Introduction
In biological systems, there are different mechanisms for reaction against the toxic effects of free radicals; nonetheless, a large amount of toxic and reactive oxygen species (ROSs) is accumulated if antioxidant systems lack sufficient capacity. This will result in oxidative stress and changes in biomolecules and different diseases [1-6]. Defence mechanisms are activated in response to oxidative stress and prevent continuing oxidation. Different parameters, including the measurement of the activity of superoxide dismutase, alkyl hydroperoxidase, and peroxidases, are used for the evaluation of the performance of the immune system against oxidative stress [7, 8]. Ferric reducing ability of plasma (FRAP) is a sensitive and accurate technique for assessing the impact of antioxidant agents, and this technique offers several advantages [9].
Due to recent advances in modern technology, the exposure of human and other biological systems has been largely increased [10,11]. Therefore, concerns are arising regarding the adverse effects of electromagnetic fields (EMFs) on human and biological systems [10-15]. Numerous studies have been conducted on such effects and have yielded contradictory results due to the complexity of such systems and biological processes [16-19]. Antioxidant agents can neutralize the adverse effects of ROSs. There are different types of antioxidants, including enzymes and minerals [20]. Oxidative stress can be mild or severe, and it can induce different cellular effects, including cellular signalling, lipid peroxidation, and damage to deoxyribonucleic acid (DNA) [21, 22].
Exposure to physical factors, such as ionizing radiation and EMFs, can induce oxidative stress. Due to their high energies, X and gamma rays have determined mechanisms and effects in the production of free radicals and oxidative stress [23]. On the other hand, the use of physical and chemical protective agents can prevent the adverse effects of such factors [24-27]. Aluminum is one of the most abundant elements in the Earth's crust, found in the form of different chemical compositions in the soil [2]. Chemical compositions, such as AlCl3, can be found in foodstuffs and drinking water [7]. The production of aluminum compositions by different industrial manufacturers and their distribution in the environment can exert adverse effects on human health [8].
Different studies have been performed on the effect of either MFs or herbal extracts on rats and cells were evaluated [27-34]. There are also recent studies on the impact of either AlCl3 or MFs on oxidative stress [35-44]. Nevertheless, some of the studies have reported contradictory outcomes regarding the oxidative stress of AlCl3. In light of the aforementioned issues, the present study aimed to assess the oxidative effect of 50 Hz MF and AlCl3, as well as the protective effect of Myrtus communis extract, on the plasma and hemoglobin of rats.
Materials and Methods
Preparation of aqueous extract of Myrtus communis leaves
For the preparation of aqueous extract, 10 g of Myrtus
communis leaves were washed with distilled water and dried at 27±2°C. The leaves were ground, and the obtained powder was soaked for 24 h at room temperature (25±2°C) by the maceration technique. Therafter, the obtained sample was filtered by a Watman paper filter. The cleared solution was sterilized, and 1 unit of the sterilized solution was injected into each mouse.
Animals
The experiment samples included six groups, each group containing eight rats. The experiment groups are mentioned in Table 1. The treatments were performed for 30 days. In the MF group, the Wistar rats were exposed to 50 Hz, 1 mT alternating MF, daily for 2 h per day. The MF was generated using a Helmholtz coil with a 30 cm radius, and the mice cages were positioned in the space in which the MF was relatively uniform. The Helmholtz coil was used to expose the rats to the alternating MF (Figure 1). For those groups that received Myrtus communis, the gavage feeding of the Myrtus communis extract was performed 2 h before receiving the AlCl3 chemical agent. In those groups receiving MF and AlCl3, the cages were positioned in the MF after the injection of AlCl3.
