







































BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijt.arakmu.ac.ir/article-1-888-en.html
Introduction
Nowadays, drug abuse is growing alarmingly in many societies. Narcotic drugs are usually prescribed for analgesia and euphoria. The most available substance for the treatment of mild depression, malaise and obesity are amphetamine and methamphetamine. However, the medical prescription of these drugs is currently limited. The term Ecstasy (XTC) refers to the chemical family of amphetamines and its derivatives. The first report of the synthesis of amphetamine was published in 1887 by Edeleano [1] in Germany and its stimulating properties were discovered almost 30 years later by Gordon Alles [2].
Amphetamine is a stimulant drug and belongs to the phenethylamine class of drugs, the structure of which is shown in Figure 1a.
The side effects of amphetamine are insomnia, fatigue and low appetite [3]. The main application of amphetamines is in the treatment of hyperactivity disorder. Amphetamine inhibits monoamine oxidase enzymes and prevents the re-absorption of catecholamines, thereby increasing the brain activity and reducing sleepiness. It has multiple isomers and derivatives, such as dexa-methamphetamine and methamphetamine with similar functions but different potencies. Methamphetamine (Figure 1b) exists in both right- and left-handed molecular forms, i.e., D-methamphetamine and L-methamphetamine [4].
D-methamphetamine has a strong stimulant effect on the brain and is chemically more effective than amphetamine [5]. Different methods for measuring amphetamines have been reported separately or simultaneously [6], including high performance liquid chromatography (HPLC) and gas chromatography (GC) [7-13].
One of the problems in the analysis of laboratory samples is the matrix effect, which directly affects the extraction recovery from different samples. In other words, the analysis of samples irrespective of the matrix is not possible. The complexity of the sample matrix, the inadequacy of the analytes concentration in the sample, and the non-conformity of the sample with the detector, cause problems with the analyses. Due to the reasons mentioned for separating the desired analytes from the sample matrix, using an appropriate preparation method is essential. The common purpose of all preparation methods is to eliminate potential interference with the measurement steps of samples, to increase the selectivity and sensitivity, and to provide a repeatable and robust method. Nowadays, the focus of these methods is to use the trace amount of sample, high selectivity in extraction, automation, and production based on low usage of organic solvents [14]. Due to the physical and chemical properties of the analytes and the type of biological tissue, various preparation methods have been introduced, of which the extraction process is the most productive one. This process is used to separate and pre-concentrate trace amounts of solids, liquids and gases.
Currently, several methods have been proposed to reduce the volume of the extraction solvent to a few microliters [15]. One of the methods for micro sample preparation is liquid phase micro-extraction. This is a sample preparation method with minimal solvent, reduced form of liquid-liquid extraction, in which only a few microlitres of the solvent is required for the condensation of analytes from various samples. This method has overcome many of the disadvantages of liquid-liquid extraction methods, i.e., using organic solvents, consuming less time and needing less specialized equipment. The advantages of this technique are high extraction speed, simplicity and low volume of organic solvent required [16]. In this study, syringe-to-syringe-dispersive liquid–phase micro-extraction (SS-DLPME) was successfully used to remove the dispersive solvent. The experimental approach was to optimize time and cost, and to increase the extraction efficiency [17].
Materials and Methods
Chemicals & Reagents: Amphetamines and methamphetamine were obtained with 99.9% purity from Sigma-Aldrich (Buchs, Switzerland). Acetonitrile (MeCN), methanol (MeOH) and 1-hexane were obtained from Merck (HPLC grade; Albany, NY, USA). The stock standard solutions of AMP and MAMP (1mg/mL) were prepared in pure methanol. Standard solutions (100ng/mL) of the analytes were prepared daily using stock standard solutions and methanol. Ultra-pure water from a Milli-Q water system (Millipore, Bedford, MA, USA) was used in all solutions.
Apparatus & Software: The HPLC system from Knauer (Berlin, Germany) was used throughout this study. It consisted of a 2850 PDA detector. ChromGate Version 3.3.1 was used to acquire data from the detector and process data, obtaining quantitative and qualitative results. A Nonpolar C18 stationary phase column (250×4 mm) with 5-nm particle size equipped with a pre-column was used for the separations process. The pH values were measured, using a Metrohm 780 pH-meter (Herisau, Switzerland). Experimental design was performed with Design Expert software version 7.
