







































BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://ijt.arakmu.ac.ir/article-1-1444-en.html
2- Department of Medical Laboratory Sciences, Faculty of Paramedical Sciences, Dezful University of Medical Sciences, Dezful, Iran
3- Department of Anatomy, Faculty of Medicine, Dezful University of Medical Sciences, Dezful, Iran
Introduction
Cisplatin is an effective drug that is prescribed for the treatment of types of cancer [1-3]. Cisplatin can cure up to 90% of cancers [4]. In addition, what has been observed about testicular cancer has up to 90% treatment power [5]. This drug is one of the best-selling anti-cancer drugs that was approved [6]. However, due to its important side effects as neurotoxicity, its administration is limited [7-9].
The risk of nephrotoxicity is between 20% and 35%, and the use of cisplatin in patients with acute kidney injury (AKI) leads to death [10-12]. This drug causes kidney toxicity in children [11, 13, 14]. Patients with AKI are clinically characterized by acute renal failure, which limits the antitumor effects of cisplatin [15, 16]. Cisplatin reabsorption by the proximal tubules is due to increased reactive oxygen species (ROS) [7, 11, 17]. Cisplatin activates apoptosis pathways and causes cell death [7, 8, 14, 17].
The structure of lipids, proteins, and DNA is destroyed under the influence of ROS. Xanthine oxidase in mitochondria and NADPH oxidase system are involved in ROS production. ROS are formed through these mechanisms in the presence of cisplatin and cause kidney damage [18, 19]. By activating hexokinase and glucose 6-phosphate dehydrogenase enzymes, cisplatin increases the production of free radicals and decreases the production of antioxidants [20, 21]. In general, antioxidant enzymes, including superoxide dismutase, glutathione peroxidase, and catalase, are inhibited by cisplatin, and then renal function is reduced [21-23]. Cisplatin activates complex signaling pathways in nephrons, and in a strong inflammatory response, nephron death and eventual kidney tissue damage occur [12, 18].
In the studies carried out so far, no effective drug that can be prescribed as a drug for the prevention or treatment of kidney damage caused by cisplatin has been presented. According to the literature, some natural compounds have been proposed as drugs with high efficiency and low toxicity to protect against AKI caused by cisplatin [15]. For instance, natural compounds, such as curcumin, ginseng, and pomegranate, as antioxidant and anti-inflammatory compounds can increase the decreased level of antioxidant enzymes caused by cisplatin and thus protect cells against oxidative stress [24]. Some researchers indicated that the administration of vitamin E and riboflavin (vitamin B2) decreases serum urea and increases the expression level of antioxidant enzymes in children with steroid-responsive nephrotic syndrome [25].
Most medicinal plants have aromatic and phenolic compounds, which are considered rich sources of antioxidant and anti-inflammatory compounds [26, 27]. These natural products can be used as supplements to reduce cisplatin-induced nephrotoxicity. Some researchers have reported the relationship between some foods and drinks and their beneficial effects against various diseases [27, 28]. Due to the important role of foods containing phenolic compounds as antioxidants and free radical scavengers, and also because of their protective roles against various diseases, including cancers, consumption of these substances is recommended [27, 29]. Yarrow belongs to the Asteraceae family, which has been used as a medicinal plant for more than 3000 years [30-32]. Yarrow contains phenolic acid and flavonoid compounds [33, 34] and is used medicinally in many cases, including spasmodic diseases, headaches, wounds [35], influenza, diabetes, asthma, and external bleeding [35-37].
Previous works indicated that Yarrow has anti-inflammatory, antioxidant, antiseptic, antispasmodic, and antibacterial properties [38-40]. Among the other therapeutic uses of this medicinal plant, its use in treating liver and biliary disorders, skin inflammation, indigestion, and bronchitis can be mentioned [41, 42]. In addition, research has demonstrated that yarrow extract reduces blood pressure due to its antioxidant activity, and as a diuretic, it reduces urinary tract infections and relieves uterine and menstrual complications [38, 43-45].
No study has so far investigated the valuable effects of yarrow as a medicinal plant containing phenolic compounds on cisplatin-induced nephrotoxicity. The present study aimed to investigate the biochemical side effects of nephrotoxicity caused by a single dose of cisplatin injection and the inflammatory response caused by the injection of this drug in various vital organs of adult male rats. In addition, the antioxidant and protective effects of Yarrow ethanoic extract on cisplatin-induced nephrotoxicity were investigated.