Table 1. Description of the 6 experiment groups evaluated in this study.
| Group number |
Description |
| 1 |
Control (without receiving any external factor) |
| 2 |
Exposed to the MF (50 Hz, 1 mT) |
| 3 |
Received AlCl3 (8 mg/kg) |
| 4 |
Exposed to MF (50 Hz, 1 mT) and AlCl3 (8 mg/kg) |
| 5 |
Received AlCl3 (8 mg/kg) and Myrtus communis extract (1.5 mg/kg, via gavage feeding) |
| 6 |
Received MF (50 Hz, 1 mT) and AlCl3 (8 mg/kg) and Myrtus communis extract (1.5 mg/kg, via gavage feeding) |

Figure 1. Helmholtz coil used in this study to expose the rats to the 50 Hz, 1 mT MF
Blood sampling
Using a heparin syringe, 1.5 mL blood samples were taken from the rats, transferred to Eppendorf tubes, and the tubes were then inserted into an ice container. All samples were centrifuged at 800 g force for 10 minutes. After the separation of blood plasma, the blood cells were washed twice using isotonic saline and three times using isotonic phosphate buffer, and each time the washings were centrifuged for 10 minutes. Finally, all samples were poured in adequate volumes in 1.5 mL Eppendorf tubes, and the tubes were transferred to a -80°C freezer.
Measurement of hemoglobin, methemoglobin, and hemichrome of red blood cells
The measurement of hemoglobin was performed using the cyanmethemoglobin technique. In this technique, the ferrous ions in hemoglobin are converted into ferric ions using ferrocyanide. Following that, the obtained methemoglobin is converted to stable cyanmethemoglobin using potassium cyanide. The maximum optical absorbance of the obtained solution is at 540 nm wavelength, and the color of the solution depends directly on the concentration of hemoglobin in the solution. When hemoglobin is oxidized, methemoglobin is produced. For the measurement of methemoglobin, the erythrocyte was hemolysed using cold hypotonic phosphate buffer. Therafter, the optical absorbance spectrum for methemoglobin was evaluated. According to equation 2, when methemoglobin is oxidized, hemichrome is produced, resulting in the hemolysis of red blood cells. For the measurement of hemichrome, the erythrocyte was hemolysed using cold hypotonic phosphate buffer. Subsequently, the optical absorbance spectrum for hemichrome was evaluated according to Equation 3.
Oxihemoglobine=119A577-39A630-89A560 (1)
Methemoglobine=28A577-307A630-55A560 (2)
Hemichrome=-133A577-114A630+233A560 (3)
Ferric reducing antioxidant power assay
The antioxidant power of plasma was measured using the Ferric Reducing Antioxidant Power (FRAP) assay. This assay is a sensitive, reliable, and accurate technique in which the antioxidant factors of a sample convert complex ferric tripyridyltriazine (FeIII-TPTZ) into the ferrous form (FeII-TPTZ). The latter one has a blue color in an acidic solution, and its maximum optical absorbance is at 593 nm. The reaction speed or reduction power of a sample has a linear trend, and the antioxidant power of plasma can be determined in micromoles using this technique or a standard curve.
Malondialdehyde assay
In this assay, thiobarbituric acid (TBA) was combined with a chemical reagent, and the solution had a color corresponding to 530-540 nm wavelengths. Then lipid peroxidation of plasma was measured. The used solutions were: TPA powder, 20 mL HOCA (5x), 500 μmolar of standard solution, 10 mL alkaline solution (10x), and 12
mL detergent solution. For this assay, 80 mL of distilled water was added to the HOCA solution, and 90 mL of distilled water was then added to the alkaline solution. 100 mg of the TBA powder was added to the HOCA solution, and 1000 mL was added to the alkaline solution. The obtained solution was heated gradually so that the powders were dissolved. The standard solutions were obtained by serial dilution of the 500 μmolar standard solution in the form of 0.78, 1.56, 3.12, 5.6, 12, 25, and 50 μmolar concentrations. To this end, the standard solution was boiled in a Bain-Marie and then cooled in the ice container and centrifuged at 10000 rpm. 200 μl of the superficial solution was transferred to a microplate, absorption reading was performed at 535 nm wavelength, and the corresponding concentration to this wavelength was calculated. Using SPSS software (version 20) and Analysis of variance (ANOVA) test, the mean values from different groups were analyzed, and the related plots were illustrated using Microsoft Excel software (version 2017).