Sample Preparation: Syringe-to-syringe dispersive liquid phase micro-extraction based on solidified floating organic drops was first proposed by Asadi et al. [18] for simultaneous measurement of albendazole and triclabendazole in water, urine, honey and milk samples. In this technique, the use of two syringes connected to each other instead of dispersive solvent was proposed [19]. For this purpose, the needle of the first syringe is removed and that of the second syringe is shortened to 5 mm, inserted into the first syringe, and the joints are sealed with PVC adhesive. In this method, the extraction solvent, being less dense than water, is injected into the syringe #1, containing the sample solution, and then the syringes #1 and #2 are attached to each other. In this step, the extraction solvent is spread through the successive injections between the syringes in the aqueous phase and the solution becomes cloudy. The mixture is then transferred to the centrifuge tube to collect the organic solvent. Finally, by placing the sample in the ice bath, the organic phase is easily collected from the solution surface, and injected into the HPLC apparatus, using a micro-syringe.
Experimental Design: We considered the following factors in designing the experiments: interactions among the factors, and relationship between the dependent and independent variables, such that we could obtain the most useful information by performing the least number of experiments [20]. This method was first presented in the 1920s by an English scientist named Fisher, and the experimental design was introduced as a means of increasing data generation from each experiment [21]. The purpose of various experimental designs was to identify the factors influencing the process and to determine their optimal levels. The variables with the greatest impact on the outcomes were determined [22]. One of the most widely used and published experimental designs in analytical literature is response surface methodology [23].
Response Surface Methodology is used to model experiments that are influenced by multiple responses. This approach is a collection of mathematical models that determine the relationship between one or more responses versus multiple independent variables [24]. This method was introduced by Box-Behnken and Wilson in 1951 [25], and is still used as one of the experimental designs, which includes such methods as central composite, Box-Behnken design, etc.
Box-Behnken Design is a second-order multivariate technique based on a three-level fractional and factorial design [25]. This is a rotatable design with central and midpoints of the edges of the space of variables. In other words, there are no combinations created from the upper and lower levels. In this design, the number of experiments is equal to: N=2k (k-1)+C, where “k” is the number of main factors and “C” is the repetitions at the central point. In this design, the number of required runs is low, which is its main advantage. Therefore, it is an important alternative to avoid performing lengthy experiments. In this method, the equation commonly used to express the relationship between the factors and the responses is as follows:
Y=β0+∑βi Xi +∑βii Xi2+∑βij Xi Xj+ε
Results
The effective parameters on the micro-extraction process are the pH of the sample, the type and volume of extraction solvent, the amount of salt and shooting time that were obtained by screening, and the optimization methods.
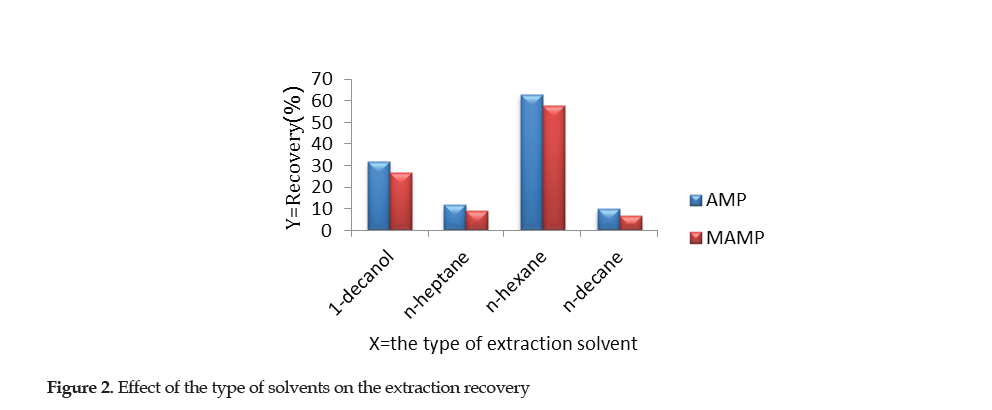
Selection of Extraction Solvents: In the SS-DLPME method, the appropriate solvent for extraction is of great importance in order to increase the efficiency. 1-decanol, n-hexane, n-heptane and n-decane were used as the solvents for the micro-extraction, the results of which are shown in Figure 2.
