Introduction
In modern times, new antibiotic-resistant bacteria have continued to emerge, increasingly posing threats to the public health [1, 2]. Nowadays, various multi-drug resistant strains of pathogenic bacteria have evolved, including Staphylococcus aureus, Salmonella species, pathogenic Shigella species, etc [3, 4]. Therefore, new antibiotic formulations are required to fight against the bacteria that are resistant to conventional antibiotics [5].
Venom components from various organisms, such as snakes [6, 7], scorpions [8, 9] and spiders [10, 11] have potential antimicrobial properties. Scorpions, poisonous arthropods, belong to Arthropoda phylum, Arachnida class and Scorpions order that are categorized into 16 families and 1500 species and subspecies worldwide [12]. The scorpion venom contains active components, such as hyaluronidase, mucopolysaccharides, serotonin, phospholipase, histamine, enzyme inhibitors and neurotoxins [13]. Small peptides, rich in cysteine and alkaline amino acids, are present in the scorpion venom that affect K+, Na+, Ca2+ and Cl- ion channels and have cytotoxic properties against cells [14].
Odontobuthus bidentatus (O. bidentatus) is a member of the Botheida family and the Odontobuthus genus can be found in six Iranian provinces (Khuzestan, Kerman, Bushehr, Fars, Ilam and Hormozgan) and in the Iraqis province of Baghdad [15]. The anti-cancerous activity of the venom of this scorpion in currently under investigation [16, under review). However, no investigation is available on the antibacterial activity of this venom. We extracted and investigated the antibacterial property of the venom from the Iranian scorpion O. bidentatus on three bacterial strains. They consisted of one Gram negative, i.e., E. coli, and two Gram positive bacteria, Staphylococcus aureus and Bacillus subtilis.
Materials and Methods
Preparation of scorpion venom: The scorpions O. bidentatus were collected from Khuzestan and Hormozgan provinces in Iran, and the venom was extracted by electrical stimulation from the telson. This structure is situated at the end of the scorpion’s tail and contains the venom glands and a sharp, curved stinger. The venomous mixture was centrifuged at 8000 g for 15 min at 4° C, and the supernatant was lyophilized and stored at -20° C for further experiments. In order to prepare the venom solution, the lyophilized powder was dissolved in a medium, and the protein concentration was determined by the Bradford method [17]. The scorpions were kept individually in glass containers under recommended laboratory conditions, fed live house flies and flour beetle larvae based on a standard protocol.
Gram-positive and Gram-negative bacterial strains: Reference bacterial strains, Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 25922), and Bacillus subtilis (ATCC 19659) were obtained from Iranian Biological Resource Center (Tehran, Iran) and were maintained on nutrient agar slants (Oxoid, Hampshire, UK) at 4° C.
Determination of minimal inhibitory concentration: The bacteria were cultured in Muller Hinton broth (Sigma Aldrich; St. Louis, MO, USA) at 37° C. The bacteria (5×105 CFU/mL) were incubated in 96-well microplate at varying concentrations of O. bidentatus crude venom (serial dilutions of 100 µg/ml of crude venom) in a final volume of 100 µL/well. Tetracycline antibiotic (50 μg/mL) and bacterial suspension were used as positive and negative controls, respectively. The microplates were incubated at 37° C and after 16 h, the Optic Density (OD) was measured at 620 nm, using a BioRad micoplate reader (Philadelphia, PA, USA). The minimal inhibitory concentration (MIC) is the lowest concentration of antimicrobial agents that causes 100% inhibition of the growth of the bacteria. The results were reported as means of three independent experiments. MIC was calculated using formula 1:

Determination of cellular viability: The colorimetric assay of 3-(4,5-Dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was used to assess the cellular viability. Similarly to the MIC assay, 5 μl of MTT dye (5 mg/mL) was added to each well after 16 h and the plate was incubated in dark at 37° C for 1 h. Then, 100 μl DMSO was added to each well and incubated in dark for another 2 h, and the absorbance was read at 595 nm. Also, the IC50 of the venom was determined using the Graphpad Prism software, v. 8. The assay was repeated in triplicate for each concentration and the cell viability was determined, using formula 2:

2.
Statistical analysis: The MTT and MIC assays were performed in triplicate and the results reported as Mean±SD. The data were analyzed using Graphpad Prism 8 Software (La Jolla, CA, USA). The venom’s activity at various concentrations was compared to that of the control group, using one-way ANOVA and Tukey’s tests.
Results
MIC assay: The O. bidentatus crude venom significantly inhibited the growth of both Gram-positive (S. aureus & B. subtilis) and Gram-negative (E. coli) bacteria. As seen in Figure 1, the MIC results indicated that the growth rates of the three bacteria were inhibited dose-dependently when treated with the venom at a range of concentrations from 6.25 µg/mL to 100 µg/mL (P<0.0001).