Discussion
In the present study, the effect of 50 Hz alternating MF and AlCl3 on oxidative stress in red blood cells of rats, and the protective effect of Myrtus communis against the adverse effects of alternating MF and AlCl3 were investigated. The results of the present research indicated that the conformation of red blood cells can be changed under the effects of the physical factor (the 1 mT alternating MF) and the chemical factor (AlCl3). As illustrated in Table 2, Figure 2, and Figure 3, the Myrtus communis extract has a significant protective effect (P˂0.05) in the groups undergoing oxidative stress (the group exposed to MF and AlCl3). It is evident also from the results (Table 1) that after the application of MF, the amount of oxyhemoglobin was significantly decreased, and the amount of production of methemoglobin, which is an index indicating oxidative stress, was markedly increased. The results of this study on the protective effects of Myrtus communis are in agreement with the results of the research by Jyoti et al. [33]. In the stated study, it was reported that the extract of Bacopa can significantly decrease the neurotoxic effects of AlCl3 in rats. One of the main results of the present study is that the oxidative stress due to AlCl3 can be enhanced when it is combined with the alternating MF. In other words, the MFs and AlCl3 have synergic effects as oxidant factors on red blood cells.
Table 2. Absorbance of oxyhemoglobin, methemoglobin, and hemichrome for different groups evaluated in this study
| MF and AlCl3 and Myrtus communis |
AlCl3 and Myrtus communis |
MF and AlCl3 |
AlCl3 |
MF |
Control |
|
| 32.07 (30.12-34.25)±1.38 |
34.96 (33.82-35.63)±0.55 |
13.10 (11.78-15.55)±1.35 |
18.02 (18.00-18.04)±0.01 |
21.03 (19.98-23.05)±1.20 |
44.91 (35.94-48.99)± 4.13 |
Oxyhemoglobin |
| 3.89 (3.50-4.09)±0.23 |
3.32 (3.00-3.55)±0.19 |
9.09 (8.30-10.15)±0.66 |
7.20 (7.00-7.40)±0.13 |
6.79 (6.00-7.85)±0.66 |
2.43 (1.74-2.89)±0.43 |
Methemoglobin |
| 1.83 (1.73-1.94)±0.07 |
1.54 (1.31-1.80)±0.18 |
5.92 (5.05-6.62)±0.51 |
4.07 (3.90-4.30)±0.12 |
3.92 (3.05-4.62)±0.51 |
0.82 (0.53-0.95)±0.15 |
Hemichrome |
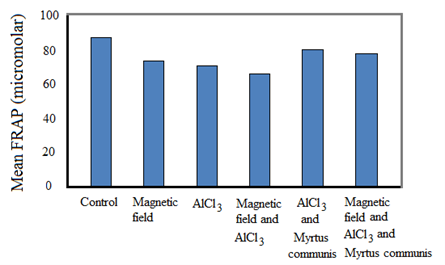
Figure 2. Mean FRAP (µmolar) for different groups evaluated in this study
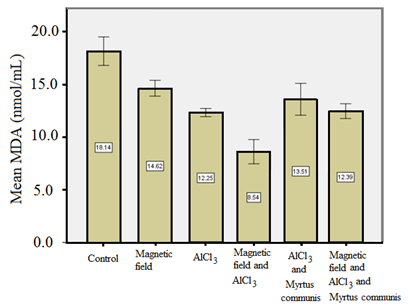
Figure 3. Mean MDA (nmol/ml) for different groups evaluated in this study
The results of the present study also pointed out that AlCl3 is an oxidant agent that can change oxyHb to methemoglobin and hemichrome. The results of the FRAP test demonstrated that AlCl3 can significantly reduce the antioxidant properties of the blood plasma of rats, compared to the control group. The MF exposure of the rats that received the AlCl3 agent can enhance the oxidative effects of AlCl3. This result is in agreement with the findings of the study by Ranjbar et al. [34], which pinpointed that exposure to Al induced oxidative stress in aluminum production workers.
Different mechanisms were reported in the literature about the effects of EMFs on live systems, including the induction of electrical current, direct effect on biological materials, discharging energy, production of free radicals, and stimulation of the cell membrane [45]. From a magnetic point of view, the human body contains organic diamagnetic compounds, which include paramagnetic molecules, such as O2, and ferromagnetic microstructures, such as Hb. As the results of the present study indicate, ferromagnetic fragments, such as Hb, which contain iron, are highly affected by external MFs due to their magnetic properties. Relatively, half of the iron in the body is contained in the Hb molecules.