After using the syringe-to-syringe method, the results indicated that n-heptane and n-decane had weak colloidal sample solution. After centrifugation, the amount of the organic phase on the surface of the solution was insignificant. Thus, the two solvents exhibited low extraction efficiency. When 1-decanol was used, the cloudy solution after centrifugation indicated that the separation of two phases was not satisfactory. N-hexane had the best extraction efficiency compared to the other solvents and was; therefore, used as the choice extraction solvent in this study. The optimal volume of n-hexane required was determined through the subsequent experimental steps.
The Effect of Ionic Strength of the aqueous solution on the extraction efficiency was studied by changing the concentration of sodium chloride from 0 to 3 molar. Based on the results, with increasing sodium chloride concentration up to 2 molar, the absorption was constant, but concentrations higher than 2 molar increased the solubility of the extraction solvent in the aqueous phase, and collecting the extraction solvent on the surface was difficult, which reduced the absorption. Therefore, the subsequent experiments were carried out without adding salt.
Response Surface Methodology was used based on the Box-Behnken design to evaluate the effect of independent variables on the response function. The independent variables were the sample pH (X1), shooting time (X2) and extraction solvent volume (X3) (Table 1).
A total of 17 experiments were run, using 17 urine samples and based on Box-Behnken design. The response surfaces plots for the analytes of AMP and MAMP are shown in Figures 3 and 4.
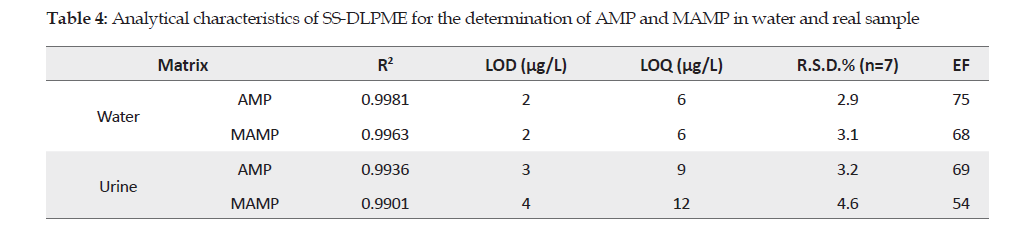
Figures of Merit: The analytical parameters of the proposed method, such as determination coefficient (R2), precision, Enrichment Factor (EF) and Limit Of Detection (LOD) were calculated under optimal conditions, the results of which are summarized in Table 4.
The linear range of calibration curve for AMP and MAMP was obtained at 5 to 100 μg/L with the determination coefficient of R2=99.8 and R2=99.6, respectively. To obtain the LOD, five analyses were performed under optimal conditions and were calculated by the equation:
LOD=3S/m, which was 2 μg/L for both AMP and MAMP
Analysis of analytes in real sample: The performance and applicability of SS-DLPME method for the extraction and determination of AMP and MAMP from the 17 urine samples were examined by spiking the standards directly into the urine samples. These samples were spiked with the standards AMP and MAMP at 25 and 50 μg/L. For each concentration, three replicates were performed, the results of which are presented in Table 4. The enrichment factor for the spiked real samples was in the range of 54-69.
Comparison of extraction methods: The parameters of the proposed method were compared with some of the methods published earlier (Table 5).
The analytical function of the developed method was comparable or better than those reported by other studies [3, 26, 27]. We observed a wide linear range, low detection limit, short analysis time, and high enrichment factor with low relative standard deviations. Instead of using solvents with higher densities than water, low-density organic solvents were selected. These solvents are generally less toxic and contain less organic materials (Table 5). Therefore, it may be suggested that the SS-DLPME technique is a sensitive, simple, rapid, highly reproducible method for the detection and measuring AMP and MAMP in biological samples.
Statistical analyses: To evaluate the method, analysis of variance (ANOVA) was used, the results of which are presented in Tables 2 and 3.
