Introduction
Multiple Sclerosis (MS) is a chronic inflammatory disease associated with the demyelination of the nerve fibers in the Central Nervous System (CNS) [1]. It is an autoimmune disease due to the pathological infiltration of mononuclear cells that lead to the demyelination of neuronal axons, gliosis in the CNS white matter, and the formation of numerous plaques in the brain and spinal cord tissue [2]. Interferons are important inflammatory mediators, believed to be involved in the mechanism and progression of the disease [3, 4]. They belong to a group of intracellular messengers called cytokines with anti-tumor, anti-viral and anti-proliferative properties [5].
Interferons also have regulatory roles in the immune system [6]. The release of interferon beta (IFN-β) is induced by cytokines, such as IL-1, IL-2, TNF-α, T-cells, macrophages and some viruses [7]. One of the pharmaceutical treatment options for MS is IFN-β [8, 9]. This agent reduces the relapse rate of MS by about 30% also lowers the incidence of neuronal lesions in the CNS [10]. The Administration of IFN-β is beneficial against the relapse of MS, but it has no effect on the initial progression [11, 12].
The role of vitamin B12 in the pathogenesis of MS has been reported over the past three decades [13]. Also, the regulatory effects of vitamin B12, such as modification of TNF-α activity in the immune system have been reported [14]. The concurrent deficiency of vitamin B12 and the regulatory mechanisms can exacerbate MS by intensifying the inflammatory processes, demyelination or retarding the remyelination process, and the management [15]. Therefore, the application of vitamin B12 may be considered as a complementary therapeutic strategy along with other treatment protocols in MS patients.
Melatonin (5-methoxy-n-acetyl-tryptamine) is a hormone secreted from the pineal gland and is synthesized from the neurotransmitter serotonin [16]. Melatonin receptors in the brain are found in the prefrontal cortex, cerebellum, hippocampus, basal ganglia, substantia nigra, nucleus accumbens, retina, and in various parts of the hypothalamus [17]. In addition, these receptors are found in such tissues as the digestive tract, adipose tissue, pancreas, ovaries, skin, lungs and heart, and lymphocytes [18, 19]. Melatonin is believed to be effective in regulating such cytokines as TNF-α, IL-8, IL-6, INF and other inflammatory mediators [20, 21].
Studies published in recent years on melatonin have suggested that melatonin may be used as a therapeutic supplement in many neurodegenerative conditions [20, 22, 23]. In patients with MS, serum melatonin levels are reduced due either to calcium deposition in the pineal gland or hypothalamic dysfunction [24, 25]. Also, melatonin plays a mediating role in the mechanisms of signal transduction by changing the ion flow through various ion channels [26]. In this study, the serum levels of INF-1β and Vitamin B12 were investigated in two groups of patients with MS.
Materials and Methods
Sample Population: The sample population consisted of 50 patients with MS referred to the Arak MS Society and subsequently to the Vali-Asr Hospital, Arak, Iran, between April 2015 and August 2017. The sample size was calculated based on the Cochrane formula.
Inclusion & Exclusion Criteria: Patients with relapsing-remitting MS based on McDonald 2 criteria, age 18-50 years old, Expanded Disability Status Scale (EDSS) between 0-5.5, completion of the consent form, and the ability to participate in the study were included. A completed consent form was received from each patient prior to participation in the study. The exclusion criteria were the use of non-interferon medications, vitamin B12, alcohol, being pregnant and/or a having a diagnosis of autoimmune disease.
Treatments: All patients were treated routinely with interferon (30µg / week / 24 weeks). They were randomly divided into a control group (n=25) that received a placebo, and a treatment group (n=25) who received one dose of melatonin (3 mg) at night an hour before bedtime for 24 weeks. Venous blood samples from both groups were collected by a nurse under sterile condition at Vali-Asr Hospital in Arak, Iran.
Biochemical Analyses: The blood samples were immediately centrifuged and the sera were kept in a freezer at -80° C. The frozen serum samples were transferred to the Immunology Laboratory at Tabriz Medical University, in which the levels of INF-1β and Vitamin B12 were assayed, using the ELISA methods. All biochemical kits were purchased from Bioassay Technology Laboratory (China) and the samples were evaluated based on the specific methodology for each assay kit. Finally, samples were read by an Elisa reading unit at an optic density of 450 nm.
Data Analyses: The recorded patients’ anthropometric data included height, weight, and age. The normality of data distribution was analyzed by Kolmogorov-Smirnov test followed by Mann-Whitney non-parametric test. The results for both groups were analyzed by SPSS V. 21 and a randomized division method.