Materials and Methods
In order to prepare Yarrow ethanolic extract, the flowers of this plant were collected from Khorramabad (Iran), dried at room temperature, and then powdered. Then, this powder was placed in 70% alcohol and placed in an Erlenmeyer flask (with continuous shaking) at a temperature of 40°C for three consecutive days. The mixture was passed through a strainer to separate the insoluble materials, and finally, the extract was dehydrated in a dry heat oven at a temperature of 50°C. Before the gavage of the animals, the dried extract was dissolved in distilled water daily to prepare the desired concentrations. In the present study, Cisplatin was obtained from Milan Pharmaceutical Company. Moreover, common kits available on the market were used to measure biochemical factors.
Animals
A total of 24 healthy adult male Wistar rats (6 weeks old and weighing 200±20 gr) were obtained from the animal house of Dezful University of Medical Sciences, Dezful, Iran, and were divided into four groups of six rats each.
Animals were housed in clean polypropylene cages with free access to standard rat chow and water under controlled conditions of humidity (65±5%), temperature (25±2°C), and air conditioning (12 h light/dark cycle).
The animal care protocol was approved by the animal ethics organizational committee (Code: IR. DUMS.REC1396.17) of Dezful University of Medical Sciences, Dezful, Iran.
Animal Grouping
First group (control group): 1 ml of normal saline was injected intra-peritoneal (IP) daily for ten days.
Second group (cisplatin group): Received an injection dose of cisplatin with a concentration of 6 mg/kg on the fifth day of the study.
Third group (Yarrow group): For 10 consecutive days, the animals received Yarrow ethanolic extract with a dose of 250 mg/kg by gavage.
Fourth group (cisplatin-Yarrow group): The animals of this group received 250 mg/kg ethanol extract of Yarrow by gavage for 10 consecutive days. They also received a single injection dose of cisplatin (i.p) with a concentration of 6 mg/kg on the fifth day of the study. The dose of cisplatin and Yarrow extract was determined according to Karwasra et al. and Eslamifar et al. [46, 47].
All animals were anesthetized with diethyl ether on the 11th day, and then blood samples were collected from the heart in heparinized vials. Serum was used to estimate biochemical parameters (urea, creatinine, uric acid, albumin, and TNFα using commercially available diagnostic kits). The kidneys of the animals were removed and frozen at -70°C to check the biochemical parameters after homogenization.
To prepare kidney tissue suspension, phosphate saline buffer (PBS) was added to the homogenized tissue. Then, it was centrifuged at 12,000 g for 15 min at 4°C. The supernatant was used to measure malondialdehyde (MDA), glutathione (GSH), glutathione peroxidase (GPX), superoxide dismutase (SOD), and catalase (CAT). In the present study, the level of MDA in kidney tissue was evaluated by the thiobarbituric acid (TBA) method [48, 49]. Absorbance at 532 nm was read by a spectrophotometer (Hitachi U-2000 Double-Beam UV/Vis spectrometer). The GSH level was determined according to Elman's [50] and Rostami et al.'s methods [51]. To measure GPX activity, tert-Butyl hydroperoxide and hydrogen peroxide (H2O2) were mixed with kidney homogenate and then read at 420 nm by ELISA [52, 53]. The SOD activity was measured by the method of Minami et al. [54] and Naserzadeh et al. [55]. To measure the level of catalase, 0.7 ml of potassium phosphate buffer (pH: 7.0), 0.1 ml of H2O2, and 100 μl of a homogenized kidney sample were mixed and its absorbance was measured at 240 nm by a spectrophotometer (Hitachi, U-2000 Double-Beam UV/Vis Spectrophotometer) was read [56]. To measure the level of TNFα in serum from the ELISA kit (Thermo Fisher Scientific, Waltham, MA, USA) was used.
Statistical Analysis
Data were analyzed for normality using the Shapiro-Wilk test. A one-way analysis of variance (ANOVA) was used to determine the parameters that indicated normal distribution. In case of significance between groups, Tukey's comparison test was used, and data were expressed as mean±standard deviation (SD). Statistical analysis was performed by GraphPad Prism (version 8.0). A statistical probability of P-value<0.05 was considered statistically significant.
