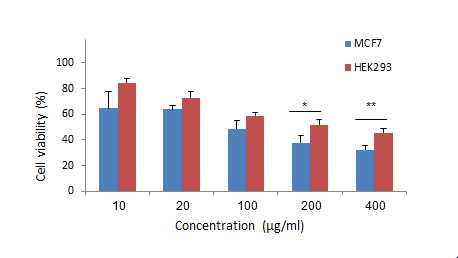
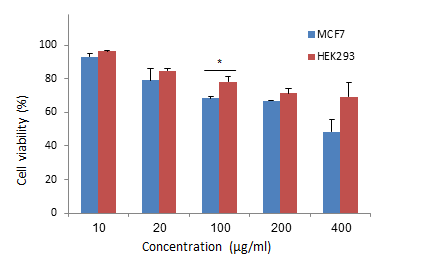
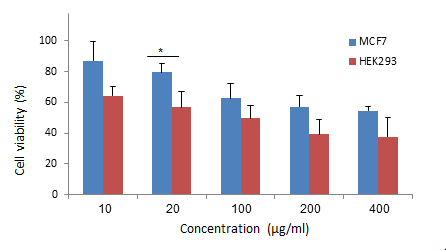
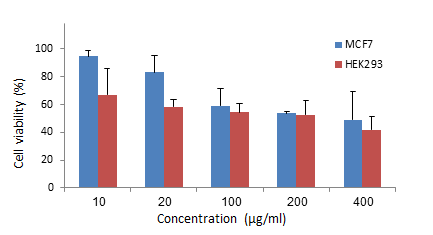
1. Nolan J, Dunne SS, Mustafa W, Sivananthan L, Kiely PA, Dunne CP. Proposed hypothesis and rationale for association between mastitis and breast cancer. Medical hypotheses. 2020;144:110057. [
DOI:10.1016/j.mehy.2020.110057] [
PMID]
2. Calderon-Montano JM, Martinez-Sanchez SM, Jimenez-Gonzalez V, Burgos-Moron E, Guillen-Mancina E, Jimenez-Alonso JJ, et al. Screening for Selective Anticancer Activity of 65 Extracts of Plants Collected in Western Andalusia, Spain. Plants. 2021;10(10). [
DOI:10.3390/plants10102193] [
PMID] [
PMCID]
3. Abu-Dahab R, Afifi F, Kasabri V, Majdalawi L, Naffa R. Comparison of the antiproliferative activity of crude ethanol extracts of nine salvia species grown in Jordan against breast cancer cell line models. Pharmacognosy magazine. 2012;8(32):319-24. [
DOI:10.4103/0973-1296.103664] [
PMID] [
PMCID]
4. Pirbalouti AG, Hashemi M, Ghahfarokhi FT. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Indust Crops Product. 2013;48:43-8. [
DOI:10.1016/j.indcrop.2013.04.004]
5. Behzad S, Pirani A, Mosaddegh M. Cytotoxic activity of some medicinal plants from Hamedan district of Iran. Iran J Pharmaceut Res. 2014;13(Suppl):199.
6. Delnavazi MR, Saiyarsarai P, Jafari-Nodooshan S, Khanavi M, Tavakoli S, Hadavinia H. Cytotoxic flavonoids from the aerial parts of Stachys lavandulifolia Vahl. Pharmaceut Sci. 2018;24(4):332-9. [
DOI:10.15171/PS.2018.47]
7. Esmaeili S, Irani M, Moazzeni Zehan H, Keramatian B, Tavakoli Harandi Z, Hamzeloo-Moghadam M. Cytotoxic activity of some ethnic medicinal plants from southwest of Iran. J Pharmacog Res. 2016;3:43-7.
8. Grbovic F, Stankovic MS, Curcic M, Dordevic N, Seklic D, Topuzovic M, et al. In Vitro Cytotoxic Activity of Origanum vulgare L. on HCT-116 and MDA-MB-231 Cell Lines. Plants. 2013;2(3):371-8. [
DOI:10.3390/plants2030371] [
PMID] [
PMCID]
9. Khallouki F, Breuer A, Akdad M, Laassri FE, Attaleb M, Elmoualij B. Cytotoxic activity of Moroccan Melissa officinalis leaf extracts and HPLC-ESI-MS analysis of its phytoconstituents. Future J Pharmaceut Sci. 2020;6(1):1-12. [
DOI:10.1186/s43094-020-00037-x]
10. Sarkhail P, Sahranavard S, Nikan M, Gafari S, Eslami-Tehrani B. Evaluation of the cytotoxic activity of extracts from six species of Phlomis genus. J Appl Pharmaceut Sci. 2017;7(2):180-4.
11. Badrhadad A, Piri K, Mansouri K. Anti-proliferative effects of some fractions of Elaeagnus angustifolia L. flower and aerial part of Nepeta crispa L. on K562 leukemic cells. Iran J Med Aromatic Plant Res. 2015;31(5):881-90.
12. Mosaddegh M, Esmaeili S, Hassanpour A, Malekmohammadi M, Naghibi F. Ethnobotanical study in the highland of Alvand and Tuyserkan, Iran. Res J Pharmacog. 2016;3(1):7-17.
13. Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant physiology and biochemistry : PPB. 2020;148:80-9. [
DOI:10.1016/j.plaphy.2020.01.006] [
PMID]
14. Kerdar T, Moradkhani S, Dastan D. Phytochemical and biological studies of scrophularia striata from Ilam. Jundishapur J Natur Pharmaceut Product. 2018;13(3):e62705. [
DOI:10.5812/jjnpp.62705]
15. Moradkhani S, Kobarfard F, Ayatollahi SA. Phytochemical Investigations on Chemical Constituents of Achillea tenuifolia Lam. Iranian journal of pharmaceutical research : IJPR. 2014;13(3):1049-54.
16. Kiani M, Firozian F, Moradkhani S. Formulation and physicochemical evaluation of toothpaste formulated with Thymus vulgaris essential oil. J Herbmed Pharmacol. 2017;6(3):130-5.
17. Hamedeyazdan S, Sharifi S, Nazemiyeh H, Fathiazad F. Evaluating antiproliferative and antioxidant activity of Marrubium crassidens. Advanc Pharmaceut Bullet. 2014;4(Suppl 1):459.
18. Shakeri A, Khakdan F, Soheili V, Sahebkar A, Rassam G, Asili J. Chemical composition, antibacterial activity, and cytotoxicity of essential oil from Nepeta ucrainica L. spp. kopetdaghensis. Indust Crop Product. 2014;58:315-21. [
DOI:10.1016/j.indcrop.2014.04.009]
19. Ayeni EA, Abubakar A, Ibrahim G, Atinga V, Muhammad Z. Phytochemical, nutraceutical and antioxidant studies of the aerial parts odoi: 10.15171/jhp.2018.12f Daucus carota L.(Apiaceae). J Herbmed Pharmacol. 2018;7(2):68-73. [
DOI:10.15171/jhp.2018.12]
20. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. International journal of cancer. 2019;144(8):1941-53. [
DOI:10.1002/ijc.31937] [
PMID]
21. Yang H, Dou QP. Targeting apoptosis pathway with natural terpenoids: implications for treatment of breast and prostate cancer. Current drug targets. 2010;11(6):733-44. [
DOI:10.2174/138945010791170842] [
PMID] [
PMCID]
22. Abdoli P, Moradkhani S, Dastan D. Comparative analysis of Nepeta crispa essential oil composition in flowering and vegetative stages. Am J Phytomed Clin Ther. 2016;4:106-12.
23. Sharifi-Rad J, Ayatollahi SA, Varoni EM, Salehi B, Kobarfard F, Sharifi-Rad M. Chemical composition and functional properties of essential oils from Nepeta schiraziana Boiss. Farmacia. 2017;65(5):802-12.
24. Shakeri A, Khakdan F, Soheili V, Sahebkar A, Shaddel R, Asili J. Volatile composition, antimicrobial, cytotoxic and antioxidant evaluation of the essential oil from Nepeta sintenisii Bornm. Indust Crop Product. 2016;84:224-9. [
DOI:10.1016/j.indcrop.2015.12.030]
25. Kahkeshani N, Hadjiakhoondi A, Navidpour L, Akbarzadeh T, Safavi M, Karimpour-Razkenari E, et al. Chemodiversity of Nepeta menthoides Boiss. & Bohse. essential oil from Iran and antimicrobial, acetylcholinesterase inhibitory and cytotoxic properties of 1,8-cineole chemotype. Natural product research. 2018;32(22):2745-8. [
DOI:10.1080/14786419.2017.1378202] [
PMID]
26. Kahkeshani N, Razzaghirad Y, Ostad SN, Hadjiakhoondi A, Shams Ardekani MR, Hajimehdipoor H. Cytotoxic, acetylcholinesterase inhibitor and antioxidant activity of Nepeta menthoides Boiss & Buhse essential oil. J Essen Oil Bear Plant. 2014;17(4):544-52. [
DOI:10.1080/0972060X.2014.929040]
27. Rodenak Kladniew B, Polo M, Montero Villegas S, Galle M, Crespo R, Garcia de Bravo M. Synergistic antiproliferative and anticholesterogenic effects of linalool, 1,8-cineole, and simvastatin on human cell lines. Chemico-biological interactions. 2014;214:57-68. [
DOI:10.1016/j.cbi.2014.02.013] [
PMID]
28. Shafaghat A, Oji K. Nepetalactone content and antibacterial activity of the essential oils from different parts of Nepeta persica. Natur Product Communicat. 2010;5(4):625-8. [
DOI:10.1177/1934578X1000500427]
29. Khalighi-Sigaroodi F, Ahvazi M, Hadjiakhoondi A, Taghizadeh M, Yazdani D, Khalighi-Sigaroodi S. Cytotoxicity and antioxidant activity of 23 plant species of Leguminosae family. Iran J Pharmaceut Res. 2012;11(1):295.
30. Ranjbaran P, Moradkhani S. Iron Chelating Activity of Nepeta Crispa Willd., an Endemic Plant in the West of Iran. Avicenna J Med Biochem. 2022;10(1):65-70. [
DOI:10.34172/ajmb.2022.09]