MTT assay: As seen in Figure 2, the crude venom significantly reduced the viability rates of E. coli, S. aureus, and B. subtilis bacteria in a dose dependent manner compared to that noted for the negative controls. The results also demonstrated that the venom significantly decreased the viability rates of both E. coli and S. aureus bacteria, respectively, at the concentrations of 6.25 µg/mL to 100 µg/mL and 3.125 µg/mL to 100 µg/mL (Figures 2A & 2B).
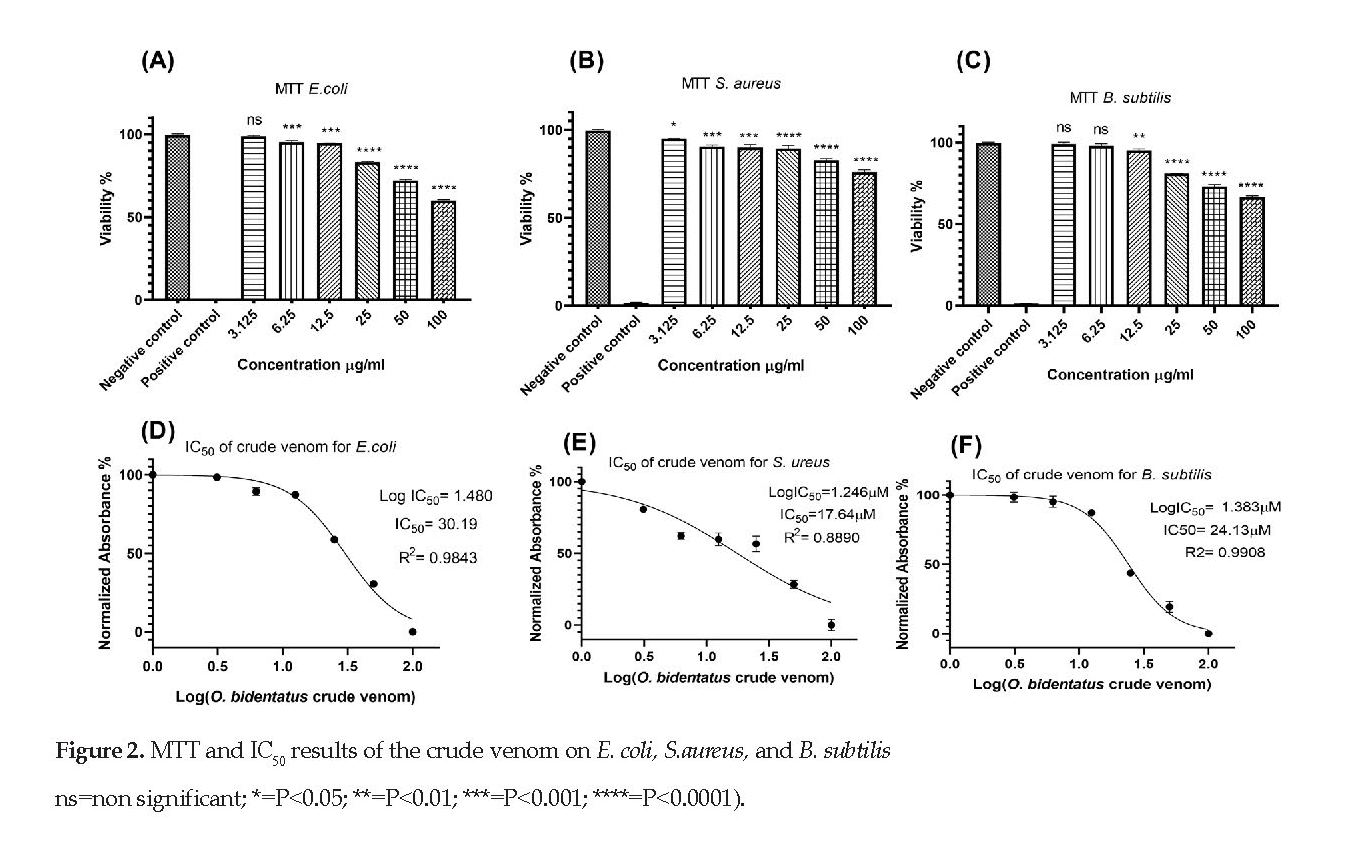
A comparable inhibition of the viability of B. subtilis required 12.5 µg/mL to 100 µg/mL of the venom (Figure 2C). The results indicated that the IC50 values for E. coli, S. aureus, and B. subtilis were 30.19, 17.64, and 24.13µM, respectively (Figures 2D, 2E & 2F).
Discussion
Scorpions are known to use their venom to disinfect themselves from bacteria and fungi [9]. Various studies have shown that scorpion venom contains active components with antimicrobial activity [18, 19]. The present study provides evidence that the crude venom extracted from O. bidentatus has antibacterial effects on all the three Gram-positive and Gram-negative bacteria tested. The data from the MIC analysis showed that the crude venom has a significant inhibitory effect on the three bacterial at almost all concentrations used. The results of MTT assay also confirmed the MIC results. According to the MTT results, the lowest IC50 of the venom was observed for S.aureus at 17.64 µM concentration.
As suggested by structure–function experiments, cationic peptides are active mostly against Gram-negative bacteria while hydrophobic peptides significantly inhibit Gram-positive bacteria [20, 21]. Interestingly, it appears that the venom of O. bidentatus contains both cationic and hydrophobic peptides, inhibiting both Gram-negative and Gram-positive strains. The antibacterial activity of some scorpions’ venoms has been investigated previously [22]. Ahmed et al. investigated the antibacterial activity of the crude venom of H. xanthopus scorpion on B. subtilis, S. typhimurium, E. faecalis and P. aeruginosa. They found that the venom inhibited the growth of all bacteria strains efficiently [22].
Erdes et al. studied the antimicrobial activity of the crude venom from Leiurus abdullahbayrami scorpion (Buthidae family) on Gram-negative (E. coli, E. aerogenes and P. aeruginosa) and Gram-positive (L. monocytogenes) bacteria. They also investigated the effects of the venom against two fungal species (C. krusei and C. albicans). The results showed that the antimicrobial effect was stronger against Gram-negative than Gram-positive bacteria [23].