Results
Effect of Yarrow Extract on Body Weight and Kidney Weight
In this research, four groups of animals (the average weight was close) were selected, then body weight and kidney weight were evaluated on the sixth day after a single dose of cisplatin. The Cisplatin group indicated a significant weight loss compared to the control group (P<0.0001). Yarrow extract treatment for 5 days before and 5 days after cisplatin injection had modulating effects on cisplatin-induced body weight loss (P<0.01, Figure 1A). However, it was observed that the average weight of the Cis-Yarrow group was significantly different from the average weight of the control group (P<0.0001, Figure 1A).
In the examination of the average kidney weight, the cisplatin group showed a significant increase compared to the control group (P<0.0001). Yarrow extract treatment for 5 days before and 5 days after cisplatin injection led to an improvement in the kidney weight of the Cis-Yarrow group compared to the cisplatin group (P<0.01); however, it was still significantly different from the control group (P<0.0001) (Figure 1B).
Ameliorative Effects of Yarrow Extract on Renal Parameters in Serum
The effects of Yarrow extract (250 mg/kg body weight, gavage) on cisplatin-induced nephrotoxicity (6 mg/kg body weight, i.p) were investigated by studying renal biochemical parameters in serum. The effect of cisplatin nephrotoxicity was evident from the high levels of serum renal markers, including creatinine, urea, and uric acid, as well as the decrease of albumin in the cisplatin group (Figure 2 A-D). The results indicate that glomerular filtration is damaged. In comparing the Cis-Yarrow group with the cisplatin group (in all three indices of creatinine, urea, and uric acid), although Yarrow extract significantly reduced cisplatin-induced nephrotoxicity (P<0.0001), it was observed that the difference was still significant (Figure 2 A-C). During the examination of the albumin level in the four studied groups, it was observed that Yarrow extract could significantly compensate for the damage caused by cisplatin (Cis-Yarrow group compared to cisplatin group, P<0.01, Figure 2 D); however, it could not completely improve the kidney damage (Cis-Yarrow group compared to the control group, P<0.01, Figure 2 D). The results demonstrated that Yarrow extract had no nephrotoxic effect.
Ameliorative Effects of Yarrow Extract on Cisplatin-induced Renal Lipid Peroxidation
Lipid peroxidation is measured based on thiobarbituric acid reactive substances (TBARS) expressed as MDA level. In this study, MDA in kidney tissue of the cisplatin group increased significantly compared to the control group (P<0.0001, Figure 3 A). Administration of Yarrow extract significantly improved the high MDA level caused by cisplatin (Cis-Yarrow group, compared to cisplatin group, P<0.0001); however, the reduction of MDA level did not reach the level of the control group, and the difference was significant (P<0.001, Figure 3 A).
In the examination of the changes in TNFα level, it was observed that Yarrow extract was able to significantly reduce the inflammation caused by cisplatin (P<0.001); however, it could not reach the level of the control group) Cis-Yarrow group, compared to the control group, P<0.0001, Figure 3 B).
Administration of Yarrow extract alone did not increase MDA and TNFα levels compared to control rats. In fact, the levels of MDA and TNFα in the control group and Yarrow group were not significantly different. Figure 3 indicates the relevant results in the four studied groups.
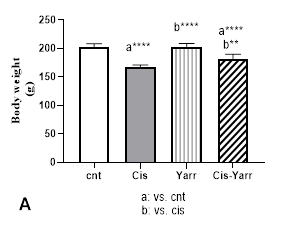
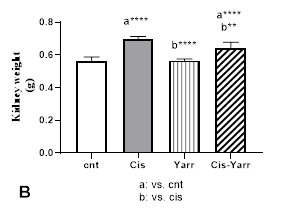
Figure 1. Therapeutic effect of Yarrow extract on A: body weight and B: kidney weight in cisplatin-induced nephrotoxicity: Nephrotoxicity in rats administered a single dose of cisplatin (6 mg/kg body weight, i.p) and Yarrow extract (250 mg/kg, gavage) was established 5 days before cisplatin treatment and continued until the end of the experiment (5 days). The results are expressed as mean±SD (n=6). The results indicate that yarrow extract improves body weight loss and kidney weight increase caused by cisplatin.