The ferromagnetic molecules, which are exposed to an external MF, are excited, and this in turn can affect the biological equilibrium of the exposed system [46]. One of the adverse effects of MFs on red blood cells is their effect on the cell membrane. With this effect, the protective role of the membrane against external damage is changed. The damage to the cell membrane can affect the structure and properties of the red blood cell due to the effect on Na-K channels and other ionic processes [47]. One of the adverse effects of an external MF is hemolysis, which occurs due to exposure to electromagnetic radiation. Oxidation-antioxidation biochemical equilibrium is a biological equilibrium, and based on the results of the present study, the oxidation-antioxidation biochemical equilibrium can be changed due to the presence of external MF and free radicals. This effect is in agreement with the study by Hashish et al. [48].
In a study by Rui and Yongjian [35], AlCl3 was administered in different doses through diet for 100 days. The results displayed a marked decrease in superoxide dismutase (SOD) activity and an increase in MDA level in the rats treated with AlCl3, suggesting the involvement of oxidative stress by AlCl3. Abd-Elghaffar et al. [36] investigated the neurotoxic oxidative damage of orally administered aluminum chloride (AlCl3) in rabbits. They reported that chronic exposure to AlCl3 resulted in encephalopathic morphopathological lesions, enhanced lipid peroxidation, and inhibition of the superoxide dismutase enzyme. Cheraghi and Roshanaei [42] performed experiments to assess the effect of curcumin on reducing the hepatotoxicity effects of AlCl3 in rats. In their study, treatments were based on intraperitoneal injections for 28 days. AlCl3 caused a notable rise in some plasma enzyme activities with decreased total protein compared to the control group. AlCl3 significantly reduced the superoxide dismutase, catalase, and glutathione levels. On the other hand, it increased the malondialdehyde (MDA) level in the liver of rats. It also caused histopathological changes in the livers. Curcumin had beneficial effects to compensate for the toxicity effects of AlCl3 [42]. These studies are in agreement with the current research in highlighting the toxic effects of AlCl3. In other words, the results of the current research and the study by Cheraghi and Roshanaei [42] pointed out that AlCl3 induced oxidative stress. Based on the results of the FRAP test (Table 3), exposing the rats to alternating MF and/or AlCl3 resulted in to decrease in FRAP. On the other hand, the use of the extract of Myrtus communis for the MF and/or AlCl3 groups increased the FRAP values.
Table 3. Mean and standard deviation values of FRAP (µmolar) and MDA (nmol/ml) for different groups were evaluated in this study
| MDA |
FRAP |
|
| 18.14±0.67 |
90.47±2.39 |
Control |
| 14.62±0.37 |
76.45±1.36 |
MF |
| 12.25±0.19 |
73.36±1.89 |
AlCl3 |
| 8.54±0.57 |
68.27±1.32 |
MF and AlCl3 |
| 13.51±0.75 |
82.52±1.01 |
AlCl3 and Myrtus communis extract |
| 12.38±0.35 |
80.22±1.44 |
MF and AlCl3 and Myrtus communis extract |
Pretreatment with Myrtus extract resulted in lower concentrations of metHb and hemichrome after exposure to EMFs, indicating the protective effects of the aqueous extract of Myrtus Communis leaves. These results are in agreement with the study by Gebicka and Banasiak, which emphasized the antioxidant effects of catechin, quercetin, and rutin, as well as the reduction of ferryl hemoglobin to methemoglobin [45]. The significant difference in the results of the FRAP test (Table 3) demonstrates that MF can produce stress in a biological system by the production of free radicals and disturbing the oxidant-antioxidant balance. Oxygen free radicals have oxidative reactions with lipids, proteins, and nucleic acids that can induce damage to cellular functions and, finally, can result in cell death. The results of the present study indicate that the Myrtus communis extract can significantly decrease the oxidative damage of 50 Hz, 1 mT alternating MF. The results are also in consonance with the findings of other studies regarding the effects of MFs on the liver [48], brain [49], kidney [50], lymphocytes, and red blood cells [51].