Results
Demographic data of the patients participating in the two groups were analyzed on SPSS V. 21, using independent t-test. The two groups were compared in terms of age, gender, weight, and height and there was no significant differences between them (Table 1).

Analyses of INF1β Data: The serum levels of INF1β were lower in patients with MS compared to the normal standard range. The results indicated that the levels of the INF1β increased significantly (P<0.004) only in patients taking a daily dose of melatonin for 24 weeks (Figure 1).
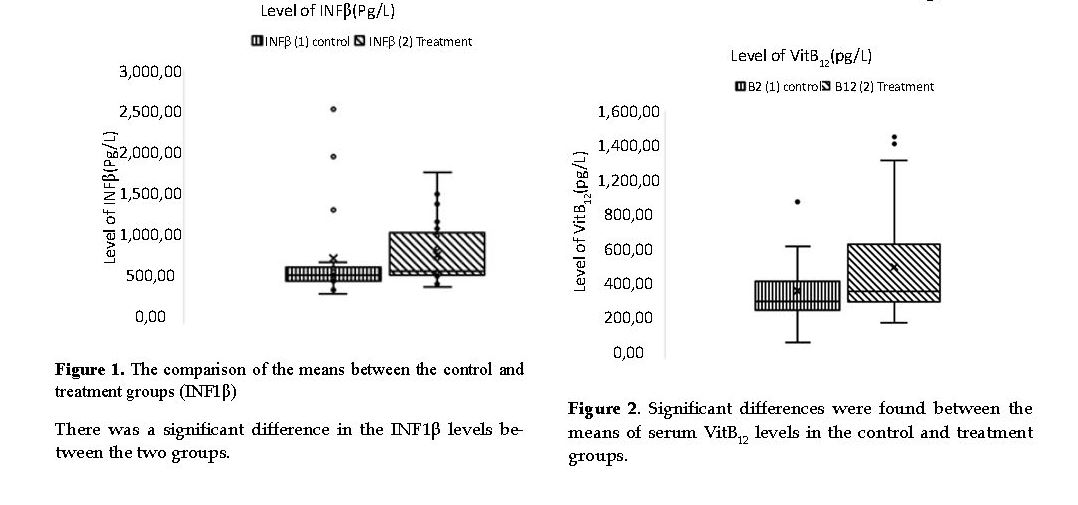
Analysis of Vitamin B12 Data: The serum level of Vitamin B12 was lower in patients with MS compared to the normal standard range. As shown in Figure 2, the serum vitamin B12 increased significantly only in patients taking melatonin over 24 weeks compared to that in the control group (P<0.004).
Discussion
The results showed that there was a significant difference between the placebo and treatment groups in the serum levels of the Vitamin B12. It is likely that melatonin influenced the higher presence of vitamin B12 in the serum. Studies on MS patients suggest that Vitamin B12 deficiency plays a role in the pathogenesis of MS [27, 28]. Miller et al. [16] and Singh et al. [17] reported that neurological disorders caused by vitamin B12 deficiency include such pathologies as demyelination and axonal degeneration [29, 30].
Also, previous studies demonstrated that the vitamin B12 levels in serum and/or CSF in MS patients decreased and there was a significant relationship between MS and vitamin B12 deficiency [31, 32]. We expected that receiving melatonin in our patients would increase the serum level of vitamin B12, because its deficiency can exacerbate the symptoms of MS by intensifying the resultant inflammatory processes, demyelination, and retarding the remyelination process and prolonging the recovery. Therefore, taking vitamin B12 in MS patients can be considered as a positive therapeutic complement along with other clinical strategies [33].
Studies have also shown that there is a direct link between vitamin B12 intake and the amount of melatonin produced in the CNS, i.e. the vitamin B12 intake increases the CNS melatonin level [34, 35]. It has been shown that in patients with MS, the serum levels of both vitamin B12 and melatonin decrease dramatically [36]. In the current study, it was assumed that the serum level of vitamin B12 in the patients receiving melatonin would change. Our results were consistent with this concept. Our results also indicated that there was a significant difference in the serum levels of INF1β between the placebo and melatonin treatment groups.
In the patients receiving melatonin, a significant increase was noted in their serum level of INF1β. Based on our findings, we may argue that there is an association between the serum melatonin and INF1β. As an effective ingredient of Cinnovex, the drug used to treat patients with recurrent MS symptoms, INF1β, reduces the recurrences and prevents the clinical exacerbations.
