Introduction
Plastic is a synthetic organic polymer formed through the polymerization of monomer units derived from oil or gas. This material has become an essential component in various industries worldwide due to its versatility and widespread applications [1, 2]. Over the years, global plastic production has surged significantly, reaching 360 million tons in 2018 compared to just 1.5 million tons in 1950. An estimated 4.8 to 12.7 million tons of this production ultimately find their way into the oceans through a variety of routes. By 2050, it is estimated that the annual production of plastics will rise to 2,000 million tons [3]. Additionally, the rapid increase in plastic production from approximately 1.7 tons annually in the 1950s to over 350 million tons in the 21st century highlights its growing importance and challenges [4]. Recent projections suggest global plastic production could reach 500 million tons by 2025 [5].
Polyvinyl chloride (PVC) is among the most commonly used synthetic plastics in a range of industrial applications, including packaging, construction, and medical devices [6]. Concerns over the effects of PVC's byproducts on the environment and human health, especially microplastics (MPs), have been raised by the increasing manufacturing and use of the material. The MPs are primarily formed through environmental processes such as weathering, mechanical abrasion, and photodegradation of plastic waste [7, 8]. The size of these particles varies from 1 μm to 5 mm, and they can be either regularly or irregularly shaped [9].
In the atmosphere, MPs can become airborne and enter the human body through inhalation, posing significant health risks [10]. Atmospheric MPs have been detected globally and are often transported in various shapes and forms. A considerable proportion of airborne MPs falls within the respirable size range of 0.1-1 μm aerodynamic diameter [11]. Humans are estimated to inhale approximately 0-3.0×10⁷ MP/NP particles per year via aerosols [12]. Studies analyzing atmospheric MPs/NPs in residential, workplace, and public transportation environments reveal that most particles are smaller than 100 μm, presenting a potential inhalation hazard [13]. There are even studies that have shown the biodistribution of inhaled micro-nano plastics in the human body. One study states that inhaled MPs can accumulate in the human respiratory system, where their biodistribution may trigger inflammatory, fibrotic, and carcinogenic responses, highlighting the urgent need to assess their potential health impacts [14].
Despite the growing awareness of the health risks associated with microplastic exposure, the specific effects on lung health remain inadequately understood. The lungs are particularly vulnerable to inhaled particles, and exposure to airborne MPs could lead to various toxicological responses, such as inflammation, oxidative stress, and tissue damage [15]. While previous research has pointed out the potential harmful effects of MPs on several organs, there is limited focus on their impact on the respiratory system, especially concerning MPs derived from PVC. Therefore, it is essential to investigate the pulmonary toxicity of PVC MPs to better understand their broader health implications. The present study examines the lung toxicity caused by subacute exposure to PVC MPs in rat models. By evaluating inflammation, oxidative damage, and other indicators of lung injury, the aim was to clarify the mechanisms behind microplastic-induced pulmonary toxicity.
Materials and Methods
Experimental design
The present study followed an experimental design, incorporating both a randomized post-test-only control group design and a true experimental design. The authors compared MDA levels, p65 NF-κB expression, and histopathological analysis of the lungs from a control group and a group of rats exposed to PVC MPs through inhalation. The whole experimental protocol received approval from the Animal Care and Use Committee of Universitas Brawijaya, Indonesia (Approval No. 81/EC/KEPK-S2/04/2025).
Experimental animals
A total of 11 female Wistar white rats (Rattus norvegicus) were obtained from the Pharmacology Laboratory, Faculty of Medicine, Universitas Brawijaya, Indonesia. The females were randomly allocated to either a control group (n=5) or a treatment group exposed to PVC microparticles (n=6). For this study, female rats were chosen because prior research suggests that they are more sensitive to toxicological exposures, making them a more accurate model for identifying treatment-related side effects [16]. The healthy rats utilized in the study were 12 to 15 weeks old, weighed 150-200 grams, and went through a regular estrous cycle. The estrous cycle stage was monitored daily using visual assessment and confirmed by vaginal smear cytology. All procedures for rat care during the study adhered to the research protocol and included measures to minimize the discomfort of the experimental animals.
Exposure to PVC microplastics per inhalation
The whole-body inhalation exposure method was carried out following the research of Trembley et al. (2022) [17], with modifications to the duration of sub-acute administration. Briefly, female rats in the estrous phase were placed in a 60×60×60 cm3 inhalation box and exposed to technical grade PVC microplastic (CV. Subur Kimia Jaya®), which was exhaled via a blower for 4 hours per day for 28 days [18]. The PVC MP was examined under a stereo microscope (Nikon® SMZ1500) with a camera attached (Onglai Fixtool 51MP®). The microplastic size used in this study was 1,061±9,09 mm, and 92% of particles had a size below 600 mm. According to the Occupational Safety and Health Administration (OSHA) recommendations, a dosage of 15 mg/m3 of PVC microplastic was used [15]. After 28 days of treatment, the rats were euthanized using deep anesthesia, and their blood was taken from the heart for examination.
