythrocyte fragility, reduced PCV and life-threatening anemia is commonly observed in mice infected with P. berghei [30]. The effect of PCV was reversed after five days of treatment with the extracts, though there were insignificant differences in the absolute values of PCV documented among the groups in our study. Further, there was no significant difference among the PCV values before and after infection. The extracts were able to reverse the decline in PCV because of their effect on the parasite. However; the PCV in the infected but untreated mice (negative controls) was much lower than those of the treated groups.
Rodent malaria causes a decline in PCV, occurring progressively 48 hours after the infection onward with no amelioration expected. The untreated mice suffered because of erythrocyte destruction, either by parasite multiplication or by destruction or entrapment in the spleen. This occurs due to the presence of many abnormal erythrocytes stimulating phagocytosis in the spleen [31]. These findings are similar to the work of Kabiru et al. who studied the anti-plasmodial effect of the methanolic extracts of Eucalyptus camaldulensis leaves [24]. These authors reported similar findings for reduced Bush mango in the infected but untreated mice.
Conclusions
The methanolic stem bark extracts of N. latifolia, and T. glaucescens exhibited comparable and significant activities against P. berghei infection in mice. Thus they could be considered as potential candidates for the development of new anti-malarial agent for clinical experiment and eventual use in humans, especially in the practice of traditional medicine.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Committee on Ethics for Medical and Scientific Research at Federal University of Technology, Minna, Nigeria. We duly observed the principles governing the use of experimental animals, set out by the university, based on the internationally accepted principles for animal use and care, as contained in the Canadian Council on Animal Care Guidelines and the review protocol.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author's contributions
Design: Eustace Bonghan Berinyuy and Emmanuel Olofu Ogbadoyi; Conduction of the lab work: Emmanuel Olofu Ogbadoyi; Supervision: Adamu Yusuf Kabiru; Participated in the study design and co-supervision: Mann Abdullahi.
Conflict of interest
The authors declare no conflict of interests in conducting this study.
Acknowledgements
The authors would like to appreciate the technical staff of Biochemistry Laboratory of the Federal University of Technology in Minna, Nigeria, for their support of this study.
References
Fan YL, Cheng XW, Wu JB, Liu M, Zhang FZ, Xu Z, et al. Antiplasmodial and anti-malarial activities of quinolone derivatives: An overview. Eur J Med Chem. 2018; 146:1-14. [DOI:10.1016/j.ejmech.2018.01.039] [PMID]
WHO. Malaria [Internet]. 2019 [Updated 2019 March 27]. Available from: https://www.who.int/news-room/fact-sheets/detail/malaria
Lawal B, Shittu OK, Kabiru AY, Jigam AA, Umar MB, Berinyuy EB, et al. Potential antimalarials from African natural products: A review.J Intercult Ethnopharmacol. 2015; 4(4):318-43. [DOI:10.5455/jice.20150928102856] [PMID] [PMCID]
Shi QW, Li LG, Huo CH, Zhang M, Wang YF. Study on natural medicinal chemistry and new drug development. Chinese Traditional and Herbal Drugs. 2010; 41(10):1583-9.
Bahekar S, Kale R. Herbal plants used for the treatment of malaria. Journal of Pharmacognosy and Phytochemistry. 2013; 1(6):141-6.
Abbah J, Amos S, Chindo B, Ngazal I, Vongtau HO, Adzu B, et al. Pharmacological evidence favouring the use of Nauclea latifolia in Malaria ethnopharmacy: Effects against nouception, inflammation and pyria in rats and mice. J Ethnopharmacol. 2010; 127(1):85-90. [DOI:10.1016/j.jep.2009.09.045] [PMID]
Lem MF, Khan Payne V, Wabo Poné J, Gertrude MT, Tchoumboue J. In vivo anthelmintic activity of Terminalia glaucescens (Combreteceae) extracts against gastrointestinal Nematodes of sheep. Br J Pharm Res. 2014; 4(18):2136-45. [DOI:10.9734/BJPR/2014/12936]
Rahman AU, Zareen S, Iqbal Choudhary M, Nadeem Akhtar M, Shujaat Sh, Ngounou FN. Some chemical constituents of Terminalia glaucescens and their enzymes inhibition activity. Zeitschrift für Naturforschung B. 2014; 60(3):347-50. [DOI:10.1515/znb-2005-0320]
Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005; 4(7):685-8. [DOI:10.5897/AJB2005.000-3127]
Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. 3rd ed. London: Chapman & Hall; 1998.
Oloyede OI. Chemical profile of unripe pulp of Carica papaya. Pak J Nutr. 2005; 4(6):379-81. [DOI:10.3923/pjn.2005.379.381]
Association of Official Analytical Chemists. Official methods of analysis of the association of official analytical chemists. 15th ed. Virginia: The Association; 1990.