Also, IFNβ is widely used as the first-line treatment in MS [37]. It also minimizes or delays the development of motor disabilities. Hence the reason for this drug to be currently one of the nine known medications that alters the clinical course of the disease [38]. IFNβ reduces the crossing of inflammatory mediators through the Blood-Brain Barrier (BBB) and subsequently increases the neuronal repairs in MS patients [39]. Therefore, melatonin may be used as an auxiliary, safe and affordable medication with minimal side effects and low toxicity risk to effectively manage the MS symptoms. Several studies have also shown that melatonin has anti-inflammatory properties and leads to the alleviation of fatigue states in MS patients [40-43]. Also, Jamshidi-Fard et al. [42] and Wurtman [43] have shown that melatonin is effective in treating the visual impairment in MS patients.
Conclusions
The level of serum melatonin was lower in MS patients than that in standard normal range. This hormone is likely to play a protective role in MS and other biological processes, such as immune responses and circadian rhythm. Also, this hormone is known to have anti-inflammatory, immunomodulatory and antioxidative effects. Further, melatonin serves to reduce oxidative and nitrous oxide (nitrosative) stresses, and can lower the serum inflammatory mediators in MS patients. The protective effects of this hormone could play an important role in the pathogenesis of the disease. In this study, the serum melatonin levels were associated with increases in the levels of INF1β and VitB12. Therefore, it can potentially be a valuable therapeutic agent in the management of this human neurological disorder.
Ethical Considerations
Compliance with ethical guidelines
The present study was registered in the Iranian Registry of Clinical Trials Code: IRCT2017072520258N55 and approved by Arak Medical University Ethics Committee with the Code of ethics confirmation: IR.ARAKMU.REC.1394.9.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author's contributions
Performed experiments, analysed data, writing original draft preparation and co-writing the paper: Masoomeh Yosefi-Fard; Review and editing original draft and Supervised the research: Gholamhassan Vaezi; Designed methodology and supervised the research also reviewed and editing original draft: Ali Akbar Maleki-Rad; Provided Multiplied Sclerosis (MS): Fardin Faraji; Reviewed and editing original draft: Vida Hojati.
Conflict of interest
The authors declared no conflict of interests.
Acknowledgements
The authors wish to thank Arak MS Society and Vali-Asr Hospital's staffs, Arak, Iran.
References
Höftberger R, Lassmann H. Inflammatory demyelinating diseases of the central nervous system. Handb Clin Neurol. 2018; 145:263-83. [DOI:10.1016/B978-0-12-802395-2.00019-5] [PMID]
Kwon MS, Noh MY, Oh KW, Cho KA, Kang BY, Kim KS, et al. The immunomodulatory effects of human mesenchymal stem cells on peripheral blood mononuclear cells in ALS patients. J Neurochem. 2014; 131(2):206-18. [DOI:10.1111/jnc.12814] [PMID]
Ontaneda D, Hyland M, Cohen JA. Multiple sclerosis: New insights in pathogenesis and novel therapeutics. Annu Rev Med. 2012; 63:389-404. [DOI:10.1146/annurev-med-042910-135833] [PMID]
Mahad DH, Trapp PBD, Lassmann PH. Pathological mechanisms in progressive multiple sclerosis. The Lancet Neurol. 2015; 14(2):183-93. [DOI:10.1016/S1474-4422(14)70256-X]
Schmeisser H, Bekisz J, Zoon KC. New function of type I IFN: Induction of autophagy. J Interferon Cytokine Res. 2014; 34(2):71-8. [DOI:10.1089/jir.2013.0128] [PMID] [PMCID]
Hertzog P, Forster S, Samarajiwa S. Systems biology of interferon responses. J Interferon Cytokine Res. 2011; 31(1):5-11. [DOI:10.1089/jir.2010.0126] [PMID]
Kasper LH, Reder AT. Immunomodulatory activity of interferon‐beta. Ann Clin Transl Neurol. 2014; 1(8):622-31. [DOI:10.1002/acn3.84] [PMID] [PMCID]
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998; 338(5):278-85. [DOI:10.1056/NEJM199801293380502] [PMID]
Prescott J, Dangi-Garimella S, Musaji N, Caffrey MK, Delmendo I, Szeszko A, et al. Current strategies in the treatment of multiple sclerosis. Am J Manag Care. 2018; 2018:3-12.
Dargahi N, Katsara M, Tselios T, Androutsou ME, de Courten M, Matsoukas J, et al. Multiple sclerosis: Immunopathology and treatment update. Brain Sci. 2017; 7(7).pii:E78. [DOI:10.3390/brainsci7070078] [PMID] [PMCID]
Stewart N, Simpson S Jr, van der Mei I, Ponsonby AL, Blizzard L, Dwyer T, et al. Interferon-β and serum 25-hydroxyvitamin D interact to modulate relapse risk in MS. Neurol. 2012; 79(3):254-60. [DOI:10.1212/WNL.0b013e31825fded9] [PMID]
Madsen C. The innovative development in interferon beta treatments of relapsing‐remitting multiple sclerosis. Brain Behav. 2017; 7(6):e00696. [DOI:10.1002/brb3.696] [PMID] [PMCID]
Wolffenbuttel BHR, Wouters HJCM, Heiner-Fokkema MR, van der Klauw MM. The many faces of cobalamin (vitamin B12) deficiency. Mayo Clinic Proceedings. Innov Qual Outcomes. 2019; 3(2):200-14. [DOI:10.1016/j.mayocpiqo.2019.03.002] [PMID] [PMCID]
Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front Nutr. 2019; 6:48. [DOI:10.3389/fnut.2019.00048] [PMID] [PMCID]
Mansueto P, Di Stefano L, D’Alcamo A, Seidita A, Adragna F, Drago G, et al. Vitamin B12 deficiency with multiple sclerosis-like neurological clinical framework. Acta Medica Mediterranea. 2012; 28:7-11.
Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2006; 38(3):313-6. [DOI:10.1016/j.biocel.2005.08.020] [PMID]
Li DY, Smith DG, Hardeland R, Yang MY, Xu HL, Zhang L, et al. Melatonin receptor genes in vertebrates. Int J Mol Sci. 2013; 14(6):11208-23. [DOI:10.3390/ijms140611208] [PMID] [PMCID]
Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin -- a pleiotropic, orchestrating regulator molecule. Prog Neurobiol. 2011; 93(3):350-84. [DOI:10.1016/j.pneurobio.2010.12.004] [PMID]
Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu Rev Pharmacol Toxicol. 2016; 56:361-83. [DOI:10.1146/annurev-pharmtox-010814-124742] [PMID] [PMCID]
Reiter RJ, Manchester LC, Tan DX. Neurotoxins: Free radical mechanisms and melatonin protection. Curr Neuropharmacol. 2010; 8(3):194-210. [DOI:10.2174/157015910792246236] [PMID] [PMCID]
Sun H, Wang X, Chen J, Gusdon AM, Song K, Li L, et al. Melatonin treatment improves insulin resistance and pigmentation in obese patients with acanthosis nigricans. Int J Endocrinol. 2018; 2018:2304746. [DOI:10.1155/2018/2304746] [PMID] [PMCID]
Hsu CN, Huang LT, Tain YL. Perinatal use of melatonin for offspring health: Focus on cardiovascular and neurological diseases. Int J Mol Sci. 2019; 20(22).pii:E5681. [DOI:10.3390/ijms20225681] [PMID] [PMCID]
Cardinali DP. Melatonin: Clinical perspectives in neurodegeneration. Front Endocrinol. 2019; 10:480. [DOI:10.3389/fendo.2019.00480] [PMID] [PMCID]
Akpinar Z, Tokgöz S, Gökbel H, Okudan N, Uğuz F, Yilmaz G. The association of nocturnal serum melatonin levels with major depression in patients with acute multiple sclerosis. Psychiatry Res. 2008; 161(2):253-7. [DOI:10.1016/j.psychres.2007.11.022] [PMID]
Tan DX, Xu B, Zhou X, Reiter RJ. Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Mol. 2018; 23(2).pii:E301. [DOI:10.3390/molecules23020301] [PMID] [PMCID]
Lee JG, Woo YS, Park SW, Seog DH, Seo MK, Bahk WM. The neuroprotective effects of melatonin: Possible role in the pathophysiology of neuropsychiatric disease. Brain Sci. 2019; 9(10).pii:E285. [DOI:10.3390/brainsci9100285] [PMID] [PMCID]
Kalita J, Misra UK. Vitamin B12 deficiency neurological syndromes: Correlation of clinical, MRI and cognitive evoked potential. J Neurol. 2008; 255(3):353-9. [DOI:10.1007/s00415-008-0660-x] [PMID]