Malondialdehyde analysis
The MDA concentration in this study was determined in accordance with the BIOXYTECH MDA-586® manufacturer's protocol. A total of 100 mg of lung tissue was finely ground in 1 mL of PBS solution and placed in a microtube. The sample was centrifuged at 14,000 rpm for 15 minutes to separate the clear supernatant. The supernatant was transferred to a new microtube and kept at 20°C. Next, 250 μL of the supernatant was pipetted into a test tube, followed by the addition of 500 μL of 40% TCA solution, 1000 μL of 1% TBA solution, and 100 μL of 1N HCL. The mixture was vortexed to ensure homogeneity. The sample was incubated at 90-95°C for 20 minutes, allowed to cool to room temperature, and then sealed with parafilm. Afterward, the supernatant was centrifuged at 4000 rpm for 10 minutes. A total of 1500 μL of the supernatant was collected and combined with 1250 dL of water. The MDA levels in the lungs were measured using a UV-Vis spectrophotometer at a wavelength (λ) of 580 nm.
Protein expression of p65 NF-κB
For protein expression, immunofluorescence staining using NF-κB p65 Antibody (sc-8008, Santa Cruz Biotechnology) and DAPI (Santa Cruz Biotechnology) was employed. The sections were first incubated with 1% BSA for 30 minutes at room temperature. They were then incubated with the primary antibody overnight at 4°C, followed by washing with BSA. Afterward, the sections were incubated with the secondary antibody for 30 minutes. Then, they were incubated with DAPI for 30 minutes, mounted on coverslips, and the immuno-fluorescence staining was evaluated with an Olympus BX51 microscope. The antibody intensity was measured using the ImageJ software.
Histopathological studies
All rats were euthanized, and the lung tissues of all groups were separated for histological studies. The organ was washed in normal saline, fixed in 10% buffer formalin, and dehydrated with alcohol. The organs were fixed and sectioned into approximately 5 μm thick slices, then stained with hematoxylin and eosin (H&E). The histopathology profile was observed under a light microscope. As for the assessed lung tissue, there is a degree of infiltration of inflammatory cells.