Lorke D. A new approach to practical acute toxicity testing. Archives of Toxicology. 1983; 54(4):275-87. [DOI:10.1007/BF01234480] [PMID]
Amos TN, Bashir L, Saba SE, Saba MA, Mohammed BM, Abdulsalam IH, et al. Phytochemicals and acute toxicity profile of aqueous and methanolic extracts of Crateva adansonii leaves in Swiss albino rats. Asian J Biochem. 2015; 10(4):173-9. [DOI:10.3923/ajb.2015.173.179]
Iyiola DA, Tijani AY, Lateef KM. Antimalarial activity of ethanolic stem bark extract of Aistonia Bosnian mice. Asian J Biol Sci. 2011; 4(3):235-43. [DOI:10.3923/ajbs.2011.235.243]
Jigam AA, Akanya HO, Dauda BEN, Ogbadoyi EO. Antiplasmodial analgesic and anti-inflammatory effects of crude Guiera senegalensis Gmel (Combretaceae) leaf extracts in mice infected with Plasmodium berghei. J Pharmacogn Phytother. 2011; 3(10):150-4. [DOI:10.5897/JPP11.033]
Nalubega R, Nyanzi SA, Nakavuma JL, Kamatenesi-Mugisha M. Ethnobotanical uses of Lantana trifolia L. and Sida cuneifolia Roxb. in mukungwe and wabinyonyi sub-counties of central Uganda. J Intercult Ethnopharmacol. 2013; 2(3):155-64. [DOI:10.5455/jice.20130809114525]
Bell D, Winstanley P. Current issues in the treatment of uncomplicated malaria in Africa. Br Med Bull. 2004; 71:29-43. [DOI:10.1093/bmb/ldh031] [PMID]
Ettebong EO, Nwafor PA, Okokon JE. In vivo antiplasmodial activities of ethanolic extract and fractions of Eleucine indica. Asian Pac J Trop Med. 2012; 5(9):673-6. [DOI:10.1016/S1995-7645(12)60105-9]
Nwadiogbu OV, Ikechukwu UR, Ikechukwu ES, Obiora A. Hepatoprotective and healthy kidney promoting potentials of methanol extract of Nauclea latifolia in alloxan induced diabetic male wistar albino rats. Asian J Biochem. 2017; 12(3):71-8. [DOI:10.3923/ajb.2017.71.78]
Fernique KK, Nathalie G, Eugène KK, Adjogoua E, Rosine AAM, Lucien KK, et al. Terminalia glaucescens Planch. Ex Benth. (Combretaceae), a Medicinal Plant of Côte d’Ivoire Pharmacopoeia: Antibacterial activity on staphylococcus and pseudomonas, acute toxicity on mice and lethal effect on vero E6 cellss. International Journal of Pharmacy & Pharmaceutical Research. 2016; 7(3):290-303.
Toma A, Deyno S, Fikru A, Eyado A, Beale A. In vivo antiplasmodial and toxicological effect of crude ethanol extract of Echinops kebericho traditionally used in treatment of malaria in Ethiopia. Malar J. 2015; 14:196. [DOI:10.1186/s12936-015-0716-1] [PMID] [PMCID]
Fentahun S, Makonnen E, Awas T, Giday M. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement Altern Med. 2017; 17(1):13. [DOI:10.1186/s12906-016-1529-7] [PMID] [PMCID]
Kabiru YA, Okolie NL, Muhammad HL, Ogbadoyi EO. Preliminary studies on the antiplasmodial potential of aqueous and methanol extracts of eucalyptus camadulensis leaf. Asian Pac J Trop Dis. 2012; 2(Suppl. 2):S809-S14. [DOI:10.1016/S2222-1808(12)60270-9]
Ettebong EO, Edwin UPM, Edet EC, Samuel EU, Ezekiel AO, Dornu TV. In vivo antiplasmodial activities of Nauclea latifolia. Asian J Med Sci. 2015; 6(3):6-11. [DOI:10.3126/ajms.v6i3.11361]
Benoit-Vical F, Valentin A, Cournac V, Pélissier Y, Mallié M, Bastide JM. In vitro antiplasmodial activity of stem and root extracts of Nauclea latifolia S.M. (Rubiaceae). J Ethnopharmacol. 1998; 61(3):173-8. [DOI:10.1016/S0378-8741(98)00036-1]
Truiti MC, Ferreira IC, Zamuner ML, Nakamura CV, Sarragiotto MH, Souza MC. Antiprotozoal and molluscicidal activies of five Brazilian plants. Brazililan Jounal of Medical and Biological Research. 2005; 38(12):1873-8. [DOI:10.1590/S0100-879X2005001200016] [PMID]
Asrade S, Mengesha Y, Moges G, Gelayee DA. In vivo antiplasmodial activity evaluation of the leaves of Balanites rotundifolia (Van Tiegh.) Blatter (Balanitaceae) against Plasmodium berghei. J Exp Pharmacol. 2017; 9:59-66. [DOI:10.2147/JEP.S130491] [PMID] [PMCID]
Okokon JE, Ettebong E, Antia BS. In vivo antimalarial activity of ethanolic leaf extract of Stachytarpheta cayennensis. Indian J Pharmacol. 2008; 40(3):111-3. [DOI:10.4103/0253-7613.42303] [PMID] [PMCID]
Seyfe S, Toma A, Esaiyas A, Debela E, Fikru A, Eyado A. Phytochemical screening and in vivo antimalarial activities of crude extracts of Lantana trifolia root and Premna oligotricha leaves in Plasmodium berghei infected mice. Journal of Medicinal Plants Research. 2017; 11(47):763-9. [DOI:10.5897/JMPR2017.6519]
Chinchilla M, Gabriela Abarca OMG, Osear Castro MBY. An in vivo model to study the anti-malaria capacity of plant extracts. Rev Biol Trop. 1998; 46(1):35-9. [PMID]








