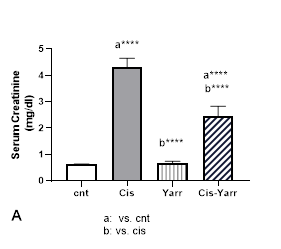
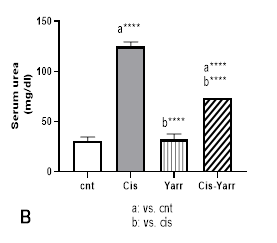
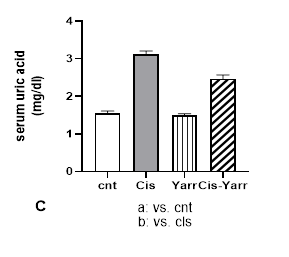
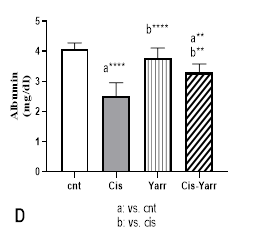
Figure 2. Effect of Yarrow extract on serum indicators of cisplatin-induced nephrotoxicity. (A: creatinine, B: urea, C: uric acid, and D: albumin). Nephrotoxicity was induced in rats by administration of a single dose of cisplatin (6 mg/kg body weight, i.p) and Yarrow extract (250 mg/kg body weight, gavage) 5 days before cisplatin treatment and until the end of the experiment (5 days later). Values are expressed as mean±SD (n=6). The results demonstrate that Yarrow extract reduces cisplatin-induced nephrotoxicity. (****P<0.0001 and **P<0.01).
Yarrow Extract Therapeutic Effects on Kidney Tissue Antioxidant Parameters
In order to evaluate the role of Yarrow extract on the level of oxidative damage caused by cisplatin, the effect of cisplatin and Yarrow extract on enzymatic and non-enzymatic antioxidant defense parameters of kidney tissue was investigated. Figure 4 indicates the effect of Yarrow treatment on changes caused by cisplatin in kidney tissue GSH levels and enzyme antioxidant parameters, including SOD, CAT, and GPX. Compared to the control group, mice injected with cisplatin showed a significant decrease in kidney tissue GSH (P<0.0001). This study demonstrated that the reduction of GSH caused by cisplatin was improved by Yarrow extract treatment (cisplatin group vs. Cis-Yarrow group, P<0.0001); however, it could not significantly increase to the level of the control group (Cis-Yarrow group compared to the control group, P<0.05). In addition, Yarrow extract alone did not significantly increase the level of glutathione in the kidney tissue compared to the control group (Figure 4 B).
The activity level of antioxidant enzymes also decreased significantly in animals treated with cisplatin compared to the control group (P<0001). Additionally, compared to the cisplatin group, the level of all antioxidant enzymes increased in the Cis-Yarrow group (SOD: P<0.001 and the level of CAT and GPx: P<0.0001). Therefore, this research indicated that gavage of Yarrow extract before and after cisplatin treatment significantly improves the reduction of antioxidant enzyme activity caused by cisplatin (Figure 4 A, C, and D).
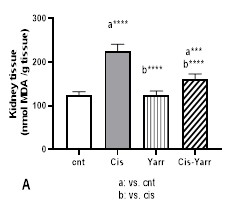
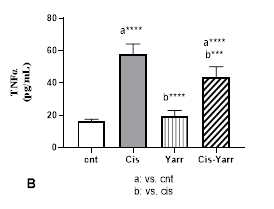
Figure 3. Yarrow effect on A: MDA and B: TNFα. As described in Figure 1, cisplatin (i.p) and Yarrow extract (gavage) were administered to rats. Values are expressed as mean ± SD (n=6). The results show that treatment with Yarrow extract improves the increase in lipid peroxidation and inflammation caused by cisplatin. (***P<0.001; ****P<0.0001).
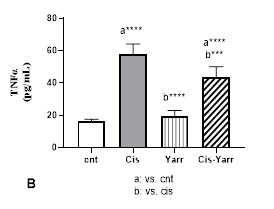
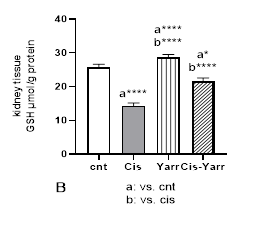
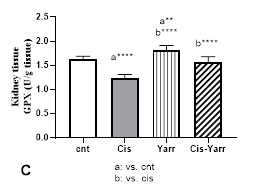
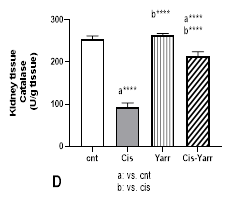
Figure 4. Effect of Yarrow extract on the reduction of antioxidant parameters caused by cisplatin in kidney tissue. (A: SOD, B: GSH, C: GPX, and D: CAT). Mice were treated with cisplatin using Yarrow extract, as described in Figure 1. Values are expressed as mean±SD (n=6). The results indicate that treatment with Yarrow extract ameliorates the decrease caused by cisplatin in SOD, catalase, GSH, and GPx (*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001).