Statistical analysis
The results of all parameters tested were evaluated by statistical analysis using SPSS (version 20) for Windows, Image J, and GraphPad Prism (version 10.2.3) software (GraphPad Co., Ltd., San Diego, CA, USA). The data were analyzed using the Mann-Whitney U test and the independent samples t-test, with a significance level set at 0.05 (p-value < 0.05).
Results
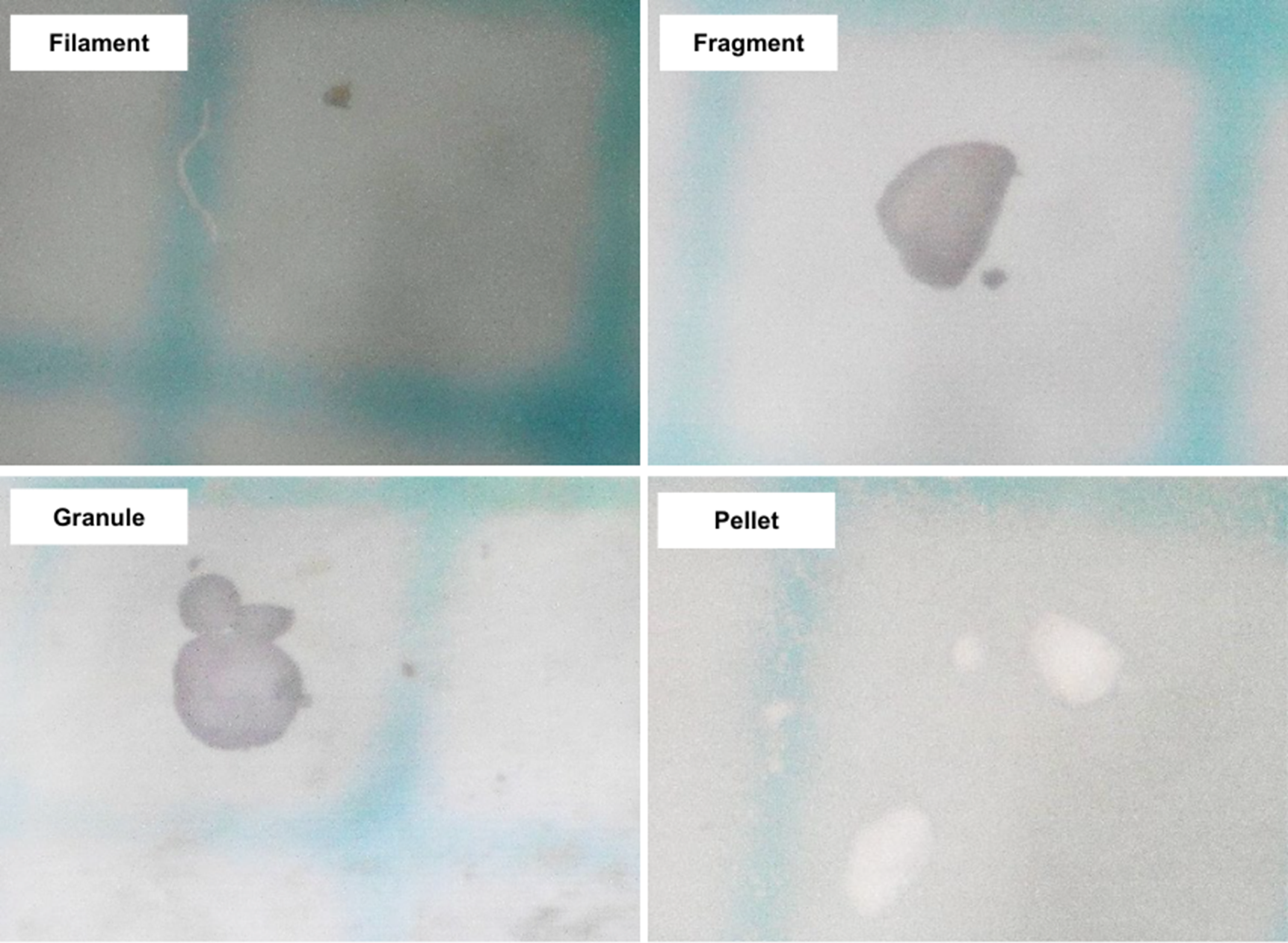
Polyvinyl chloride microplastic characterisation
In this study, a total of 459 PVC particles were used, characterized by two main properties: size and shape. The size of the PVC particles varied, ranging from <100 μm to >1000 μm, with the most dominant group being between 100 μm and 200 μm. The average size of all the samples was 1065.751 ± 9090.988 μm. In addition to size, the samples were categorized into four shapes: filament, fragment, granule, and pellet. The number of particles for each shape was 18, 376, 17, and 48, respectively. The most dominant shape was the fragment, with a total of 376 particles (Figure 1).
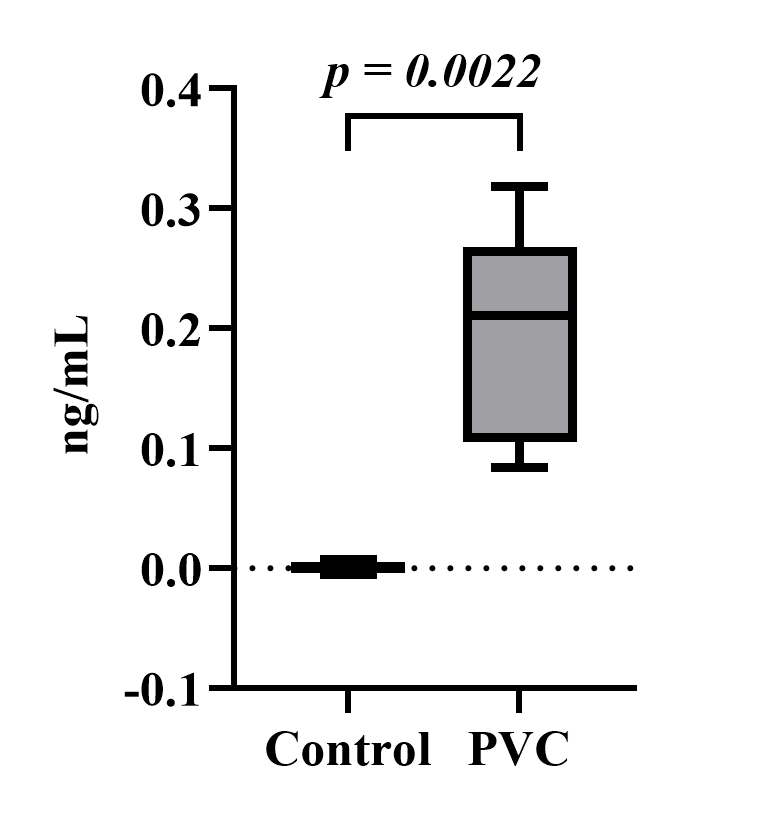
Pulmonary MDA concentration
The MDA levels in the lungs in this study were measured using a spectrophotometer with an absorbance of 580 nm. (Figure 2) compares the average MDA concentration levels in the lung organs between the control and PVC groups. The PVC group has a higher average MDA concentration of 0.197866921 ng/mL than the control group, which is 0.000875517 ng/mL. The Shapiro-Wilk normality test displayed normal results for the control group (p=0.706) and the PVC group (p=0.849). The Levene’s homogeneity test indicated non-homogeneous results (p=0.008). Then, the Mann-Whitney test was selected, and a value was obtained (p=0.002). It can be determined that a significant difference in MDA levels exists between the observation groups.
Figure 2. Effects of polyvinyl chloride exposure on MDA tissue levels in PVC-induced lung damage in Wistar rats. The data are presented as means ± SD (Malondialdehyde: MDA and Polyvinyl Chloride: PVC).
