Introduction
Cisplatin is a chemotherapy agent used to treat various types of cancers originated from testes, ovarian, head, neck, and lungs [
1]. However, its side effects, including neurotoxicity, ototoxicity, and nephrotoxicity, limit cisplatin’s application as an anti-cancer agent [
2]. These side effects have been linked to the generation of Reactive Oxygen Species (ROS) by this drug [
3]. Cisplatin’s mechanism of action is to form crosslinks within Deoxyribonucleic Acid (DNA) strands. This could result in genotoxicity, which further complicates the treatment [
4,
5]. Studies have reported that cisplatin interacts with DNA, Ribonucleic Acid (RNA), and various proteins leading to its cytotoxicity [
6-
9], which is associated with oxidative stress, cellular apoptosis, inhibition of DNA synthesis, cell cycle modulation, and DNA-adduct formation [
8].
Antioxidants could be used to protect against the generation of ROS; hence, inhibiting the oxidizing process and preventing further cellular damage [
10]. Therefore, using an antioxidant agent to minimize the production of ROS by cisplatin may bring greater success to anticancer therapy. Studies have suggested that specific components found in certain plants have antioxidant activities. Among those are flavonoids, including taxifolin and catechin, that have been linked to rich antioxidant activity [
11-
13]. These plants’ constituents demonstrate their antioxidant potentials by scavenging free radicals. Also, these components are able to chelate trace elements and inhibit enzymes, both of which are capable of generating free radicals [
14]. Also, terpenes have been considered as antioxidant sources. For instance, germacrene D is a sesquiterpene with an extra cyclic methyl moiety, which is believed to be involved in free radical scavenging activities [
15].
Previous studies have identified high antioxidant activity in several Pinaceae members due to their rich polyphenolic profiles [
16,
17]. Pinus eldarica Medw. (Pinaceae) also known as “Tehran pine” is endemic to many regions in Iran. Its bark extract has been shown to be a source of polyphenolic compounds, such as catechin and taxifolin [
18]. Also, the volatile oil from P. eldarica is a rich source of multiple terpenes, including germacrene D and β-caryophyllene [
19]. More recent studies have indicated that the polyphenolic compounds may contribute to antioxidant activity through different mechanisms, such as hydrogen donation and free radicals scavenging [
10]. The structural forms of terpenes have also provided these compounds with antioxidant property.
Due to the high content of polyphenolic and terpenes found in the bark and needle of P. eldarica, we aimed to investigate the cytoprotective effects of its products against cisplatin-induced cytotoxicity in Hepatoma G2 (HepG2) cell line.
Materials and Methods
Plant Collection: The needles and bark of P. eldarica were collected in September 2019 from Isfahan, Iran supervised and approved by Isfahan University of Medical Sciences. The collected samples were authenticated by the Department of Pharmacognosy, School of Pharmacy, Isfahan University of Medical Sciences.
Volatile oil preparation: A total of 3750 g leaves was collected. Leaves were cut into small pieces (an average of 2 cm each). Using the hydrodistillation Clevenger method, 100 g of the dried and powdered needles were hydrodistilled in 1L water for 4 h. The volatile oil thus obtained was stored in a sealed vial at 4°C until further experiments [
20].
Extract preparation: Approximately 20 g of the collected bark was powdered. Using maceration method, the powder was mixed in water and methanol at the ratio of 30:70. Next, the mixture was shaken for 30 minutes and kept in dark for 24 h. The mixture was shaken for 30 minutes, and the extract was filtered and freeze-dried. The obtained Bark Hydroalcoholic Extract of Pinus eldarica (BHAEPE) was kept at 4°C to ensure that the main components would remain stable until further experiments [
21].
Volatile oil analysis: The Needle Volatile Oil from Pinus eldarica (NVOPE) was analyzed using Agilent technologies 7890A Gas Chromatograph-Mass Spectrometer (GC-MS). This device was equipped with a 5975C mass detector. The following procedure was followed to analyze 0.1μl of NVOPE sample: split ratio of 1:10, helium carrier gas flow rate was set at 1.9ml/min with 17.7lbf/in2 as the pressure, injection site’s temperature was set at 280°C. The column’s temperature was set to rise 4°C per minute from the initial 60°C until it reached 280°C. HP-5 MS capillary column was used (30m x 0.25 mm; film thickness 0.25 μm). Mass spectra’s ionized potential was 70eV, the ionizing source temperature and ionization current were 230°C and 750 μA, respectively [
20]. A computer matched the result of the analysis with its database (NIST and Wiley 275L). To calculate the Kovats index, the retention time of n-alkanes (C9-C20) was determined using the above-mentioned technique. The components of NVOPE were identified using their Kovats index and compared with the standard [
22].