There are also recent studies on the effect of MFs on oxidative stress. Ghodbane et al. [37] reviewed the biological effects of static MFs on free radical generation, oxidative stress, apoptosis, genotoxicity, and cancer. They concluded that exposure to static MFs causes oxidative stress, damage in ion channels, changes in cell morphology and expression of different genes and proteins, as well as cell apoptosis and proliferation. Contrary to our study, Balind et al. [38] introduced the beneficial effects of ELF-MF (50 Hz, 0.5 mT) on a model of global cerebral ischemia in the brain of gerbils.
Sharpe Martyn et al. [39] studied the effects of oscillating MFs on the mitochondrial electron transport chain by measuring the consumption of oxygen (O2) in isolated rat liver mitochondria, normal human astrocytes, several human brain tumor cells, and O2 generation/consumption of plant cells by an O2 electrode. The results suggested that variable fields could be therapeutically efficacious in brain cancers, such as glioblastoma, and diffuse intrinsic pontine glioma through selective disruption of the electron flow in immobile electron transport chain complexes [39]. Schuermann and Mevissen [40] summarized key experimental findings on oxidative stress related to animal and cell exposure to EMFs. Based on that review, many animal and cell studies illustrated increased oxidative stress due to exposure to radiofrequency and ELF-MF. Wang and Zhang [41] reviewed reported studies about the impact of MFs on the levels of reactive oxygen species. There were discrepancies regarding the increasing or decreasing levels of reactive oxygen species in human, mouse, and rat cells and tissues due to MF type, intensity, frequency, exposure time, and assay time-point, as well as different biological samples examined in different studies.
In a study by Quesnel‑Galván et al. [43], the male Wistar rats were subjected to an experimental model of chronic unpredictable mild stress, and the antioxidant status of the cerebrum and cerebellum was estimated after 14 days. Their results demonstrated a significant increase in the catalase activity and reduced concentration of glutathione, which showed a partial restoration in the cerebrum antioxidant system. Coballase-Urrutia et al. [44] demonstrated the effects of static MFs (0.8 mT) in a restraint-stressed animal model, based on changes in different markers of oxidative damage. The results indicated a significant increase in nitric oxide, MDA, and advanced oxidation protein products, as well as a decrease in superoxide dismutase, glutathione, and glycation end products in the plasma of the restraint stress model. It was proposed that exposure to weak-intensity static MFs could offer a complementary therapy by attenuating oxidative stress. The results of several studies align with the findings of the present research, which indicate the oxidative effects of alternating MFs. The results of our study emphasized the oxidative effects of EMF and aluminum chloride on male Wistar rats and the protective antioxidant properties of the aqueous extract of Myrtus Communis leaves.
Acknowledgement
This study was supported by the Research Council of Arak University of Medical Sciences under Grant Number 2226 (Ethical Code: IR.ARAKMU.REC.1394.96).