Samy et al. investigated the antibacterial activity of venoms from snake, scorpions (Buthotus hottenota and Buthus martensii Karsch) and bees [6]. They showed that the scorpion venoms significantly inhibited the growth of S. Aureus, Proteus mirabilis, Proteus vulgaris, Enterobacter aerogenes, Pseudomonas aeruginosa and E. Coli bacteria [6]. Salama and Geasa studied the antimicrobial activity of venoms from three Egyptian scorpions (Leuirus quinquestriatus, Androctonus amoreuxi and Androctonus australis) [24]. In their study, the antimicrobial activities of these venoms against four Gram-positive and Gram-negative bacteria (Bacillus cereus, Bacillus subtillis, Citrobacter freundi and Klibsella pneumonia) were evaluated. They showed that L. quinquestriatus venom had a significant antibacterial effect against B. subtillis and C. freundi. In contrast, A. amoreuxi and A. australis venoms did not have a noticeable effect on the tested bacteria [24].
Our study demonstrated for the first time the potential activity of the venom from O. bidentatus scorpion against the three bacterial strains. To this date, no other study has been conducted on the antibacterial activity of this venom. The findings of this study may be useful in future investigations to uncover the antibacterial mechanisms of O. bidentatus venom.
Limitations
This study focused on the antibacterial effects of the whole venom of O. bidentatus scorpion. In future studies, we recommend that the venom’s fractions be investigated to explore the most effective antibacterial component(s).
Conclusions
The findings suggest that the venom of O. bidentatus scorpion has antibacterial properties against S. Aureus, B. subtilis and E. Coli. Also, our results offer preliminary clues toward the development of new antibacterial drugs and agents with high therapeutic potentials for use in animals and humans. The findings may provide a foundation for future investigations on the putative antimicrobial properties of similar toxins and will expand our toxicology knowledge applicable to the design and development of new agents generated from them.
Ethical Considerations
Compliance with ethical guidelines
Scorpions (Odontobuthus bidentatus) were collected with permission of the Ministry of Health, Govt. of Iran and their venom were extracted in the Razi Institute with required permission.
Funding
The present paper was extracted from the PhD. dissertation of the first author, Department of Biology, Faculty of Basic Sciences, Razi University, Kermanshah.
Author's contributions
Conceptualization and methodology: Hani Keshavarz Alikhani, Jamil Zargan; Investigation: Hani Keshavarz Alikhani, Ashkan Haji Noor Mohammadi, Ahmad heydari, Mohammad Hosseinpour; Writing – original draft: Hani Keshavarz Alikhani; Writing – review & editing: Abbas Hajizadeh; Funding acquisition and Resources: All author; Supervision: Jamil Zargan, Ali Bidmeshkipour.
Conflict of interest
The authors declared no conflicts of interest.
Acknowledgements