Extract analysis: Folin-Ciocalteu reagent was used to analyze the Total Phenolic Content (TPC) of BHAEPE. This test was done using Blainski’s method with some modifications [
23], with gallic acid as the standard. BHAEPE and gallic acid samples were first dissolved in 10 ml of ethanol. Water was then added to a volume of 100 ml for each sample. Next, 20 μl of the blank sample (ethanol mixed with water) along with gallic acid and BHAEPE samples were separately mixed with 1.58ml water. Then, 100μl of F-C reagent was added to the mixture. After 8 minutes, 300μl sodium bicarbonate was added to the samples. The samples were kept at room temperature in the dark for 3 h. The absorbance of the samples was read at 765 nm. The result was presented as mg of gallic acid equivalents/g (mg GAE/g) to BHAEPE ’s weight using the following formula:
(TPC=c.V/m)
In this formula, TPC shows the total phenolic content of the sample that is obtained by multiplying BHAEPE ’s concentration obtained from gallic acid calibration curve (c) by the volume of BHAEPE used (V) divided by the BHAEPE mass (m).
Cell culture: HepG2 cell line was acquired from Rooyan Institute (Isfahan, Iran). Cells were cultured in Dulbecco’s Modified Eagle’s medium F12 and non-essential amino acids, 10% fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells were grown in a T25 flask. The conditions inside the incubator (Memert, D91126, Germany) were set at 37°C, <5% carbon dioxide, and 95% humidity. In order to detach cells from the flask, first cells were rinsed with sterile phosphate buffer saline. Then, 1 ml of trypsin was added. The flask was kept inside the incubator for 3 minutes. Then, 2 ml cell culture medium was added to the flask. Detached cells were centrifuged (Hermle, Z206A, Germany) at 1800 rpm for 5 minutes and the cells were isolated for further experiments.
DPPH test: A solution 4 μg/ml of 2,2-Diphenyl-1-picrylhydrazyl (DDPH) was made up at 4 μg/ml by dissolving 0.2 g DPPH powder in 50 ml of methanol. A 96-well plate was used to perform this test. Gallic acid (100μl) as the positive control and the desired concentrations of BHAEPE and NVOPE were mixed with 100 μl of DPPH solution. In order the samples with DPPH agent, the culture plate was left in the dark at room temperature for 30 minutes. The samples’ absorbance was read on an ELISA Reader (BioTek Instruments, Inc, 140213C, USA) at 517 nm. The DPPH solution was used as the negative control and the methanol-water mixture was marked as the blank. Using the following equation, the antioxidant activity of the samples was calculated:
.jpg)
Cell viability test: To determine the IC50 of cisplatin and investigate the safety and protective effects of BHAEPE and NVOPE on HepG2 cell line, we performed 3-(4,5-dimethyl-thiazolyl-2)-2,5-diphenyl tetrazolium bromide (MTT) assay with minor modifications [
24]. After detaching the cells from the culture flask, a total of 5×104 cells in the suspension were counted using Neubauer chamber counting grid. The cells were then incubated in a 96-well plate. Next, various concentrations of BHAEPE, NVOPE, and cisplatin were added to the predetermined wells and the plate was incubated at 37°C for 48 h. The medium was then removed and MTT reagent was added to each well and incubated for 3-4 h. DMSO was then added to each well and the absorbance was read at 570 nm using an ELISA Reader. The same media for cells was used as the blank. Cells that received nothing other than cell culture media were assigned as the negative control. To investigate the protective effects of BHAEPE and NVOPE against cisplatin and to keep them from cross interaction, cells were first incubated for 3 h with BHAEPE or NVOPE at predetermined concentrations in assigned wells and then cisplatin was added to the wells.
Statistical analysis: The data are presented as Mean±SD. The statistical significance of the data was tested by one-way analysis of variance with Tukey’s multiple comparison post hoc test using GraphPad Prism 8 (Graph Pad Software, Inc., CA, USA). Statistically significance was set at P<0.05.
Results
Analyses of volatile oil & extracts: The P. eldarica’s needles yielded 1.5 ml volatile oil. As shown in
Figure 1, the main components of the oil were germacrene D (35%), β-Caryophyllene (18%) and δ-Cadinene (4%). The P. eldarica bark yielded 22.54 g of extract. The Folin-Ciocalteu test revealed that 1g of BHAEPE had a total phenolic content equivalent to 371±6 mg of gallic acid.
.jpg)
DPPH results: Greater antioxidant activity was observed for BHAEPE as its concentration was increased. A direct association between the antioxidant activity and BHAEPE concentration was established at concentrations below 20 μg/ml. At concentrations above 20 μg/ml, BHAEPE did not have improved antioxidant activity. There was no correlation between NVOPE’s antioxidant activity and samples’ concentrations. Moreover, NVOPE produced the maximum antioxidant activity at 300 μg/ml dose.
Effects of cisplatin, BHAEPE & NVOPE: Varying concentrations of cisplatin were incubated with the cells to determine the IC50 of this agent. Cisplatin at 15μM was selected as the optimal IC50 concentration over 48-hour incubation to investigate the protective effects of BHAEPE and NVOPE. Neither BHAEPE nor NVOPE demonstrated cytotoxic effects at any of the concentrations used. Cell growth (P<0.05) was observed on high BHAEPE concentrations (50, 75, or 100 μg/ml) compared to the negative control (
Figure 2).
.jpg)
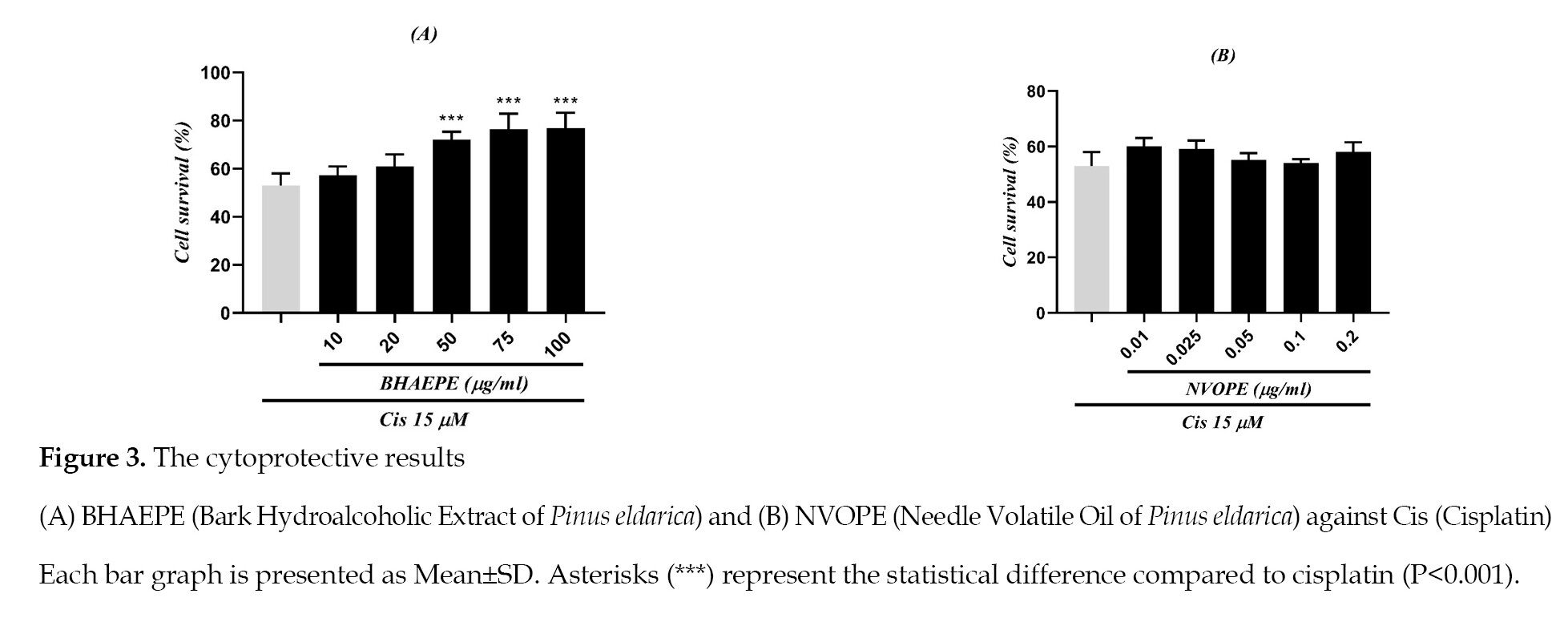
None of the used concentrations of NVOPE and the low concentrations of BHAEPE (10 and 20 μg/ml) provided protection for cells against cisplatin (P<0.001). However, BHAEPE showed cytoprotective effects against cisplatin at high concentrations (50, 75. and 100 μg/ml) (
Figure 3).
Discussion
This study investigated the cytoprotective effects of the bark and needles oil of P. eldarica against cisplatin-induced cytotoxicity in hepatoma G2 (HepG2) cell line. Our results show that the main component of NVOPE was germacrene D and that none of NVOPE’s concentrations exerted cytoprotective effects against cisplatin. By comparing BHAEPE and NVOPE, it was evident that BHAEPE provided higher antioxidant capacity as compared to NVOPE. The maximum antioxidant capacities of BHAEPE and NVOPE were 20 μg/ml dose and former at 300 μg/ml, respectively. Also, based on Folin-ciocalteu test, BHAEPE was considered as a source of polyphenolic compounds. Previous reports have indicated that other members of Pinaceae family are also rich in polyphenols [
25,
26]. A study has suggested that the total phenolic content of 100g of butanolic bark extract from both Pinus roxburghii and Pinus wallichiana is equivalent to 893±0.15 mg, and 591±2.31 mg of gallic acid, respectively [
26]. Moreover, low doses of BHAEPE did not protect the cells against cisplatin. However, BHAEPE provided cytoprotective effects at higher concentrations. Also, neither BHAEPE nor NVOPE provided cytotoxic effects in HepG2 cells against cisplatin (
Figure 2).
The mitogenic and anti-aging effects of P. maritime bark extract (Pycnogenol®) have previously been reported, suggesting that the effects were due to the radical scavenging activity of polyphenolic contents; i.e., mainly proanthocyanidins in Pycnogenol® [
27]. Since P. eldarica and P. maritime are both members of Pinaceae family and have similar phenolic profiles [
18], the mitogenic effects seen at high concentrations of BHAEPE may be associated with the high amounts of phenolic compounds. Although a study has reported the cytotoxic activity of the volatile oil from Pinus heldreichii, Pinus peuce, and Pinus mugo needles in HeLa, CaCo-2 and MCF-7 cell lines [
28], our results suggest that NVOPE did not provide cytotoxic effects in HepG2 cells. However, NVOPE at 0.05 μg/ml produced lower cell viability than other concentrations, even though the difference was statistically insignificant (
Figure 2).
There have been reports of different volatile oil yields and constituents depending on the seasons when samples were collected [
29,
30]. Furthermore, other factors such as environmental temperature, and age of the tree could affect the volatile oil, suggestive of fluctuations in the yields of volatile oil and constituents by various reports [
29,
31]. Our data from a research under publication suggest that BHAEPE has mitogenic effects at higher doses on HUVECs cell line. Moreover, NVOPE and BHAEPE have had cytoprotective effects on these cells against cisplatin cytotoxicity [
32]. Since HUVECs is a normal cell line, but HepG2 is a cancerous cell line, there might be unknown mechanisms to explain how the antioxidant capacity differs for protecting normal versus cancer cell lines. The finding is of interest since the objective in chemotherapy is to kill cancer cells without harming the normal cells. Cisplatin acts as an anti-tumor drug via multiple mechanisms, one of which being through oxidative stress [
8].
Finally, different mechanisms of cytotoxicity other than oxidative stress might exist through which cisplatin interacts with normal versus malignant cells. It could be speculated that the antioxidant capacity of BHAEPE and NVOPE were not capable of interacting with cisplatin’s other cytotoxic mechanisms; therefore, they did not protect the cells at certain concentrations. In this study only certain concentrations of both BHAEPE and NVOPE were used to elaborate their cytotoxicity/protectivity against cisplatin in HepG2 cells. Nevertheless, other cytotoxicity methods could be used to investigate the potential of the volatile oil and bark extract of P. eldarica against cisplatin in this cell line. Further investigations are required to uncover the unclear mechanisms of radical scavenging effects of these compounds in different cell lines.
Conclusions
Our experimental findings suggest that neither BHAEPE nor NVOPE caused any cytotoxicity in HepG2 cells. Hence, these compounds may be considered safe with the cell line used in this study. Moreover, neither NVOPE or low doses of BHAEPE could not protect cells against cisplatin-induced cytotoxicity, but BHAEPE at high doses provided promising cytoprotective effects. The antioxidant properties of P. eldarica might have differing effects in protecting normal cells versus malignant cells.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee, Isfahan University of Medical Sciences, Isfahan, Iran (Ethics Code: IR.MUI.RESEARCH.REC.1398.463).