References
- Scassellati SG, Moretti M, Villarini M, Fatigoni C, Pasquini R. Evaluation of genotoxic and/or co-genotoxic effects in cells exposed in vitro to extremely-low frequency electromagnetic fields. Ann Ig. 2003;16(1-2):321-40. [PMID: 15554538]
- Sigel A, Sigel H, Sigel RK. Interrelations between essential metal ions and human diseases. Springer Nuture; 2013. [Link]
- Farina M, Lara F, Brandao R, Jacques R, Rocha J. Effects of aluminum sulphate on erythropoiesis in rats. Toxicol Lett. 2002;132(2):131-9. [DOI: 10.1016/s0378-4274(02)00077-2] [PMID: 12044547]
- Ja-Liang L, Yu-Jen Y, Sun-Shen Y, Mei-Ling L. Aluminum utensils contribute to aluminum accumulation in patients with renal disease. Am J Kidney Dis. 1997;30(5):653-8. [DOI: 10.1016/s0272-6386(97)90489-3] [PMID: 9370180]
- Al-Hashem F. Camel's milk protects against aluminum chloride-induced toxicity in the liver and kidney of white albino rats. Am J Biochem Biotechnol. 2009;5(3):127-36. [DOI:10.3844/ajbbsp.2009.98.109]
- Karimpour Malekshah A, Torabizadeh Z, Naghshwar F. Developmental toxicity of aluminum from high doses of AlCl3 in mice. J. Applied Res. 2005;5(4):575-9. [Link]
- Kloppel H, Fliedner A, Kordel W. Behaviour and ecotoxicology of aluminium in soil and water--review of the scientific literature. Chemosphere. 1997;35(1-2):353-63. [DOI: 10.1016/s0045-6535(97)00161-6] [PMID: 9232003]
- Kongerud J, Søyseth V. Respiratory disorders in aluminum smelter workers. J Occup Environ Med. 2014;56(5 Suppl):60-70. [DOI: 10.1097/JOM.0000000000000105] [PMID: 24806727]
- Tahara H. Osteomalacia and vitamin D deficiency in hemodialyzed patients. Clinl Calcium. 2004;14(9):42-5. [PMID: 15577108]
- Niu Q, Yang Y, Zhang Q, Niu P, He S, Di Gioacchino M, et al. The relationship between Bcl-gene expression and learning and memory impairment in chronic aluminum-exposed rats. Neurotox Res. 2007;12(3):163-9. [DOI: 10.1007/BF03033913] [PMID: 17967740]
- Somova L, Missankov A, Khan M. Chronic aluminum intoxication in rats: dose-dependent morphological changes. Methods Find Exp Clin Pharmacol. 1997;19(9):599-604. [PMID: 9500123]
- Yokel RA. Toxicity of gestational aluminum exposure to the maternal rabbit and offspring. Toxicol Appl Pharmacol. 1985;79(1):121-33. [DOI: 10.1016/0041-008x(85)90374-6] [PMID: 4049399]
- Rifat S, Eastwood M, McLachlan DC, Corey P. Effect of exposure of miners to aluminium powder. Lancet. 1990;336(8724):1162-5. [DOI: 10.1016/0140-6736(90)92775-d] [PMID: 1978033]
- Krewski D, Yokel RA, Nieboer E, Borchelt D, Cohen J, Harry J, et al. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10(Suppl 1):1-269. [DOI: 10.1080/10937400701597766] [PMID: 18085482]
- Radziun E, Wilczyńska JD, Książek I, Nowak K, Anuszewska E, Kunicki A, et al. Assessment of the cytotoxicity of aluminium oxide nanoparticles on selected mammalian cells. Toxicol In Vitro. 2011;25(8):1694-700. [DOI: 10.1016/j.tiv.2011.07.010] [PMID: 21835238]
- Sharma DR, Wani WY, Sunkaria A, Kandimalla RJ, Verma D, Cameotra SS, et al. Quercetin protects against chronic aluminum-induced oxidative stress and ensuing biochemical, cholinergic, and neurobehavioral impairments in rats. Neurotox Res. 2013;23(4):336-57. [DOI: 10.1007/s12640-012-9351-6] [PMID: 22918785]
- Di Lazzaro V, Capone F, Apollonio F, Borea PA, Cadossi R, Fassina L, et al. A consensus panel review of central nervous system effects of the exposure to low-intensity extremely low-frequency magnetic fields. Brain Stimul. 2013;6(4):469-76. [DOI: 10.1016/j.brs.2013.01.004] [PMID: 23428499]