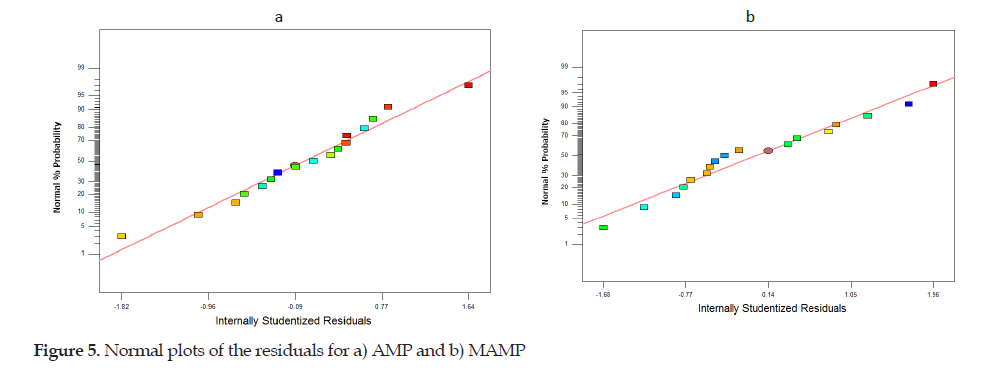
The two important parameters were P-value and lack of fit in the Tables. The P-value indicates the factors’ effect versus the AMP and MAMP extraction efficiency. Factors with P-values less than 0.05 had the greatest effect on the extraction, using the SS-DLPME methodology. According to the Tables, the P-value obtained for the lack of fit of AMP and MAMP indicated that the models were well suited for the responses. Also, the normal plots of residual in Figure 5a and 5b indicated the adequacy and accuracy of the models.
The determination of coefficients R2 (AMP: 0.97; MAMP: 0.98) and R2 adjusted (AMP: 0.94; MAMP: 0.95) demonstrated a good relationship between the experimental results and the models. This model was obtained according to the effective term of the extraction recovery for AMP and MAMP as follows:
Discussion
Considering the growing consumption of stimulant drugs, such as AMP and MAMP, among people especially the youth, the advantage of having a simple, sensitive, practical and efficient laboratory method for the evaluation of biological samples is of considerable significance. The advantages of detecting various chemicals in urine samples are as follows: a) the concentration of many drugs are as high as 10 fold in urine compared to those detected in the serum; b) lack of proteins in urine eliminates the chance of interaction or binding with the drugs; c) drugs remain in the urine usually longer than that in the serum, making it a readily accessible matrix to detect most drugs; and d) the fact that taking urine samples from individuals is non-invasive, adds to the advantages.
This study was conducted to extract the trace amount of AMP and MAMP in urine by a novel micro-extraction method, which is rapid, inexpensive, highly sensitive and efficient yet environmentally friendly. The proposed method provides a simple, non-invasive and easily available technique that is comparable to other popular methods, which are also sensitive for similar purposes. Our method increases the chance of individuals for participation in various research studies with a focus on detecting AMP and MAMP in their biological samples. The followings are the interpretation of the results and comparisons with the findings of similar published studies conducted earlier:
Extraction efficiency: In three previous studies, conducted between 2004 and 2010 based on existing methods, the extraction recovery of amphetamines in urine samples ranged from 0.17 to 22.7 µg/L with the LOD values of 0.02 to 8.2 µg/L and the extraction time of 20-30 minutes [3, 26, 27]. Our extraction recovery rates were 17.2 to 22.7 µg/L with the LOD being 0.8-8.2 µg/L while the extraction time taking only five minutes. These findings suggest that the proposed method is an efficient, sensitive and rapid approach. Also, the method is economically preferable over the older techniques while using small amounts of the solvent, making it an environmentally desirable approach. These advantages were achieved due to the application of rapid LC, MS and SPE technologies.
Response Surface Method: Prior to developing our approach, we used the response surface methodology, based on the Box-Behnken method [25]. This enabled us to evaluate the interactions among the variables, such as pH, shooting time, the solvent volume, and the response surface (Table 1). The statistical analyses of the assessments ultimately helped us bring greater improvements to the proposed method.
Other features: Overall, the proposed method is advantageous over the existing protocols for the detection of amphetamines in biological samples, owing to its features, such as simplicity, high sensitivity, speed of detection, low cost and being environmentally friendly. This method compares favorably well with other current approaches used for the detection of drugs with similar mechanisms of action to amphetamines. Lastly, the proposed method has the potential to be used by the offices of medical examiner and forensic medicine experts as a rapid, low cost and sensitive means of evaluating and tracking AMP and MAMP in human biological samples.
Recommendations for Future Studies: The authors recommend that future studies on amphetamines or similar drugs be conducted on larger sample sizes, typically 400 or greater. Also, the data would be more clinically informative if subjects’ homogeneity is strictly observed. Thus, we recommend that human subjects be voluntarily recruited and grouped into adults aged 20-60 years old, men versus women, and healthy subjects versus drug addicts. Prior to the study, it would clinically be very informative if the subjects are screened for the status of their liver and kidney health, and the duration and type of drug abused.