Funding
This article was extracted from a PharmD dissertation by Amin Sharifan and was funded by Isfahan University of Medical Sciences (Grant No.: 398609).
Author's contributions
All authors equally contributed to the experiments and preparation of the drafts of this manuscript.
Conflict of interest
The authors declare no conflicts of interest in conducting this research.
Acknowledgments
We wish to thank the management and staff of Isfahan University of Medical Sciences for supporting this study.
References
- Al-Eitan LN, Alzoubi KH, Al-Smadi LI, Khabour OF. Vitamin E protects against cisplatin-induced genotoxicity in human lymphocytes. Toxicol In Vitro. 2020; 62:104672. [DOI:10.1016/j.tiv.2019.104672] [PMID]
- Pasetto LM, D’Andrea MR, Brandes AA, Rossi E, Monfardini S. The development of platinum compounds and their possible combination. Crit Rev Oncol Hematol. 2006; 60(1):59-75. [DOI:10.1016/j.critrevonc.2006.02.003] [PMID]
- Oun R , Moussa YE , Wheate NJ . The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018; 47(19):6645-53. [DOI:10.1039/C8DT90088D] [PMID]
- Huang H, Zhu L, Reid BR, Drobny GP, Hopkins PB. Solution structure of a cisplatin-induced DNA interstrand cross-link. Science. 1995; 270(5243):1842-5. [DOI:10.1126/science.270.5243.1842] [PMID]
- Muggia FM, Bonetti A, Hoeschele JD, Rozencweig M, Howell SB. Platinum antitumor complexes: 50 years since Barnett Rosenberg’s discovery. J Clin Oncol. 2015; 33(35):4219-26. [DOI:10.1200/JCO.2015.60.7481] [PMID]
- Onoa GB, Cervantes G, Moreno V, Prieto MJ. Study of the interaction of DNA with cisplatin and other Pd(II) and Pt(II) complexes by atomic force microscopy. Nucleic Acids Res. 1998; 26(6):1473-80. [DOI:10.1093/nar/26.6.1473] [PMID] [PMCID]
- Melnikov SV, Söll D, Steitz TA, Polikanov YS. Insights into RNA binding by the anticancer drug cisplatin from the crystal structure of cisplatin-Modified ribosome. Nucleic Acids Res. 2016; 44(10):4978-87. [DOI:10.1093/nar/gkw246] [PMID] [PMCID]
- Kumar S, Tchounwou PB. Molecular mechanisms of cisplatin cytotoxicity in acute promyelocytic leukemia cells. Oncotarget. 2015; 6(38):40734-46. [DOI:10.18632/oncotarget.5754] [PMID] [PMCID]
- Nazari A, Mirian M, Aghaei M, Aliomrani M. 4-Hydroxyhalcone effects on cisplatin-induced genotoxicity model.Toxicol Res (Camb). 2021; 10(1):11-7. [DOI:10.1093/toxres/tfaa091] [PMID]
- Zhu M, Huang Y, Wang Y, Shi T, Zhang L, Chen Y, et al. Comparison of (poly) phenolic compounds and antioxidant properties of pomace extracts from kiwi and grape juice. Food Chem. 2019; 271:425-32. [DOI:10.1016/j.foodchem.2018.07.151] [PMID]
- Firuzi O, Mladenka P, Petrucci R, Marrosu G, Saso L. Hypochlorite scavenging activity of flavonoids. J Pharm Pharmacol. 2004; 56:801-7. [DOI:10.1211/0022357023556] [PMID]
- Rao ChV, Vijayakumar M. Protective effect of (+)‐catechin against gastric mucosal injury induced by ischaemia‐Reperfusion in rats. J Pharm Pharmacol. 2007; 59(8):1103-7. [DOI:10.1211/jpp.59.8.0007] [PMID]
- Aliomrani M, Sepand MR, Mirzaei HR, Kazemi AR, Nekonam S, Sabzevari O. Effects of phloretin on oxidative and inflammatory reaction in rat model of cecal ligation and puncture induced sepsis. Daru. 2016; 24(1):15. [DOI:10.1186/s40199-016-0154-9] [PMID] [PMCID]
- Majumdar S, Srirangam R. Potential of the bioflavonoids in the prevention/treatment of ocular disorders. J Pharm Pharmacol. 2010; 62(8):951-65. [DOI:10.1211/jpp.62.08.0001] [PMID] [PMCID]
- Casiglia S, Bruno M, Bramucci M, Quassinti L, Lupidi G, Fiorini D, et al. Kundmannia sicula (L.) DC: A rich source of germacrene D. J Essent oil Res. 2017; 29(6):437-42. [DOI:10.1080/10412905.2017.1338625]