The authors wish to appreciate the Biology Department of Imam Hossein University, Tehran, Iran, for supporting this study.
References
Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Call of the wild: Antibiotic resistance genes in natural environments. Nat Rev Microbiol. 2010; 8(4):251-9. [DOI:10.1038/nrmicro2312] [PMID]
Andersson DI, Hughes D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat Rev Microbiol. 2010; 8(4):260-71. [DOI:10.1038/nrmicro2319] [PMID]
Avakh Majalan P, Hajizade A, Nazarian Sh, Pourmand MR, Amiri Siyavoshani K. Investigating the prevalence of Shigella species and their antibiotic resistance pattern in children with acute diarrhea referred to selected hospitals in Tehran, Iran. J Appl Biotechnol Rep. 2018; 5(2):70-4. [DOI:10.29252/JABR.05.02.06]
Klemm EJ, Wong VK, Dougan G. Emergence of dominant multidrug-resistant bacterial clades: Lessons from history and whole-genome sequencing. Proc Natl Acad Sci. 2018; 115(51):12872-7. [DOI:10.1073/pnas.1717162115] [PMID] [PMCID]
Brogden NK, Brogden KA. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals? Int J Antimicrob Agents. 2011; 38(3):217-25. [DOI:10.1016/j.ijantimicag.2011.05.004] [PMID] [PMCID]
Perumal Samy R, Gopalakrishnakone P, Thwin MM, Chow TKV, Bow H, Yap EH, et al. Antibacterial activity of snake, scorpion and bee venoms: A comparison with purified venom phospholipase A2 enzymes. J Appl Microbiol. 2007; 102(3):650-9. [DOI:10.1111/j.1365-2672.2006.03161.x] [PMID]
Shittu LAJ, Bankole M, Ahmed T, Bankole MN, Shittu RK, Saalu CL, et al. Antibacterial and antifungal activities of essential oils of crude extracts of sesame radiatum against some common pathogenic micro-organisms. Iran J Pharmacol Ther. 2007; 6(2):165-70. http://ijpt.iums.ac.ir/article-1-135-en.html
Conde R, Zamudio FZ, Rodrı́guez MH, Possani LD. Scorpine, an anti‐malaria and anti‐bacterial agent purified from scorpion venom. FEBS Lett. 2000; 471(2-3):165-8. [DOI:10.1016/S0014-5793(00)01384-3]
Torres‐Larios A, Gurrola GB, Zamudio FZ, Possani LD. Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus. Eur J Biochem. 2000; 267(16):5023-31. [DOI:10.1046/j.1432-1327.2000.01556.x] [PMID]
Kozlov SA, Vassilevski AA, Feofanov AV, Surovoy AY, Karpunin DV, Grishin EV. Latarcins, antimicrobial and cytolytic peptides from the venom of the spider Lachesana tarabaevi (Zodariidae) that exemplify biomolecular diversity. J Biol Chem. 2006; 281(30):20983-92. [DOI:10.1074/jbc.M602168200] [PMID]
Kuhn-Nentwig L, Dathe M, Walz A, Schaller J, Nentwig W. Cupiennin 1d*: The cytolytic activity depends on the hydrophobic N‐terminus and is modulated by the polar C‐terminus. FEBS Lett. 2002; 527(1-3):193-8. [DOI:10.1016/S0014-5793(02)03219-2]
Chowell G, Díaz-Dueñas P, Bustos-Saldaña R, Alemán Mireles A, Fet V. Epidemiological and clinical characteristics of scorpionism in Colima, Mexico (2000-2001). Toxicon. 2006; 47(7):753-8. [DOI:10.1016/j.toxicon.2006.02.004] [PMID]