- Morabito C, Guarnieri S, Fanò G, Mariggiò MA. Effects of acute and chronic low frequency electromagnetic field exposure on PC12 cells during neuronal differentiation. Cell Physiol Biochem. 2011;26(6):947-58. [DOI: 10.1159/000324003] [PMID: 21220925]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys. 2010;99(6):818-36. [DOI: 10.1097/HP.0b013e3181f06c86] [PMID: 21068601]
- Focke F, Schuermann D, Kuster N, Schär P. DNA fragmentation in human fibroblasts under extremely low frequency electromagnetic field exposure. Mutat Res. 2010;683(1):74-83. [DOI: 10.1016/j.mrfmmm.2009.10.012] [PMID: 19896957]
- Verkasalo PK, Pukkala E, Kaprio J, Heikkila KV, Koskenvuo M. Magnetic fields of high voltage power lines and risk of cancer in Finnish adults: nationwide cohort study. BMJ. 1996;313(7064):1047-51. [DOI: 10.1136/bmj.313.7064.1047 ] [PMID: 8898595]
- Feychting M. Health effects of static magnetic fields-a review of the epidemiological evidence. Prog Biophys Mol Biol. 2005;87(2):241-6. [DOI: 10.1016/j.pbiomolbio.2004.08.007] [PMID: 15556662]
- Verschaeve L. Evaluations of international expert group reports on the biological effects of radiofrequency fields. In: Eksim A, editor. Wireless Communication Networks-Recent Advances. In Tech; Rijeka, Croatia: 2012: 523-546. [Link]
- Lagroye I, Percherancier Y, Juutilainen J, De Gannes FP, Veyret B. ELF magnetic fields: animal studies, mechanisms of action. Prog Biophys Mol Biol. 2011;107(3):369-73. [DOI: 10.1016/j.pbiomolbio.2011.09.003] [PMID: 21914452]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47-95. [DOI: 10.1152/physrev.00018.2001] [PMID: 11773609]
- Sen S, Chakraborty R, Sridhar C, Reddy Y, De B. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int J Pharm Sci Rev Res. 2010;3(1):91-100. [Link]
- Zhao W, Diz D, Robbins M. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007; 80(1): 23-31. [DOI: 10.1259/bjr/18237646] [PMID: 17704323]
- Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327(1):48-60. [PMID: 22182453] [DOI: 10.1016/j.canlet.2011.12.012]
- Krisko A, Leroy M, Radman M, Meselson M. Extreme anti-oxidant protection against ionizing radiation in bdelloid rotifers. Proc Natl Acad Sci USA. 2012;109(7):2354-7. [DOI: 10.1073/pnas.1119762109] [PMID: 22308443]
- Getoff N. Vitamin-induced intracellular electrons are the mechanism for their well-known beneficial effects: A review. Nutrition. 2013;29(4):597-604. [DOI: 10.1016/j.nut.2012.09.012] [PMID: 23306138]
- Xu Y, Parmar K, Du F, Price BD, Sun Y. The radioprotective agent WR1065 protects cells from radiation damage by regulating the activity of the Tip60 acetyltransferase. Int J Biochem Mol Biol. 2011;2(4):295-302. [PMID: 22187663]
- Veerapur V, Prabhakar K, Parihar VK, Kandadi MR, Ramakrishana S, Mishra B, et al. Ficus racemosa stem bark extract: a potent antioxidant and a probable natural radioprotector. Evid Based Complement Alternat Med. 2009;6(3):317-24. [DOI: 10.1093/ecam/nem119 ][PMID: 18955240]
- Jyoti A, Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology. 2006;27(4):451-7. [DOI: 10.1016/j.neuro.2005.12.007] [PMID: 16500707]
- Ranjbar A, Khani-Jazani R, Sedighi A, Jalali-Mashayekhi F, Ghazi-Khansari M, Abdollahi M. Alteration of body total antioxidant capacity and thiol molecules in human chronic exposure to aluminum. Toxicol Environ Chem. 2008;90(4):707-13. [DOI:10.1080/02772240701660650]
- Rui D, Yongjian Y. Aluminum chloride induced oxidative damage on cells derived from hippocampus and cortex of ICR mice. Brain Res. 2010;1324:96-102. [DOI: 10.1016/j.brainres.2010.02.024] [PMID: 20156420]