- Ma CH, Wang Q, Liu M. Evaluation of antioxidant activity of pine polyphenols from three Pinaceae species in vitro. Southwest China J Agric Sci. 2016; 29(5):1063-7. https://www.cabdirect.org/cabdirect/abstract/20173004192
- Apetrei CL, Tuchilus C, Aprotosoaie AC, Oprea A, Malterud KE, Miron A. Chemical, antioxidant and antimicrobial investigations of Pinus cembra L. Bark and needles. Molecules. 2011; 16(9):7773-88. [DOI:10.3390/molecules16097773] [PMID] [PMCID]
- Iravani S, Zolfaghari B. Phytochemical analysis of Pinus eldarica bark. Res Pharm Sci. 2014; 9(4):243-50. [PMID] [PMCID]
- Afsharypuor S, San’aty F. Essential oil constituents of leaves and fruits of Pinus eldarica Medw. J Essent oil Res. 2005; 17(3):327-8. [DOI:10.1080/10412905.2005.9698920]
- Matsuo AL, Figueiredo CR, Arruda DC, Pereira FV, Scutti JA, Massaoka MH, et al. α--Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem Biophys Res Commun. 2011; 411(2):449-54. [DOI:10.1016/j.bbrc.2011.06.176] [PMID]
- Minaiyan M, Ghannadi A, Etemad M, Mahzouni P. A study of the effects of Cydonia oblonga Miller (Quince) on TNBS-induced ulcerative colitis in rats. Res Pharm Sci. 2012; 7(2):10310. [PMID] [PMCID]
- Babushok VI, Linstrom PJ, Zenkevich IG. Retention indices for frequently reported compounds of plant essential oils. J Phys Chem Ref Data. 2011; 40(4):043101. [DOI:10.1063/1.3653552]
- Blainski A, Lopes GC, de Mello JC. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013; 18(6):6852-65. [DOI:10.3390/molecules18066852] [PMID] [PMCID]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2):55-63. [DOI:10.1016/0022-1759(83)90303-4]
- Metsämuuronen S, Sirén H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem Rev. 2019; 18(3):623-64. [DOI:10.1007/s11101-019-09630-2]
- Maimoona A, Naeem I, Saddiqe Z, Ali N, Ahmed G, Shah I. Analysis of total flavonoids and phenolics in different fractions of bark and needle extracts of Pinus roxburghii and Pinus wallichiana. J Med Plants Res. 2011; 5(13):2724-8. https://academicjournals.org/journal/JMPR/article-abstract/278774016776
- Liu FJ, Zhang YX, Lau BH. Pycnogenol enhances immune and haemopoietic functions in senescence-accelerated mice. Cell Mol Life Sci. 1998; 54(10):1168-72. [DOI:10.1007/s000180050245] [PMID]
- Basholli-Salihu M, Schuster R, Hajdari A, Mulla D, Viernstein H, Mustafa B, et al. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm Biol. 2017; 55(1):1553-60. [DOI:10.1080/13880209.2017.1309555] [PMID] [PMCID]
- Sharma S, Adams JP, Sakul R, Martin EM, Ricke SC, Gibson KE, et al. Loblolly pine (Pinus taeda L.) essential oil yields affected by environmental and physiological changes. J Sustain For. 2016; 35(6):417-30. [DOI:10.1080/10549811.2016.1192046]
- Llusià J, Peñuelas J, Alessio GA, Estiarte M. Seasonal contrasting changes of foliar concentrations of terpenes and other volatile organic compound in four dominant species of a Mediterranean shrubland submitted to a field experimental drought and warming. Physiol Plant. 2006; 127(4):632-49. [DOI:10.1111/j.1399-3054.2006.00693.x]
- Sporek M. Volatile Oil Content of Scots Pine Needles (Pinus sylvestris L.). Chemistry-Didactics-Ecology-Metrology. 2016; 21:141-7. [DOI:10.1515/cdem-2016-0013]
- Sharifan A, Etebari M, Zolfaghari B, Aliomrani M. Investigating the effects of bark extract and volatile oil of Pinus eldarica against cisplatin-induced genotoxicity on HUVECs cell line. Toxicol Res. 2021; 10(2):223-31. [DOI:10.1093/toxres/tfab006] [PMID] [PMCID]








































.jpg)
.jpg)
.jpg)