Discussion
Cisplatin is used as an effective and functional antitumor drug; nevertheless, it is associated with complications of AKI in 30% of patients [11, 57]. The side effects of this anti-cancer drug are the inhibition of the mitochondrial respiratory complex in renal tubular cells and, as a result, the production of ROS, which ultimately leads to kidney tissue damage.
The ROS production and oxidative stress lead to lipid peroxidation, changes in the antioxidant system, and gene expression [58-60]. Research has demonstrated that dysregulation in kidney tissue and function is associated with the production of oxidative stress and increased inflammation. It has been reported that in cisplatin-induced nephrotoxicity, apoptosis occurs in tissue [61, 62].
Researchers introduced some compounds that improve the side effects caused by cisplatin, including vitamin E and vitamin C [24, 63-65], selenium [66], some plant compounds [26], nitric oxide modifiers, some factors interfering with metabolic pathways with cisplatin and anti-apoptotic [67-70] and melatonin [63].
Eslamifar et al. showed the ameliorating effects of gallic acid on cisplatin-induced nephrotoxicity in male Wistar rats [49]. In another study, Eslamifar et al. evaluated the acute vascular damage caused by cisplatin in vital organs and the protective effect of yarrow on these damages [47].
Previous studies have indicated that ferulic acid compounds, similar to phenolic acid, have a protective role against nephrotoxicity and hepatotoxicity caused by cisplatin [71, 72]. In addition, the researchers reported that the antioxidant property of Carrichtera annua ethanolic extract counteracts liver and kidney poisoning caused by cisplatin [73].
Yarrow, as a medicinal plant with antioxidant properties, contains phenolic acid that can eliminate free radicals and oxidative damage in tissues. Numerous studies have reported that the Yarrow plant has significant antioxidant and anti-inflammatory activities and can inhibit tissue damage in oxidative conditions [44, 47, 74-76].
Research has demonstrated the protective role of Yarrow against methotrexate-induced nephrotoxicity [77], cyclophosphamide-induced testicular toxicity [78], and cisplatin-induced ocular toxicity [79].
The ROS production, oxidative stress, MDA accumulation, and reduction of antioxidant enzyme activity caused by cisplatin injection are due to the production of active free radicals in the kidney tissue. The GSH is a ROS scavenger and plays an important role in maintaining cell immunity. The protective role of two antioxidant enzymes, CAT and GPX, is through H2O2 decomposition. Moreover, SOD plays a protective role by changing the form of superoxide anion. Therefore, it can be said that SOD, CAT, and GPX are antioxidant enzymes that are essential for protecting and improving kidney function [80].
A decrease in the activity of antioxidant enzymes in the kidney tissue, an increase in the level of creatinine, urea, and uric acid, a decrease in serum albumin, as well as an increase in the level of MDA and TNFα indicate kidney damage caused by cisplatin [22, 80-82].
In the present study, nephrotoxicity was induced by the injection of a single dose of cisplatin (6 mg/kg bw, i.p). According to findings, kidney damage was confirmed by changes in kidney damage parameters, including creatinine, urea, and uric acid, an increase in MDA, a decrease in albumin, an increase in TNFα level as an inflammatory factor, and a reduction in tissue antioxidant indices.
This study confirmed that the use of Yarrow extract significantly improves kidney function by improving enzymatic and non-enzymatic antioxidant markers. Moreover, the antioxidant and anti-inflammatory properties of Yarrow against cisplatin-induced nephrotoxicity were confirmed; these properties can play a key role in modulating toxicity caused by oxidant compounds [44, 83, 84]. These preclinical findings show Yarrow extract as a "nephroprotective" agent against cisplatin toxicity in male Wistar rats.
Conclusions
Administration of cisplatin, as a drug with high effectiveness, is a common method for cancer treatment; nevertheless, the side effects of this drug, including nephrotoxicity, have limited its use.