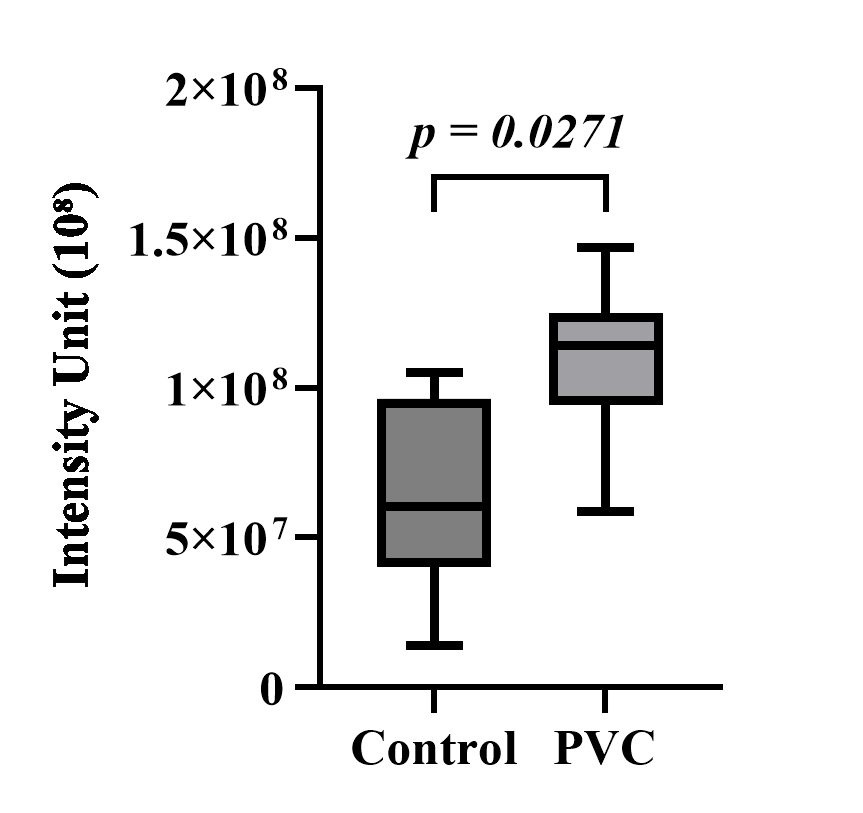
PVC exposure and its impact on p65 NF-κB expression
Immunofluorescence images of lung tissue were obtained for both the control and PVC groups. Each group was divided into three images consisting of DAPI, p65 NF-κB, and a merge of the two (Figure 3A). Next, the intensity of the FITC green light was measured in five fields of view for each sample and averaged. The results indicated that the expression of p65 NF-κB in the PVC group was higher, with a value of 110,228,199 iu, compared to the control group’s 63,658,687.5 iu (Figure 3B). The measured intensity of p65 NF-κB expression was then analyzed using the SPSS (version 25) software. The Shapiro-Wilk normality test indicated normal distribution for the control group (p=0.809) and the PVC group (p=0.301). The Levene’s homogeneity test showed homogeneity (p=0.637). Subsequently, an independent T-test was conducted, yielding a p-value of 0.027. Although some overlap in error bars was observed, indicative of biological variation in the inflammatory response, the statistical analysis confirmed a significant difference between the groups. It can be determined that a significant difference in p65 NF-κB expression exists between the observed groups.
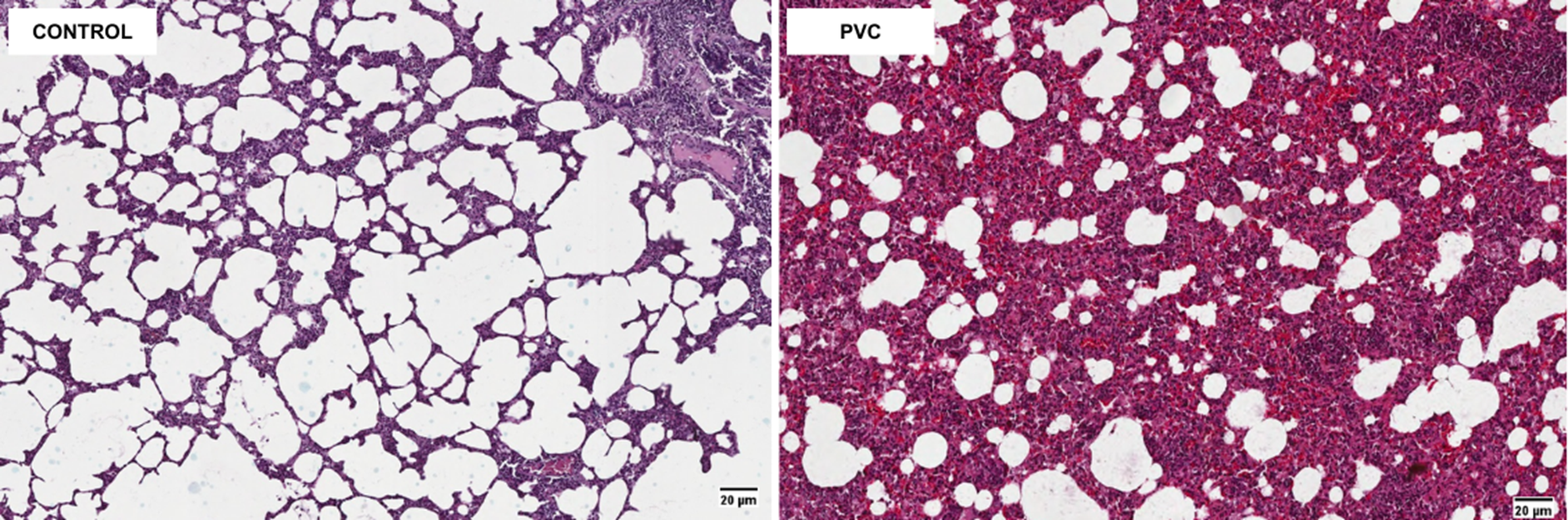
Figure 3A. Histopathology of rat lung (H&E stain, × 20).