Conclusions
The novel SS-DLPME method proposed by this study provides a model for the extraction and measurement of AMP, MAMP and other drugs with similar mechanisms of action in human biological samples. Also, this technique may be used as an appropriate, rapid, efficient and sensitive method in forensic medicine to monitor the above drugs. This method is selective for extracting, pre-concentrating and determining the amphetamines levels in human urine samples. Further refinement of this method versus such variables as age, gender, liver and kidney health, and the duration and type of drug abused, awaits future, well designed studies in human subjects.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted consistent with the guidelines set by the Forensic Medicine Laboratories across Markazi Province in Iran.
Funding
This paper was extracted from the doctoral thesis of the first author at the Department of Chemistry, Faculty of Science, Islamic Azad University, Arak, Iran.
Author's contributions
Both authors contributed equally to the preparation of this manuscript.
Conflict of interest
The authors declared no conflict of interest.
References
1.Allen A, Cantrell TS. Synthetic reductions in clandestine amphetamine andmethamphetamine laboratories: A review. Forensic Sci Int. 1989; 42(3):183-99. [DOI:10.1016/0379-0738(89)90086-8]
2.Allen A, Kiser W. Methamphetamine from ephedrine: I. Chloroephedrines and aziridines. J Forensic Sci. 1987; 32(4):953-62. [DOI:10.1520/JFS12406J]
3.Xiong J, Chen J, He M, Hu B. Simultaneous quantification of amphetamines, caffeine and ketamine in urine by hollow fiber liquid phase micro-extraction combined with gas chromatography-flame ionization detector. Talanta. 2010; 82(3):969-75. [DOI:10.1016/j.talanta.2010.06.001] [PMID]
4.Philips SR. Amphetamine, p-hydroxyamphetamine and β-phenethylamine in mouse brain and urine after (−)‐and (+)‐deprenyl administration. J Pharm Pharmacol. 1981; 33(11):739-41. [DOI:10.1111/j.2042-7158.1981.tb13920.x] [PMID]
5.Cook JD, Schanberg SM. The effects of methamphetamine on behavior and on the uptake, release and metabolism of norepinephrine. Biochem Pharmacol. 1970; 19(Suppl 1):1162-79. [DOI:10.1016/0006-2952(70)90377-1]
6.Leardi R. Experimental design in chemistry: A tutorial. Anal Chim Acta. 2009; 652(1-2):161-72. [DOI:10.1016/j.aca.2009.06.015] [PMID]
7.Bruce R, Maynard W. Determination of amphetamine and related amines in blood by gas chromatography. Anal Chem. 1969; 41(7):977-9. [DOI:10.1021/ac60276a003] [PMID]
8.Chen L, Yu Y, Duan G. Wang X, Shen B, Xiang P. Simultaneous determination of selegiline, desmethylselegiline, R/S-methamphetamine, and R/S-amphetamine on dried urine spots by LC/MS/MS: Application to a pharmacokinetic study in urine. Front Chem. 2019; 7:248. [DOI:10.3389/fchem.2019.00248] [PMID] [PMCID]
9.Marquet P, Lacassie E, Battu C, Faubert H, Lachâtreab G. Simultaneous determination of amphetamine and its analogs in human whole blood by gas chromatography-mass spectrometry. J Chromatogr B: Biome Sci Applic. 1997; 700(1-2):77-82. [DOI:10.1016/S0378-4347(97)00318-6]
10.Nakahara Y, Takahashi K, Shimamine M, Takeda Y. Hair analysis for drug abuse: I. Determination of methamphetamine and amphetamine in hair by stable isotope dilution gas chromatography/mass spectrometry method. J Forensic Sci. 1991; 36(1):70-8. [DOI:10.1520/JFS13007J]
11.Nakashima K, Suetsugu K, Yoshida K, Akiyama S, Uzu S, Imai K. High performance liquid chromatography with chemi-luminescence detection of methamphetamine and its related compounds using 4-(N, N-dimethylaminosulphonyl)- 7-fluoro-2, 1, 3-benzoxadiazole. Biomed Chromatogr. 1992; 6(3):149-54. [DOI:10.1002/bmc.1130060311] [PMID]
12.Skender L, Karačić V, Brčić I, Bagarićb A. Quantitative determination of amphetamines, cocaine, and opiates in human hair by gas chromatography/mass spectrometry. Forensic Sci Int. 2002; 125(2-3):120-6. [DOI:10.1016/S0379-0738(01)00630-2]
13.Woźniak MK, Wiergowski M, Aszyk J, Kubica P, Namieśnik J, Biziuk M. Application of gas chromatography-tandem mass spectrometry for the determination of amphetamine-type stimulants in blood and urine. J Pharm Biomed Anal. 2018; 148:58-64. [DOI:10.1016/j.jpba.2017.09.020] [PMID]
14.Chiang JS, Huang SD. Simultaneous derivatization and extraction of amphetamine and methylenedioxyamphetamine in urine with headspace liquid-phase micro-extraction followed by gas chromatography-mass spectrometry. J Chromatogr A. 2008; 1185(1):19-22. [DOI:10.1016/j.chroma.2008.01.038] [PMID]
15.Pena-Pereira F, Lavilla I, Bendicho C. Miniaturized preconcentration methods based on liquid-liquid extraction and their application in inorganic ultratrace analysis and speciation: A review. Spectrochimica Acta Part B: Atomic Spectroscopy. 2009; 64(1):1-15. [DOI:10.1016/j.sab.2008.10.042]
16.Rezaee M, Yamini Y, Faraji M. Evolution of dispersive liquid-liquid micro-extraction method. J Chromatogr A. 2010; 1217(16):2342-57. [DOI:10.1016/j.chroma.2009.11.088] [PMID]
17.Montgomery DC. Design and analysis of experiments. Toronto: John Wiley & Sons; 2017. https://books.google.com/books?id=Py7bDgAAQBAJ&dq=Montgomery+DC.+Design+and+analysis+of+experiments.&source=gbs_navlinks_s
18.Asadi M, Dadfarnia S, Haji Shabani AM. Simultaneous extraction and determination of albendazole and triclabendazole by a novel syringe to syringe dispersive liquid phase microextraction-solidified floating organic drop combined with high performance liquid chromatography. Anal Chim Acta. 2016; 932:22-8. [DOI:10.1016/j.aca.2016.05.014] [PMID]
19.Koosha E, Ramezani M, Niazi A. Syringe-to-syringe-dispersive liquid-phase micrextraction combined with flame atomic absorption spectrometry for pre-concentration and determination of cobalt with the aid of experimental design. Int J Environ Anal Chem. 2018; 98(6):506-19. [DOI:10.1080/03067319.2018.1480764]
20.Ebrahimi H, Leardi R, Jalali, M. Experimental design in analytical chemistry - Part I: theory. J AOAC Int. 2014; 97(1):3-11. [DOI:10.5740/jaoacint.SGEEbrahimi1] [PMID]
21.Hinkelmann K, Kempthorne O. Design and analysis of experiments: Advanced experimental design. New Jersey: Wiley; 2005. [DOI:10.1002/0471709948]
22.Dejaegher B, Vander Heyden Y. The use of experimental design in separation science. Acta Chromatogr. 2009; 21(2):161-201. [DOI:10.1556/AChrom.21.2009.2.1]
23.Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008; 76(5):965-77. [DOI:10.1016/j.talanta.2008.05.019] [PMID]
24.Hanrahan G, Lu K. Application of factorial and response surface methodology in modern experimental design and optimization. Crit Rev Anal Chem. 2006; 36(3-4):141-51. [DOI:10.1080/10408340600969478]
25.Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandão GD, et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal Chim Acta. 2007; 597(1):179-86. [DOI:10.1016/j.aca.2007.07.011] [PMID]
26.Cháfer-Pericás C, Campíns-Falcó P, Herráez-Hernández R. Application of solid-phase micro-extraction combined with derivatization to the determination of amphetamines by liquid chromatography. Anal Biochem. 2004; 333(2):328-35. [DOI:10.1016/j.ab.2004.05.056] [PMID]
27.Chia KJ, Huang SD. Simultaneous derivatization and extraction of amphetamine-like drugs in urine with headspace solid-phase micro-extraction followed by gas chromatography-mass spectrometry. Anal Chim Acta. 2005; 539(1-2):49-54. [DOI:10.1016/j.aca.2005.03.018]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |















.png)