Gwee MCE, Nirthanan S, Khoo HE, Gopalakrishnakone P, Manjunatha Kini R, Cheah LS. Autonomic effects of some scorpion venoms and toxins. Clin Exp Pharmacol Physiol. 2002; 29(9):795-801. [DOI:10.1046/j.1440-1681.2002.03726.x] [PMID]
Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, Valdivia HHF, Possania LD. Scorpion venom components that affect ion-channels function. Toxicon. 2013; 76:328-42. [DOI:10.1016/j.toxicon.2013.07.012] [PMID] [PMCID]
Lourenço WR, Pézier A. Taxonomic consideration of the genus Odontobuthus vachon (Scorpiones, Buthidae), with description of a new species. Rev Suisse Zool. 2002; 109(1):115-25. [DOI:10.5962/bhl.part.79581]
Keshavarz Alikhani H, Bidmeshkipour A, Zargan J. Cytotoxic and apoptotic induction effects of the venom of Iranian scorpion (Odontobuthus bidentatus) in the Hepatocellular carcinoma cell line (HepG2). Int J Pept Res Ther. 2020. [DOI:10.1007/s10989-020-10029-3]
Kruger NJ. The Bradford method for protein quantitation. In: Walker JM, editor. The Protein Protocols Handbook, Springer Protocols Handbooks. Totowa, NJ: Humana Press; 2009. 17-24. [DOI:10.1007/978-1-59745-198-7_4]
Gordon YJ, Romanowski EG, McDermott AM. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res. 2005; 30(7):505-15. [DOI:10.1080/02713680590968637] [PMID] [PMCID]
Brown KL, Hancock REW. Cationic host defense (antimicrobial) peptides. Curr Opin Immunol. 2006; 18(1):24-30. [DOI:10.1016/j.coi.2005.11.004] [PMID]
Tossi A, Sandri L, Giangaspero A. Amphipathic, α‐helical antimicrobial peptides. Pept Sci. 2000; 55(1):4-30. [DOI:10.1002/1097-0282(2000)55:1<4::AID-BIP30>3.0.CO;2-M]
Dathe M, Wieprecht T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta (BBA) Biomembr. 1999; 1462(1-2):71-87. [DOI:10.1016/S0005-2736(99)00201-1]
Ahmed U, Mujaddad-ur-Rehman M, Khalid N, Fawad SA, Fatima A. Antibacterial activity of the venom of Heterometrus xanthopus. Indian J Pharmacol. 2012; 44(4):509-11. [DOI:10.4103/0253-7613.99332] [PMID] [PMCID]
Erdeş E, Doğan TS, Coşar İ, Danışman T, Kunt KB, Şeker T, et al. Characterization of Leiurus abdullahbayrami (Scorpiones: Buthidae) venom: Peptide profile, cytotoxicity and antimicrobial activity. J Venom Anim Toxins Incl Trop Dis. 2014; 20:48. [DOI:10.1186/1678-9199-20-48] [PMID] [PMCID]
Salama W, Geasa N. Investigation of the antimicrobial and hemolytic activity of venom of some Egyptian scorpion. J Microbiol Antimicrob. 2014; 6(1):21-8. [DOI:10.5897/JMA2013.0286]










































 , Jamil Zargan *2
, Jamil Zargan *2 

 , Ali Bidmeshkipour1
, Ali Bidmeshkipour1 

 , Ashkan Haji Nour Mohammadi3
, Ashkan Haji Nour Mohammadi3 

 , Mohammad Hosseinpour3
, Mohammad Hosseinpour3 

 , Ahmad Heydari4
, Ahmad Heydari4 

 , Abbas Hajizadeh3
, Abbas Hajizadeh3 