Figure 3B. Effects of polyvinyl chloride exposure on p65 NF-κB inflammatory expression in the lung tissue of Wistar rats. The data are presented as means ± SD.
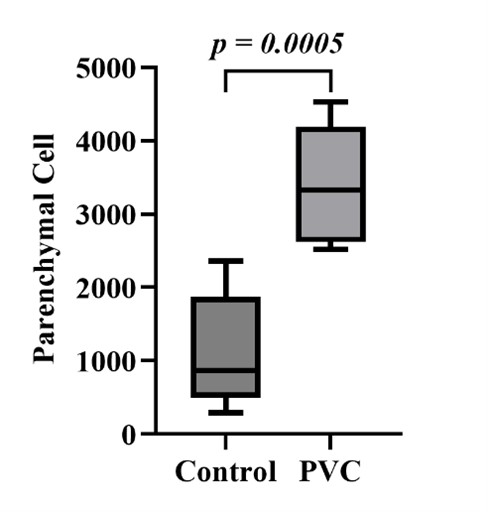
3.4. Pulmonary histopathological examination
The histopathology of lung tissue between the control group and the PVC group is shown in Figure 4A. The PVC group experienced a more extensive inflammatory process compared to the control group, as indicated by the thickening of the alveolar epithelium and the widespread distribution of inflammatory cells. The number of inflammatory cell infiltrates in the PVC group was significantly higher, with 3,406 cells compared to the control group’s 1,109.34 cells (Figure 4B). The Shapiro-Wilk normality test revealed normal distributions for the control group (p=0.487) and the PVC group (p=0.700). The Levene’s homogeneity test indicated homogeneity (p=0.936). Subsequently, an independent T-test was performed, yielding a p-value of 0.000. It can be determined that there is a significant difference in the level of inflammatory cell infiltration between the observed groups.
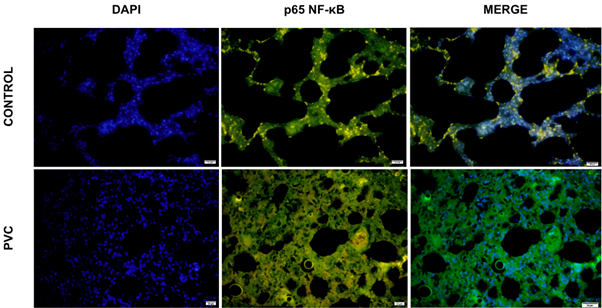
Figure 4A. Immunofluorescence results of p65 NF-κB expression in lung tissue (IF, × 40).
Figure 4B. Effects of polyvinyl chloride exposure on inflammatory cell infiltration in the lung tissue of Wistar rats. The data are presented as means ± SD.
Discussion
In many toxicological studies involving the inhalation route, the lungs are one of the primary organs analyzed. As a vital organ in the human respiratory system, the lungs have various essential functions, including gas exchange, blood pH regulation, air filtration, and moisture control [19]. The lungs also serve as a "port of entry" for various agents from the external environment into the body, including MPs like PVC [20]. The present study demonstrates the significant pulmonary toxicity induced by subacute inhalation exposure to PVC MPs in rat models. The findings are marked by increased MDA levels, upregulation of p65 NF-κB expression, and pronounced histopathological changes in lung tissue.