The present work revealed the modulating effect of yarrow extract as a medicinal plant with antioxidant properties in rat kidney tissue. According to the findings, yarrow extract can provide a promising therapeutic perspective in reducing oxidative stress and ultimately reducing the severity of cisplatin-induced nephrotoxicity. Since cisplatin can cause acute kidney damage by affecting the reabsorption function of kidney tissue, the results of this study can be placed in an important position. Although the results of the present study indicate that Yarrow extract protects kidney tissue from cisplatin-induced toxicity by preventing oxidative stress, further studies are necessary to show whether Yarrow extract may also affect the "efficacy" of cisplatin therapy. In the absence of such evidence, despite the clear renal protection of yarrow extract, a definitive assessment of the potential therapeutic importance of this plant as an aid in chemotherapy cannot be practical.
Compliance with Ethical Guidelines
Ethical approval of all experimental protocols was approved by the animal ethics organizational committee of Dezful University of Medical Sciences, Dezful, Iran (Code: IR. DUMS.REC1396.17), and all procedures were performed according to the relevant guidelines and regulations.
Availability of Data and Material
Data used and/or analyzed in this study are available by email to Z.E. as the corresponding author upon reasonable request.
The authors declare that there is no conflict of interest.
This work was supported by a minimal research grant (Dezful University of Medical Sciences, Dezful, Iran, Tracking Code: 99044). The funders had no role in the different phases of the study.
Z.E. and S.S. designed the initial idea of the research. Z.E., S.S., and R.G. performed experimental protocols. "R.G. and Z.E. wrote the main text of the manuscript. All authors reviewed and approved the final manuscript.
Acknowledgement
The authors appreciate the assistance of the staff of the research laboratories at Dezful University of Medical Science (Iran) for their support and assistance in conducting this research.
References
1. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364-78.] [doi: 10.1016/j.ejphar.2014.07.025] [pmid: 25058905]
2. Hartmann JT, Fels LM, Knop S, Stolte H, Kanz L, Bokemeyer C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide-based combination chemotherapy with or without amifostine in patients with solid tumors. nvest New Drugs. 2000;18:281-9. [doi: 10.1023/a:1006490226104] [pmid: 10958599]
3. Hartmann JT, Lipp H-P. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4(6):889-901. [doi: 10.1517/14656566.4.6.889] [pmid: 12783586
4. Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307-20. [doi: 10.1038/nrd1691] [pmid: 15789122]
5. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265-79. [doi: 10.1038/sj.onc.1206933] [pmid: 14576837]
6. Siddik ZH. Biochemical and molecular mechanisms of cisplatin resistance. Clinically Relevant Resistance in Cancer Chemotherapy (Cancer treat res). 2002;112:263-84. [doi: 10.1007/978-1-4615-1173-1_13] [pmid: 12481720]
7. Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2(11):2490-518. [doi: 10.3390/toxins2112490] [pmid: 22069563]
8. McSweeney KR, Gadanec LK, Qaradakhi T, Ali BA, Zulli A, Apostolopoulos V. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers. 2021;13(7):1572. [doi: 10.3390/cancers13071572] [pmid: 33805488]
9. Quintanilha JCF, Saavedra KF, Visacri MB, Moriel P, Salazar LA. Role of epigenetic mechanisms in cisplatin-induced toxicity. Crit Rev Oncol Hematol. . 2019;137:131-42. [doi: 10.1016/j.critrevonc.2019.03.004] [pmid: 31014509]
10. Pierson‐Marchandise M, Gras V, Moragny J, Micallef J, Gaboriau L, Picard S, et al. The drugs that mostly frequently induce acute kidney injury: a case− noncase study of a pharmacovigilance database. Br J Clin Pharmacol. 2017;83(6):1341-1349. [doi: 10.1111/bcp.13216] [pmid:28002877]
11. Tang C, Livingston MJ, Safirstein R, Dong Z. Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat Rev Nephrol. 2023;19(1):53-72. [doi: 10.1038/s41581-022-00631-7] [pmid: 36229672]
12. Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019;26(1):25. [doi: 10.1186/s12929-019-0518-9] [pmid:30866950]
13. Ruggiero A, Ariano A, Triarico S, Capozza MA, Romano A, Maurizi P, et al. Cisplatin-induced nephrotoxicity in children: what is the best protective strategy? J Oncol Pharm Pract. 2021;27(1):180-6. [doi: 10.1177/1078155220961550] [pmid: 32990190]
14. Tang Q, Wang X, Jin H, Mi Y, Liu L, Dong M, et al. Cisplatin-induced ototoxicity: Updates on molecular mechanisms and otoprotective strategies. Eur J Pharm Biopharm. 2021;163:60-71. [doi: 10.1016/j.ejpb.2021.03.008] [pmid: 33775853]
15. Fang C-y, Lou D-y, Zhou L-q, Wang J-c, Yang B, He Q-j, et al. Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol Sin. 2021;42(12):1951-1969. [doi: 10.1038/s41401-021-00620-9] [pmid: 33750909]
16. Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019;88:102925. [doi: 10.1016/j.bioorg.2019.102925] [pmid: 31003078]
17. Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31(1):15-25. [doi: 10.1007/s40620-017-0392-z] [pmid: 28382507]
18. Zhang J, Ye Z-w, Tew KD, Townsend DM. Cisplatin chemotherapy and renal function. Adv Cancer Res. 2021;152:305-27. [doi: 10.1016/bs.acr.2021.03.008] [pmid: 34353441]
19. Yousef MI, Hussien HM. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem Toxicol. 2015;78:17-25. [doi: 10.1016/j.fct.2015.01.014] [pmid: 25640527]
20. Chirino YI, Hernández-Pando R, Pedraza-Chaverrí J. Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol. 2004;4(1):20. [doi: 10.1186/1471-2210-4-20] [pmid: 15458572]
21. Mapuskar KA, Steinbach EJ, Zaher A, Riley DP, Beardsley RA, Keene JL, et al. Mitochondrial superoxide dismutase in cisplatin-induced kidney injury. Antioxidants. 2021;10(9):1329. [doi: 10.3390/antiox10091329] [pmid: 34572961]
22. Allameh H, Fatemi I, Malayeri AR, Nesari A, Mehrzadi S, Goudarzi M. Pretreatment with berberine protects against cisplatin-induced renal injury in male Wistar rats. Naunyn-Schmiedeberg's Arch Pharmacol. 2020;393(10):1825-1833. [doi: 10.1007/s00210-020-01877-3] [pmid:32410067]
23. Zhang Y, Chen Y, Li B, Ding P, Jin D, Hou S, et al. The effect of monotropein on alleviating cisplatin-induced acute kidney injury by inhibiting oxidative damage, inflammation and apoptosis. Biomed Pharmacother. 2020;129:110408. [doi: 10.1016/j.biopha.2020.110408] [pmid: 32574971]
24. Ridzuan NR, Rashid NA, Othman F, Budin SB, Hussan F, Teoh SL. Protective role of natural products in cisplatin-induced nephrotoxicity. Mini Rev Med Chem. 2019;19(14):1134-1143. [doi: 10.2174/1389557519666190320124438] [pmid: 30894108]
25. Mathew J, Kabi B, Rath B. Anti‐oxidant vitamins and steroid responsive nephrotic syndrome in Indian children. J Paediatr Child Health. 2002;38(5):450-454. [doi: 10.1046/j.1440-1754.2002.00016.x] [pmid: 12354259]
26. Hooshyar N, Sedighi M, Hooshmand M, Valizadeh R, Ebrahimi S, Khosravifarsani M, et al. Mechanistic impact of medicinal plants affecting cisplatin-induced nephrotoxicity; an overview. Immunopathol Persa. 2019;5(1):e07. [doi: 10.15171/ipp.2019.07]
27. Abd Rashid N, Abd Halim SAS, Teoh SL, Budin SB, Hussan F, Ridzuan NRA, et al. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed Pharmacother. 2021;144:112328. [doi: 10.1016/j.biopha.2021.112328] [pmid: 34653753]
28. Sun W, Shahrajabian MH. Therapeutic potential of phenolic compounds in medicinal plants—Natural health products for human health. Molecules. 2023;28(4):1845. [doi: 10.3390/molecules28041845] [pmid: 36838831]
29. Mahomoodally M, Aumeeruddy M, Rengasamy KR, Roshan S, Hammad S, Pandohee J, et al. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin Cancer Biol. 2021:69:140-149. [doi: 10.1016/j.semcancer.2019.08.009] [pmid: 31412298]
30. Radušiene J, Gudaityte O. Distribution of proazulenes in Achillea millefolium sl wild populations in relation to phytosociological dependence and morphological characters. Plant Genetic Resources. 2005;3(2):136-143. [doi:10.1079/PGR200568]
31. Huseynova H. New distribution areas of some species of plants on the southern part of the Caspian coast. Biosyst Diversity. 2023;31(1):123-130. [doi: 10.15421/012313]
32. Zakeri S, Gorji N, Moeini R, Memariani Z. