Introduction
Cadmium is a heavy metal found in soil and plants, and used in industries as an anti-corrosion agent, stabilizer in PVC products, paint products, battery manufacturing, and in nuclear power plants as a neutron absorber [
1]. This metal is toxic and hazardous to human and animal health, and no physiological properties and functions are present to neutralize it in the human body [
1,
2]. Cadmium poisoning is a global health concern reported in many parts of the world, causing numerous deaths each year [
3]. The cadmium toxicity occurs more frequently in people who are exposed to this metal during their careers, e.g. painting, soldering, welding, and burying industrial wastes. Therefore, cadmium poisoning is considered an occupational hazard putting at least 512,000 workers in the United States at health risk annually [
4]. The hazardous effects of cadmium on the body organs are extensive, such as the liver, kidneys, bones, hematopoietic and respiratory disorders [
5-
7].
The kidneys are one of the main target organs for cadmium accumulation and toxicity, and the resultant damages mainly include the destruction of glomeruli and the proximal tubules. The cadmium accumulation in the epithelial cells of proximal tubules results in impaired reabsorption of urine components, causing subsequent polyuria, urinary excretion of low molecular weight proteins, bicarbonaturia, glucosuria, and phosphaturia. Kidneys damage caused by acute and chronic exposure to this heavy metal has been reported in epidemiological and experimental studies [
8,
9]. So far, several mechanisms have been proposed for the renal toxicity due to acute and chronic cadmium poisoning, including oxidative stress and attenuation of antioxidant defenses in the kidneys [
10]. Although the use of cadmium in industries is limited to minimize the pollution and poisoning, there is still a long way to go for the complete control and prevention. Thus, cadmium poisoning remains a threat to the public health, especially in developing countries. Currently, research is still ongoing to find appropriate methods and agents to combat the toxic effects of cadmium.
Montelukast is an antagonist of leukotriene CysLT1 receptors, with anti-inflammatory and antioxidant properties. It is currently used as an effective drug in the prevention and treatment of asthma and allergic rhinitis. Experimental studies have demonstrated the anti-inflammatory and antioxidant effects of montelukast in various animal organs, including the lungs and kidneys of rats with sepsis [
11-
16]. Also, an earlier study [
13] has suggested that Montelukast is able to protect the rat kidneys against Ecoli-induced oxidative damages by inhibiting neutrophil infiltration, enhancing oxidant-antioxidant balance, regulating the production of inflammatory mediators, while being useful in the treatment of pyelonephritis in rats [
13]. The protective effects of Montelukast against oxidative stress models caused by ischemic-reperfusion damage to the small intestine, liver, and kidneys have also been demonstrated in rat models [
14-
16].
The efficacy of Montelukast in preventing the cadmium-induced kidney damages at the cellular level has previously been shown [
17]. Thus, this study was planned to investigate the specific role of Montelukast antioxidant properties that result in the renal tissue protection, in comparison to vitamin E as a potent antioxidant, both in vivo and in vitro.
Materials and Methods
This study was conducted in two sections, a) an in vivo section, using Wistar rats, and b) an in vitro section, using a human embryonic kidney cell line, i.e., HEK293.
In vivo section
Laboratory animals: In this section, 42 male rats of Wistar breed, weighing 200-250 grams were randomly divided into seven equal groups of six rats each. They were kept under an optimal laboratory conditions of temperature at 22±2°C and lighting cycle of 12 hours light and dark, with the relative humidity set at 40%-60%.
Experimental design
Group 1: (control) received 0.5 mL of normal saline Intraperitoneally (IP) for 5 days.
Group 2: (cadmium control) received cadmium chloride at 3 mg/kg/day IP, for 5 days [
18].
Group 3: (Montelukast control) received this drug at 20 mg/kg/day orally (PO) for 5 days [
19,
20].
Group 4: (Montelukast treatment) received cadmium chloride at 3 mg/kg/day IP and Montelukast concurrently at 20 mg/kg/day PO (one hour before receiving cadmium chloride doses) for 5 days.
Group 5: (Montelukast pre-treatment) received Montelukast at 20 mg/kg/day PO for 5 days, followed an hour later by cadmium chloride at 3 mg/kg/day IP for 5 days.
Group 6: (Vitamin E control) received vitamin E Intravenously (IV) at 150 mg/kg/day, for 5 days [
21].
Group 7: (vitamin E treatment) concurrently received both cadmium chloride at a dose of 3 mg/kg/day and vitamin E at 150 mg/kg/day, IP for 5 days.
Collection of blood & tissue samples: Blood sampling was performed one day after the last treatment in each group. For this purpose, after anesthesia with ketamine and xylazine (10 and 80 g/kg, IP, respectively), a blood sample was withdrawn from each rat in a plain test tube by cardiac puncture and centrifuged at 3000 rpm for 10 min. The serum samples were separated and stored at -70°C until the subsequent oxidant-antioxidant and biochemical tests. After euthanasia, the rats’ left kidneys were removed and used to prepare tissue extracts.
Preparation of tissue extracts: To prepare the extracts from the kidney tissue, each sample was homogenized in Phosphate Buffered Saline (PBS) at pH of 7.4, using a homogenizer, at 3000 rpm for one minute. The homogenized samples were centrifuged for 20 minutes at 10,000 rpm at 4°C. Finally, the supernatants were transferred to labeled microtubes and stored at -70°C until the subsequent tests were performed.
Serum biochemical analyses: The serum markers relevant to renal function, including urea, creatinine, total protein, albumin, calcium, and phosphorus were measured by a calorimetric method, using a biochemical autoanalyzer (BT1500, Biotecnica, Italy) and appropriate commercial kits (Pars Azmoun, Iran). The serum sodium and potassium levels were measured by flame photometry (410 Clinical Flame Photometer, Sherwood Scientific, UK).
Measurement of oxidant & antioxidant indices: Measurement of Glutathione Peroxidase (GPX), Super Oxide Dismutase (SOD), and Total Antioxidant Capacity (TAC) in the tissue and serum samples were performed, using Randox commercial kits (UK). Also, the concentrations of Malondialdehyde (MDA) and Nitric Oxide (NO) were determined spectrophotometrically [
22,
23].
In vitro study
Cell line: The HEK293 cell line was purchased from Tehran Pasteur Institute Cell Bank. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and 1% Pen-Strep, and incubated at 37°C, 95% humidity and 5% CO2.
In vitro groups
Group 1 (control): no treatment performed on the cells.
Group 2: The cells were treated with cadmium chloride at IC50 concentration (120 μM) and incubated for 24 h [
17].
Group 3: The cells were treated with a maximum concentration of Montelukast (100 μM) without any toxic effects on the cells, and incubated for 24 h [
17].
Group 4: The cells were simultaneously treated with both CdCl2 and Montelukast at IC50 and therapeutic concentrations, respectively, and incubated for 24 h.
Group 5: The cells were initially treated with Montelukast at therapeutic concentration. After 24 h of incubation, CdCl2 and Montelukast were simultaneously added to the medium at IC50 and therapeutic concentrations, respectively, and incubated for 24 h.
Group 6: The cells were treated with a therapeutic concentration of vitamin E (100 μM) and incubated for 24 h [
17].
Group 7: The cells were simultaneously treated with both CdCl2 and vitamin E at IC50 and therapeutic concentrations, respectively, and incubated for 24 h.
The determination of IC50 for CdCl2 and therapeutic concentrations of Montelukast and vitamin E has been described in details by a previous study [
17]. Briefly, HEK293 cells were cultured at 1×104 cells per well in 96-well plates for 24 h. Subsequently, the cells were separately incubated with the following compounds for 24 h:
• Cadmium Chloride (CdCl2) at 10, 50, 100, 150, 200, or 500 μM
• Montelukast at 10, 50, 100, 150, 200, or 250 μM
• Vitamin E at 10, 50, 100, 150, or 200 μM
Following the above treatmen, the survival rates of the cells were measured, using the MTT assay.
Cell extract preparation: After 24 h of treatment, the cells were detached from the flask, using trypsin/EDTA (Gibco, USA) and centrifuged at 1500 rpm for 5 min, and the pelleted cells were rinsed twice with PBS. Subsequently, 500µL of cold lysis buffer was added to the pelleted cells and kept on ice for 30 minutes after resuspension of the pellets. The samples were centrifuged at 10,000 rpm for 15 min at 4°C. Finally, the resulting supernatant was used to measure protein levels and the activity of antioxidant enzymes.
Protein concentration: The amounts of protein in the cell samples were measured using the Bradford method [
24].
Oxidant & antioxidant assessments in cell culture: The oxidant and antioxidant evaluations of GPX, TAC, MDA and NO were performed based on the protocols described in the in vivo section.
Statistical analyses: The results of both in vivo and in vitro experiments were expressed as Means±SD. Data analysis was performed using the SPSS v. 16. The data were analyzed by one-way Analysis of Variance (ANOVA) and LSD post hoc test. In this study, a P<0.05 was considered statistically significant.
Results
In vivo study
Serum biochemical assessment: The mean serum urea, creatinine, and potassium levels in the cadmium chloride control group (group 2) increased significantly compared to the normal control group (P<0.05;
Table 1).

In both Montelukast treatment and pre-treatment (groups 4 and 5), and the group treated with vitamin E (group 7), the concentrations of the previously mentioned analytes decreased compared to those in group 2, which were statistically significant for group 5 and 7 (P<0.05).
Evaluation of serum oxidant and antioxidant markers: The serum Nitric Oxide (NO) and Malondialdehyde (MDA) levels increased significantly in the CdCl2 control group (group 2) compared to those for the normal controls (P<0.05;
Table 2).
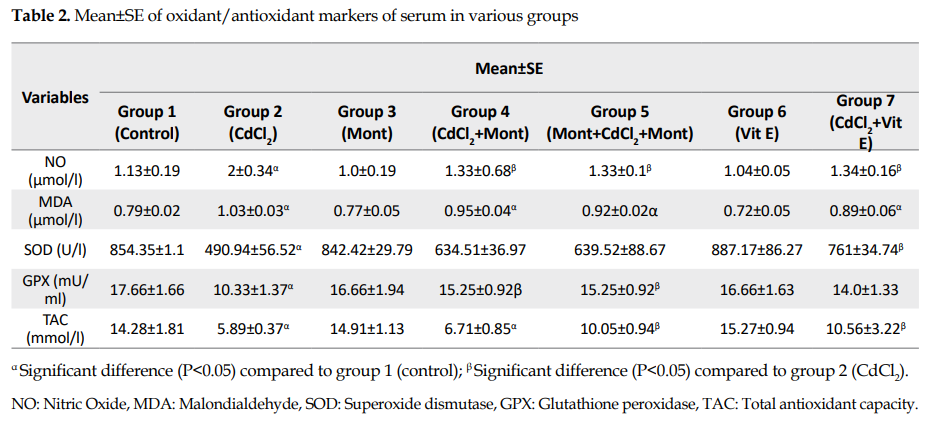
The means of NO and MDA levels in Montelukast treatment and pretreatment groups (groups 4 and 5) were lower than those in group 2, with the difference being significant for NO only (P<0.05). There was no significant difference in NO and MDA concentrations among groups 4, 5 and 7 (vitamin E group). In addition, no significant difference was found between Montelukast and vitamin E controls (groups 3 & 6), respectively, compared to the normal control group in terms of the serum markers.
The mean serum activity for Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPX) enzymes decreased significantly in the CdCl2 controls (group 2) compared to those for the normal controls (P<0.05;
Table 2). In groups 4, 5, and 7 (treatment and pretreatment with Montelukast, and treatment with vitamin E, respectively), the mean activity of SOD and GPX was higher than those in group 2. The increases in SOD and GPX were significant in groups 4 and 5 in terms of GPX and in group 7 in terms of SOD levels (P<0.05). There was no significant difference in the activity of the two enzymes among groups 4, 5, and 7. Moreover, no significant difference was found between the control groups of Montelukast (group 3) and vitamin E (group 6), compared to the normal controls for the levels of the above-mentioned enzymes.
The mean serum Total Antioxidant Capacity (TAC) decreased significantly in the CdCl2 controls (group 2) and Montelukast treatment (group 4) compared to those for the normal controls (P<0.05;
Table 2). The mean TAC for the Montelukast pretreatment and vitamin E treatment (groups 5 and 7) was significantly higher than those for group 2 (P<0.05). There was no significant difference for TAC between groups 4, 5, and 7 and between the control groups of Montelukast (group 3) and vitamin E (group 6) compared to that for the normal controls.
Evaluations of oxidant and antioxidant indices in kidney tissue: The mean NO concentration in the kidney tissue for the control group of CdCl2 (group 2) was significantly higher than that in the normal controls (P<0.05;
Table 3).
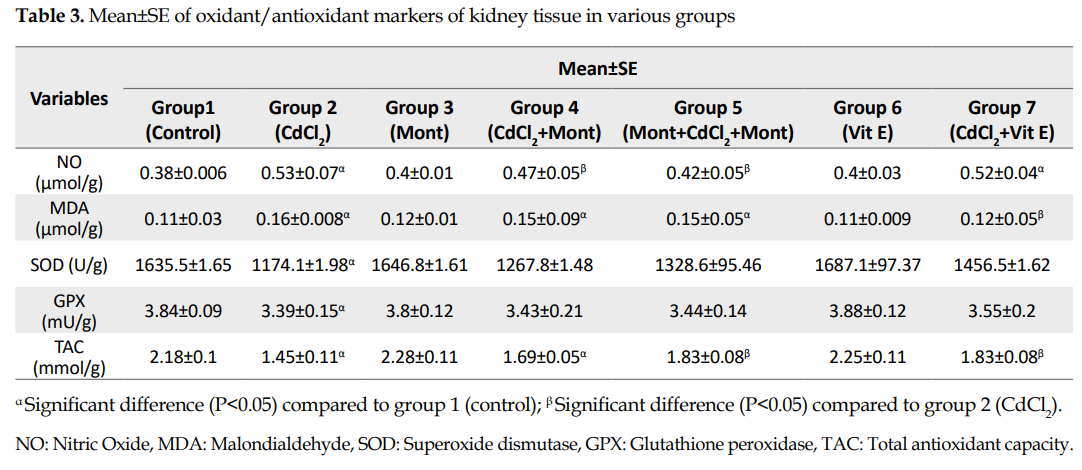
The mean NO concentration in pretreatment and treatment groups with Montelukast (groups 4 and 5) decreased significantly compared to that in group 2 (P<0.05) while in group 7, no significant difference was found compared to that in group 2. Also, there was no significant difference in NO between groups 4 and 5. The mean in group 7 was significantly higher than that in group 5 (P<0.05). In addition, no significant difference was found for the NO concentration between the control groups of Montelukast (group 3) and vitamin E (group 6) compared to that in the normal controls.
The mean concentration of MDA in the kidney tissue for the control group of CdCl2 (group 2) was significantly higher than that in the normal control group (P<0.05;
Table 3). There was no significant difference in groups 4 and 5 (treatment and pretreatment with Montelukast) compared to that in group 2. However, the concentration of MDA in vitamin E group (group 7) decreased significantly compared to that in group 2 (P<0.05). Also, there was no significant difference for MDA level between the control groups of Montelukast (group 3) and vitamin E (group 6), compared to the that in the normal controls.
The activities of SOD and GPX enzymes in the kidney tissue samples were significantly reduced in the control group of CdCl2 (group 2) compared to those for the normal controls (P<0.05;
Table 3). In groups 4, 5, and 7 (treatment and pretreatment with Montelukast and treatment with vitamin E), the mean activities for both enzymes increased compared to that in group 2, although the differences were not statistically significant. There was no significant difference in the activity of the two enzymes among groups 4, 5, and 7. Additionally, no significant difference was found in groups 3 and 6, in terms of SOD and GPX activities compared to those for the normal controls.
The Total Antioxidant Capacity (TAC) of the kidney tissue sample was significantly reduced in groups 2, 4, and 5 compared to that of the normal controls (P<0.05;
Table 3). The mean TAC for the pretreatment group with Montelukast and vitamin E treatment (groups 5 & 7) was significantly increased compared to that in group 2 (P<0.05). Also, no significant difference was found between groups 3 and 6, compared to that for the normal controls.
In vitro study
Evaluation of oxidant and antioxidant indices for HEK293 cells: The evaluation of oxidant and antioxidant capacities for HEK293 cells in culture showed that the mean GPX and SOD activities and TAC concentration in cadmium chloride group (group 2) were significantly lower than that in the normal controls, while the MDA and NO concentrations were significantly higher (P<0.05;
Table 4).

The mean GPX and SOD activities and TAC concentration in Montelukast treatment and pretreatment (groups 4 and 5) were significantly higher than that in group 2 (P<0.05). Also, the mean concentrations of MDA and NO in groups 4 and 5 were significantly lower than those in group 2 (P<0.05). There were no significant differences for GPX and SOD activities and TAC, NO, and MDA concentrations among the groups 4, 5, and 7 (vitamin E). In addition, no significant differences were found in terms of the indices between the control groups of Montelukast (group 3) and vitamin E (group 6) compared to those for the normal controls (
Table 4).
Discussion
In the present study, acute poisoning was induced in rats by the intraperitoneal injection of cadmium at a dose of 3mg/kg for 5 consecutive days. The serum examination showed that the major markers of renal dysfunction, i.e., serum urea, creatinine, and potassium concentrations increased significantly in the cadmium chloride group compared to the normal controls. Cadmium nephrotoxicity has been proven in numerous experimental and clinical studies [
9,
25-
29]. Research has shown that cadmium binds to albumin in the blood and enters the liver and kidneys. In the kidneys, after glomerular filtration, this heavy metal is absorbed and accumulates in the apical cells of the proximal tubules, causing structural and cellular damages [
30]. Additionally, in a previous study by the current authors, cadmium treatment resulted in decreased nuclear density and fragmentation, decreased cell adhesion and integrity, increased apoptosis, and decreased viability in human kidney cells [
17].
In our experiments, acute cadmium chloride toxicity altered all oxidant and antioxidant indices in serum and kidney tissue compared to that in the control group. The concentrations of MDA and NO in our experiments significantly increased while TAC, SOD, and GPX activities were significantly reduced. These changes are suggestive of oxidative stress in serum and kidney tissue secondary to exposure to cadmium. So far, several mechanisms have been proposed for the renal toxicity due to acute or chronic cadmium poisoning, including the role of oxidative stress and suppression of antioxidant defenses caused by this heavy metal in the kidneys and other organs [
10]. On the other hand, oxidative stress plays an important role in causing kidney damages, especially acute injuries and inflammation [
31-
33]. Free radicals, such as superoxide and hydroxyl radicals, react easily with the molecular components of nephrons and lead to structural and/or functional damages [
34].
In our study, the exposure of kidney cells to CdCl2 resulted in a significant increase in MDA and NO concentrations, as oxidation markers, and a significant decrease in the activity of antioxidant enzymes, SOD, GPX and TAC. Our findings were consistent with those of other studies regarding the oxidative effects of cadmium, especially in kidney tissue and cells [
25,
26,
35,
36]. Most of the damages caused by cadmium toxicity in cell cultures were due to increased cellular Reactive Oxygen Species (ROS) load [
37,
38]. In 2009, Liu et al. showed that changes in ROS-induced gene expression due to acute cadmium toxicity at the cell culture level were more significant than that of the chronic toxicity [
38]. This may be due to the development of compensatory mechanisms, such as metallothionein and glutathione, in chronic cadmium toxicity. Studies have shown that interference with cellular antioxidant enzymes and the production of ROS are the major mechanisms of cadmium-induced cellular apoptosis [
39,
40].
Another study that investigated cadmium treatment in HEK293 cells observed a significant increase in the release of lactate dehydrogenase and MDA from the cells and a significant decrease in antioxidant enzyme activities and glutathione level along with DNA damage and reduced cell viability [
41].
In the current study, the oral administration of Montelukast at a dose of 20 mg/kg per day in both the treatment and pretreatment groups resulted in decreased serum urea, creatinine, and potassium concentrations in rats receiving cadmium. In comparison with the two forms of Montelukast administrations, the pretreatment form was more effective in reducing the renal toxicity of cadmium. These effects can probably be related to the anti-inflammatory and antioxidant properties of Montelukast, which will be discussed below. Given the irreversibility of most kidney injuries and complications induced by acute cadmium toxicity, it seems that prevention of these injuries is preferable to treatment. Further, the prophylactic administration of Montelukast for a longer time leads to an increase in the level of the drug in the blood and provides a greater efficacy in preventing the renal complications. In many studies, Montelukast has been used preventively for long periods, especially to protect the kidneys [
42].
Earlier studies have shown that Montelukast has protective effects against a variety of kidney injuries. In our previous study, in vitro treatment of HEK293 cells with Montelukast, in both treatment and pretreatment forms improved the effects against cadmium and enhanced the cell viability. After treatment with Montelukast in both forms and both studied days (first and third days after the last treatment), the survival rate was significantly increased. Also, the cells’ morphology, nuclear density, and status appeared healthier with fewer cell apoptosis than those in cadmium control group [
17]. In another study investigation the anti-inflammatory properties, it was found that the administration of Montelukast prevented tissue damage and oxidative stress in the kidney, liver, and lung following experimental sepsis and significantly reduced the urea and creatinine levels in the rat serum [
11]. Further, another study investigated the acute pyelonephritis induced by the injection of E-coli into the rat kidney medulla, and found that the blood creatinine, and urea levels increased. In that study, the intraperitoneal administration of Montelukast improved the inflammatory status of the kidneys [
13].
Similarly, Sener et al. [
16] showed that treatment with Montelukast in the ischemia-reperfusion model had protective effects on renal tissue and significantly improving the function. Also, Montelukast, at a dose of 10 mg/kg daily for up to one month, has reduced the pituitary gland damage caused by cadmium in rats by lowering its toxic and oxidative effects [
43]. In the current study, the administration of Montelukast was effective in reducing the oxidative stress caused by acute cadmium poisoning in rats. In comparison with the two forms of treatment and pretreatment, the antioxidant effects were more pronounced in the pretreatment form. The administration of Montelukast as a pretreatment (for 5 days before cadmium exposure and then for 5 days simultaneously with cadmium) caused a significant decrease in the NO concentration in the kidney tissue and serum, and a significant increase in serum GPX and TAC compared to those found in the untreated group. However, the effects of treatment with Montelukast (for 5 days simultaneously with cadmium) on the oxidant and antioxidant markers were low and the only significant change was reduced the NO concentration in the serum and kidney tissue, compared to that found for the untreated group.
The effect of Montelukast administration in the pretreatment and treatment forms on lowering the effects of cadmium oxidative stress in kidney cells showed that both forms of administration improved the efficacy, such that the concentrations of MDA and NO in the two groups were significantly lower while the TAC, SOD, and GPX levels were significantly higher compared to those in the CdCl2 exposed untreated group. The Montelukast treatment and pretreatment had the same effect on all of the markers, except for the SOD activity, which was significantly higher in the pretreatment than in that the treatment group. In addition, a comparison of Montelukast with vitamin E treatment on kidney cells showed that the effects of both treatments on the oxidative stress markers were similar and there was no significant difference between Montelukast pretreatment and vitamin E treatment, except for the MDA level. Therefore, it may be concluded that the effects of Montelukast treatment, especially the pretreatment, in protecting kidney cells against oxidative stress caused by cadmium, are similar to that of vitamin E, as a potent antioxidant. A study by Coskun et al. examined the protective effect of Montelukast on vital organ damage caused by experimental sepsis [
11]. They showed that administration of Montelukast after cecal ligation and puncture increased the survival of rats compared to those in the untreated group, while it had supportive effects on other tissues including the kidneys.
Following surgery in rats, the amount of SOD activity and GSH concentration in kidney tissue decreased and Myeloperoxidase (MPO) activity increased, which were adjusted by taking Montelukast administration. In addition, treatment with Montelukast for 10 days reduced the amount of oxidants and damages in the liver caused by methotrexate [
44]. Montelukast also reduced the carbon tetrachloride-induced lipid peroxidation and cell infiltration in liver tissue by blocking the leukotriene receptors [
45]. In a 2012 study by Beytur et al. [
42], the beneficial and therapeutic effects of Montelukast against cisplatin toxicity on testicular tissue, serum testosterone levels, and sperm motility, and serum oxidant and antioxidant balance were determined. Furthermore, the protective effect of Montelukast on models of oxidative stress due to ischemia-reperfusion injury of the small intestine [
14], liver [
15], and kidney [
16] has also been demonstrated in rats.
Conclusions
The findings of this study demonstrated that the antioxidant properties of Montelukast can be considered as one of its main protective mechanisms against cadmium nephrotoxicity. This was established by the decrease in the oxidative stress indices and increase in the antioxidant capacity following experiments in both rats and human cell culture models. Moreover, due to the irreversibility of most cadmium-induced structural and functional damages in the kidneys, it is likely that the long-term prophylactic administration of Montelukast provides greater efficacy and protection in cases of occupational cadmium toxicity.
Ethical Considerations
Compliance with ethical guidelines
All the animal experiments were approved by the Institutional Animal Care and Use Committee of Shahid Chamran University of Ahvaz, and were implemented in strict accordance with approved guidelines (Ethics Code: 98/3/6/35765).
Funding
This research was funded by Shahid Chamran University of Ahvaz (Grant No.: SCU.VC99.199).
Author's contributions
All authors contributed fairly equally to the experiments and preparation of the drafts of this manuscript.
Conflict of interest
The authors declare no conflict of interest with any internal or external entity in conducting this study.
Acknowledgements
The authors would like to thank the research vice chancellor of Shahid Chamran University of Ahvaz for financial support of the research project.
Refrences:












