The elevated MDA levels observed in this study indicate heightened oxidative stress, as MDA is a key lipid peroxidation biomarker and is commonly used to assess cellular damage caused by oxidative stress [21]. The MDA, a byproduct of lipid peroxidation, forms when reactive oxygen species (ROS) target polyunsaturated fatty acids in cell membranes, causing cellular damage and dysfunction. The exposure to PVC MPs likely triggers ROS production, either through direct interactions with lung epithelial cells or via the release of toxic additives, exacerbating oxidative stress [22]. This oxidative imbalance disrupts cellular homeostasis, causing membrane damage, protein oxidation, and DNA damage, which in turn catalyzes inflammatory processes. As inflammation progresses, it amplifies tissue injury, contributing to the impairment of lung function [23]. The elevated MDA levels in response to PVC exposure reflect the extent of this oxidative damage and serve as a clear indication of the harmful effects of MPs on lung tissue. Similarly, a study by Kang et al. (2024) [24] reported that subchronic exposure to polystyrene MPs via intratracheal instillation at a dose of 15 mg/kg resulted in elevated levels of MDA, a marker of lipid peroxidation, in rats, which further demonstrated the role of microplastic exposure in driving oxidative stress and related pathophysiological effects.
The NF-κB, as a transcription factor, regulates the expression of pro-inflammatory cytokines and chemokines, playing a key role in immune cell recruitment and the persistence of inflammation in the lung. Its upregulation suggests that microplastic exposure serves as a potent inflammatory stimulus, exacerbating pulmonary injury. In mammals, the NF-κB transcription factor family consists of five members: p65 (RelA), RelB, Rel, NF-κB1 (p105/p50), and NF-κB2 (p100/p52). Among these, p65 often forms a dimer with p50, creating the p65/p50 complex—the most common and transcriptionally active form of NF-κB. This dimer plays a central role in regulating inflammatory and immune responses [25, 26]. Furthermore, p65 features a highly active transactivation domain at its C-terminal, enabling efficient induction of target gene expression upon activation [27]. The p65/p50 transcription factor is also recognized as the primary mediator of the canonical NF-κB pathway, which can be activated by oxidative stress [28, 29]. The significant increase in p65 NF-κB expression observed in this context reflects the activation of a critical inflammatory pathway, further linking oxidative stress and inflammation to tissue damage.
Our findings demonstrate that exposure to PVC MPs induces a significant increase in the expression of the p65 NF-κB protein, highlighting the activation of a critical inflammatory signaling pathway, which aligns with previous research by Cao et al. (2023) [30] that revealed polystyrene MPs cause lung damage primarily through the activation of the NF-κB signaling cascade and emphasized that microplastic exposure leads to a pronounced upregulation of p65 expression, playing a central role in initiating and sustaining pro-inflammatory responses. Similarly, Woo et al. (2023) [31] found that exposure to smaller-sized polypropylene nanoplastics (NPs) induces lung inflammation through a p38-mediated NF-κB pathway driven by mitochondrial damage. This sustained inflammatory state exacerbates pulmonary damage and potentially leads to chronic respiratory conditions [32]. These findings underscore the need to explore further the mechanisms by which different types of MPs activate NF-κB pathways, as PVC and polystyrene MPs appear to induce p65 upregulation directly. At the same time, polypropylene NPs involve mitochondrial dysfunction and p38 signaling, highlighting the importance of understanding the physicochemical properties of MPs, such as size, surface charge, and polymer type, in determining their inflammatory potential.
Histopathological examination revealed an increased infiltration of parenchymal lung cells in PVC-exposed tissues, which is caused by oxidative stress and the inflammatory response, which can lead to alterations indicating an acute inflammatory response and impaired lung function [33]. These alterations indicate an acute inflammatory response and impaired lung function, raising concerns about the potential for chronic conditions such as fibrosis or chronic obstructive pulmonary disease (COPD) with prolonged exposure [34]. Additionally, the persistent oxidative stress induced by exposure may contribute to cellular damage, further promoting inflammation and tissue remodeling, which are key drivers of chronic lung diseases.
Conclusions
Subacute exposure to PVC MPs in rat models induces significant pulmonary toxicity, characterized by inflammatory responses, oxidative stress, and histopathological damage. These findings highlight the need for greater attention to MPs’ environmental and health risks, particularly those derived from frequently used materials, such as PVC. Future studies should focus on understanding the underlying molecular mechanisms of PVC microplastic toxicity and exploring effective strategies for mitigating exposure and reducing the environmental burden of plastic pollution.
Data Access and Responsibility
The corresponding author had full access to all the data in this study and takes full responsibility for the integrity and accuracy of the data analysis.
Ethical Considerations
This research has received ethical legislation number: No.81/EC/KEPK-S2/04/2025.
Authors' Contributions
MRA was responsible for conceptualization, data curation, formal analysis, investigation, methodology, writing the original draft, and reviewing and editing the manuscript. ARM contributed to the investigation, methodology, and the review and editing of the writing. TA was responsible for conceptualization, supervision, and writing review and editing. HWS handled funding acquisition, writing the original draft, reviewing and editing the manuscript, and supervision. DN was involved in data curation, writing, reviewing, and editing the manuscript.
Acknowledgement
The authors are grateful to the Faculty of Medicine, Universitas Brawijaya, for their support of this study.
Conflict of Interests
The authors declare that they have no conflict of interest regarding the publication of this article.
Funding
This study was supported by the Faculty of Medicine at Universitas Brawijaya, Indonesia, under grant No. 2617 I 49 / UN10.F08/ PN/ 2023 from the Research and Community Service Agency.
References
- Sharma A, Kumari S, Chopade RL, Pandit PP, Rai AR, Nagar V, et al. An assessment of the impact of structure and type of microplastics on ultrafiltration technology for microplastic remediation. Sci Prog. 2023;106(2). [DOI: 10.1177/00368504231176399] [PMID: 37321675]
- Kapoor A, Raghunathan M, Lal B, Kumar P, Srivastava N, Devnani GL, et al. Sustainable valorization of waste plastic into nanostructured materials for environmental, energy, catalytic and biomedical applications: A review. Chemosphere. 2024;364:143279. [DOI: 10.1016/j.chemosphere.2024.143279]
- Prabhu PP, Pan K, Krishnan JN. Microplastics: global occurrence, impact, characteristics and sorting. Front Mar Sci. 2022;9. [DOI:10.3389/fmars.2022.893641]
- Borriello L, Scivicco M, Cacciola NA, Esposito F, Severino L, Cirillo T. Microplastics, a global issue: human exposure through environmental and dietary sources. Foods. 2023;12(8):3396. [DOI: 10.3390/foods12183396] [PMID: 37761106]
- Osman AI, Hosny M, Eltaweil AS, Omar S, Elgarahy AM, Farghali M, et al. Microplastic sources, formation, toxicity and remediation: a review. Environ Chem Lett. 2023;12:2129–69. [DOI: 10.1007/s10311-023-01593-3] [PMID: 37362012]
- Lewandowski K, Skórczewska K. A Brief review of poly(vinyl chloride) (PVC) recycling. Polymers.2022;14(15):3035. [DOI: 10.3390/polym14153035] [PMID: 35893999]
- Wu P, Huang J, Zheng Y, Yang Y, Zhang Y, He F, et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol Environ Saf. 2019;184:109612. [DOI: 10.1016/j.ecoenv.2019.109612]
- Luo D, Chu X, Wu Y, Wang Z, Liao Z, Ji X, et al. Micro- and nano-plastics in the atmosphere: A review of occurrence, properties and human health risks. J Hazard Mater. 2024;465:133412. [DOI: 10.1016/j.jhazmat.2023.133412]
- Winiarska E, Jutel M, Zemelka-Wiacek M. The potential impact of nano- and microplastics on human health: Understanding human health risks. Environ Res. 2024;251(Pt2):118535. [DOI: 10.1016/j.envres.2024.118535]
- Yao X, Luo XS, Fan J, Zhang T, Li H, Wei Y. Ecological and human health risks of atmospheric microplastics (MPs): a review. Environ Sci Atmos. 2022;2(5):921–42. [DOI:10.1039/d2ea00041e]
- Rahman A, Sarkar A, Yadav OP, Achari G, Slobodnik J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci Total Environ. 2021;757:143872. [DOI: 10.1016/j.scitotenv.2020.143872]
- Zhang Q, Xu EG, Li J, Chen Q, Ma L, Zeng EY, et al. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ Sci Technol. 2020;54(7):3740-51. [DOI: 10.1021/acs.est.9b04535] [PMID: 32119774]
- Torres-Agullo A, Karanasiou A, Moreno T, Lacorte S. Airborne microplastic particle concentrations and characterization in indoor urban microenvironments. Environ Pollut. 2022;308:119707. [DOI: 10.1016/j.envpol.2022.119707] [PMID: 35803441]
- Zuri G, Karanasiou A, Lacorte S. Human biomonitoring of microplastics and health implications: A review. Environ Res. 2023;237(Pt1):116966. [DOI: 10.1016/j.envres.2023.116966] [PMID: 37634692]
- Cary CM, Seymore TN, Singh D, Vayas KN, Goedken MJ, Adams S, et al. Single inhalation exposure to polyamide micro and nanoplastic particles impairs vascular dilation without generating pulmonary inflammation in virgin female Sprague Dawley rats. Part Fibre Toxicol. 2023;20(1):16. [DOI: 10.1186/s12989-023-00525-x] [PMID: 37088832]
- Pohjanvirta R, Miettinen H, Sankari S, Hegde N, Lindén J. Unexpected gender difference in sensitivity to the acute toxicity of dioxin in mice. Toxicol Appl Pharmacol. 2012;262(2):167–76. [DOI: 10.1016/j.taap.2012.04.032] [PMID:22564538]