Dardiotis E, Arseniou S, Sokratous M, Tsouris Z, Siokas V, Mentis AA, et al. Vitamin B12, folate, and homocysteine levels and multiple sclerosis: A meta-analysis. Mult Scler Relat Disord. 2017; 17:190-7. [DOI:10.1016/j.msard.2017.08.004] [PMID]
Miller JW. Vitamin B12 deficiency, tumor necrosis factor-alpha, and epidermal growth factor: A novel function for vitamin B12? Nutr Rev. 2002; 60(5 Pt 1):142-4. [DOI:10.1301/00296640260093805] [PMID]
Singh NN. Vitamin B-12 associated neurological diseases [Internet]. 2018. [Updated 2018 October 22]. Available from: https://emedicine.medscape.com/article/1152670-overview
Reynolds EH. Multiple sclerosis and vitamin B12 metabolism. J Neuroimmunol. 1992; 40(2-3):225-30. [DOI:10.1016/0165-5728(92)90137-A]
Kocer B, Engur S, Ak F, Yilmaz M. Serum vitamin B12, folate, and homocysteine levels and their association with clinical and electrophysiological parameters in multiple sclerosis. J Clin Neurosci. 2009; 16(3):399-403. [DOI:10.1016/j.jocn.2008.05.015] [PMID]
Khosravi-Largani M, Pourvali-Talatappeh P, Rousta AM, Karimi-Kivi M, Noroozi E, Mahjoob A, et al. A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalSci. 2018; 10:37-44. [DOI:10.1016/j.ensci.2018.01.007] [PMID] [PMCID]
Nozari E, Ghavamzadeh S, Razazian N. The effect of vitamin B12 and folic acid supplementation on serum homocysteine, anemia status and quality of life of patients with multiple sclerosis. Clin Nutr Res. 2019; 8(1):36-45. [DOI:10.7762/cnr.2019.8.1.36] [PMID] [PMCID]
Bagur MJ, Murcia MA, Jiménez-Monreal AM, Tur JA, Bibiloni MM, Alonso GL, et al. Influence of diet in multiple sclerosis: A systematic review. Adv Nutr. 2017; 8(3):463-72. [DOI:10.3945/an.116.014191] [PMID] [PMCID]
Emamgholipour S, Hossein-Nezhad A, Sahraian MA, Askarisadr F, Ansari M. Evidence for possible role of melatonin in reducing oxidative stress in multiple sclerosis through its effect on SIRT1 and antioxidant enzymes. Life Sci. 2016; 145:34-41. [DOI:10.1016/j.lfs.2015.12.014] [PMID]
Kieseier BC. The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs. 2011; 25(6):491-502. [DOI:10.2165/11591110-000000000-00000] [PMID]
La Mantia L, Vacchi L, Di Pietrantonj C, Ebers G, Rovaris M, Fredrikson S, et al. Interferon beta for secondary progressive multiple sclerosis. 2012. Cochrane Database Syst Rev. 2012; 1:CD005181. [DOI:10.1002/14651858.CD005181.pub3] [PMID]
Kay M, Hojati Z, Dehghanian F. The molecular study of IFNβ pleiotropic roles in MS treatment. Iran J Neurol. 2013; 12(4):149-56. [PMID] [PMCID]
Alimoradian A, Shamsi M, Faraji F, Ahmadi M, Sayyedi SE. [Evaluation of the serum melatonin levels in the treatment of patients with multiple sclerosis (Persian)]. Journal of Arak University of Medical Sciences. 2018; 21(2):55-64.
Beriwal N, Namgyal T, Sangay P, Al Quraan AM. Role of immune-pineal axis in neurodegenerative diseases, unraveling novel hybrid dark hormone therapies. Heliyon. 2019; 5(1):e01190. [DOI:10.1016/j.heliyon.2019.e01190] [PMID] [PMCID]
Jamshidi Fard AR, Rafipour H, Faraji F. [Effects of melatonin on visual functioning of patients with multiple sclerosis (Persian)]. Journal of Arak University of Medical Sciences. 2013; 16(5):8-18.
Wurtman R. Multiple sclerosis, melatonin and neurobehavioral diseases. Front Endocrinol. 2017; 8:280. [DOI:10.3389/fendo.2017.00280] [PMID] [PMCID]










































 , Gholamhassan Vaezi2
, Gholamhassan Vaezi2 
 , Ali Akbar Maleki-Rad3
, Ali Akbar Maleki-Rad3 
 , Fardin Faraji4
, Fardin Faraji4 
 , Vida Hojati2
, Vida Hojati2 