- Abd-Elghaffar SK, El-Sokkary GH, Sharkawy AA. Aluminum-induced neurotoxicity and oxidative damage in rabbits: protective effect of melatonin. Neuro Endocrinol Lett. 2005;26(5):609-16. [PMID: 16264393]
- Ghodbane S, Lahbib A, Sakly M, Abdelmelek H. Bioeffects of static magnetic fields: oxidative stress, genotoxic effects, and cancer studies. BioMed Res Int. 2013:2013:602987. [DOI: 10.1155/2013/602987] [PMID: 24027759]
- Rauš Balind S, Selaković V, Radenović L, Prolić Z, Janać B. Extremely low frequency magnetic field (50 Hz, 0.5 mT) reduces oxidative stress in the brain of gerbils submitted to global cerebral ischemia. PLoS One. 2014;9(2):e88921. [DOI: 10.1371/journal.pone.0088921] [PMID: 24586442]
- Sharpe MA, Baskin DS, Pichumani K, Ijare OB, Helekar SA. Rotating magnetic fields inhibit mitochondrial respiration, promote oxidative stress and produce loss of mitochondrial integrity in cancer cells. Front Oncol. 2021:11:768758. [DOI: 10.3389/fonc.2021.768758] [PMID: 34858847]
- Schuermann D, Mevissen M. Manmade electromagnetic fields and oxidative stress-biological effects and consequences for health. Int J Mol Sci. 2021;22(7):3772. [DOI: 10.3390/ijms22073772] [PMID: 33917298]
- Wang H, Zhang X. Magnetic fields and reactive oxygen species. Int J Mol Sci. 2017;18(10):2175. [DOI: 10.3390/ijms18102175] [PMID: 29057846]
- Cheraghi E, Roshanaei K. The protective effect of curcumin against aluminum chloride induced oxidative stress and hepatotoxicity in rats. Pharm Biomed Res. 2019;5(1):11-18. [DOI:10.18502/pbr.v5i1.761]
- Quesnel‑Galván LR, Torres-Durán PV, Elías-Viñas D, Verdugo-Díaz L. Effect of extremely low frequency magnetic ields on oxidative balance in rat brains subjected to an experimental model of chronic unpredictable mild stress. BMC Neurosci.. 2021;22(1):52. [DOI: 10.1186/s12868-021-00656-x] [PMID: 34488631]
- Coballase-Urrutia E, Navarro L, Ortiz JL, Verdugo-Díaz L, Gallardo JM, Eugenia Hernández M, et al. Static magnetic fields modulate the response of different oxidative stress markers in a restraint stress model animal. Biomed Res Int. 2018;2018:3960408. [DOI: 10.1155/2018/3960408][PMID: 29888261]
- Gebicka L, Banasiak E. Flavonoids as reductants of ferryl hemoglobin. Acta Biochim Pol. 2009;56(3):509-13. [PMID: 19774231]
- Yamaguchi-Sekino S, Sekino M, Ueno S. Biological effects of electromagnetic fields and recently updated safety guidelines for strong static magnetic fields. Magn Reson Med Sci. 2011;10(1):1-10. [PMID: 21441722] [DOI: 10.2463/mrms.10.1]
- Black M, Soo L, Papstein V. Effects of low frequency magnetic fields on Na,K-ATPase activity. Bioelectrochem Bioenerg. 1995;38(2):267-273. [DOI:10.1016/0302-4598(95)05032-4]
- Hashish A, El-Missiry M, Abdelkader H, Abou-Saleh R. Assessment of biological changes of continuous whole body exposure to static magnetic field and extremely low frequency electromagnetic fields in mice. Ecotoxicol Environ Saf. 2008;71(3):895-902. [DOI: 10.1016/j.ecoenv.2007.10.002] [PMID: 17996303]
- Bediz CS, Baltaci AK, Mogulkoc R, Öztekin E. Zinc supplementation ameliorates electromagnetic field-induced lipid peroxidation in the rat brain. Tohoku J Exp Med. 2006;208(2):133-40. [DOI: 10.1620/tjem.208.133] [PMID: 16434836]
- Kula B, Sobczak A, Kuśka R. Effects of static and ELF magnetic fields on free-radical processes in rat liver and kidney. Electro Magnetobiol. 2000;19(1):99-105. [DOI:10.1081/JBC-100100300 ]
- Zmyslony M, Rajkowska E, Mamrot P, Politanski P, Jajte J. The effect of weak 50 Hz magnetic fields on the number of free oxygen radicals in rat lymphocytes in vitro. Bioelectromagnetics. 2004;25(8):607-12. [DOI: 10.1002/bem.20045] [PMID: 15515035]











