- Trembley JH, So SW, Nixon JP, Bowdridge EC, Garner KL, Griffith J, et al. Whole-body inhalation of nano-sized carbon black: a surrogate model of military burn pit exposure. BMC Res Notes. 2022;15(1):275. [DOI: 10.1186/s13104-022-06165-2] [PMID: 35953874]
- Kania N, Mayangsari E, Setiawan B, Nugrahenny D, Tony F, Wahyuni ES, et al. The effects of Eucheuma cottonii on signaling pathway inducing mucin synthesis in rat lungs chronically exposed to particulate matter 10 (PM 10) coal dust. J Toxicol. 2013;2013:528146. [DOI: 10.1155/2013/528146] [PMID: 24228027]
- Schneider JL, Rowe JH, Garcia-de-Alba C, Kim CF, Sharpe AH, Haigis MC. The aging lung: Physiology, disease, and immunity. Cell. 2021;184(8):1990-2019. [DOI: 10.1016/j.cell.2021.03.005] [PMID: 33811810]
- Zha H, Li Q, Wang Q, Zhang Y, Lu H, Li L. Hazardous potential assessment of airborne and foodborne polyvinyl alcohol and polyhydroxyalkanoates nanoplastics: comparison with polyvinyl chloride nanoplastics. Chem Eng J. 2024;485(17):150122. [DOI:10.1016/j.cej.2024.150122]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13-30. [DOI: 10.1016/j.ab.2016.10.021] [PMID: 27789233]
- Das A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci Total Environ. 2023;895:165076. [DOI: 10.1016/j.scitotenv.2023.165076] [PMID: 37391150]
- Kadac-Czapska K, Ośko J, Knez E, Grembecka M. Microplastics and oxidative stress—current problems and Prospects. Antioxidants. 2024;13(5):579. [DOI: 10.3390/antiox13050579] [PMID: 38790684]
- Kang H, Huang D, Zhang W, Wang JY, Liu Z, Wang Z, et al. Inhaled polystyrene microplastics impaired lung function through pulmonary flora/TLR4-mediated iron homeostasis imbalance. Sci Total Environ. 2024;946:174300. [DOI: 10.1016/j.scitotenv.2024.174300] [PMID: 38936707]
- Capece D, Verzella D, Flati I, Arboretto P, Cornice J, Franzoso G. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. 2022;43(9):757-75. [DOI: 10.1016/j.it.2022.07.004]
- Deka K, Li Y. Transcriptional regulation during aberrant activation of NF-κB signalling in cancer. Cells. 2023;12(5):788. [DOI:10.3390/cells12050788]
- Gnanaskandan S, Srikanth P. Nuclear Factor Kappa B p65: a possible biomarker for persistent inflammation in HIV-1 infection? Cureus. 2024;16(10):e71308. [DOI: 10.7759/cureus.71308]
- Mishra V, Banga J, Silveyra P. Oxidative stress and cellular pathways of asthma and inflammation: Therapeutic strategies and pharmacological targets. Pharmacol Ther. 2018;181:169-82. [DOI: 10.1016/j.pharmthera.2017.08.011] [PMID: 28842273]
- Lingappan K. NF-κB in oxidative stress. Curr Opin Toxicol. 2018;7:81-86. [DOI: 10.1016/j.cotox.2017.11.002] [PMID: 29862377]
- Cao J, Xu R, Geng Y, Xu S, Guo M. Exposure to polystyrene microplastics triggers lung injury via targeting toll-like receptor 2 and activation of the NF-κB signal in mice. Environ Pollut. 2023;320:121068. [DOI: 10.1016/j.envpol.2023.121068]
- Woo JH, Seo HJ, Lee JY, Lee I, Jeon K, Kim B, et al. Polypropylene nanoplastic exposure leads to lung inflammation through p38-mediated NF-κB pathway due to mitochondrial damage. Part Fibre Toxicol. 2023 ;20(1). [DOI: 10.1186/s12989-022-00512-8] [PMID: 36624477]
- Narala VR, Narala SR, Aiya Subramani P, Panati K, Kolliputi N. Role of mitochondria in inflammatory lung diseases. Front Pharmacol. 2024;15:1433961. [DOI: 10.3389/fphar.2024.1433961]
- Bezerra FS, Lanzetti M, Nesi RT, Nagato AC, Silva CP e., Kennedy-Feitosa E, et al. Oxidative stress and inflammation in acute and chronic lung injuries. Antioxidants. 2023;12(3):548. [DOI: 10.3390/antiox12030548] [PMID: 36978796]
- Rodrigues S de O, da Cunha CMC, Soares GMV, Silva PL, Silva AR, Gonçalves-De-albuquerque CF. Mechanisms, pathophysiology and currently proposed treatments of chronic obstructive pulmonary disease. Pharmaceuticals. 2021;14(10):979. [DOI: 10.3390/ph14100979] [PMID: 34681202]










































 , Athaya Rahmanardi Muhammad1
, Athaya Rahmanardi Muhammad1 

 , Triwahju Astuti2
, Triwahju Astuti2 

 , Hikmawan Wahyu Sulistomo *3
, Hikmawan Wahyu Sulistomo *3 

 , Dian Nugrahenny4
, Dian Nugrahenny4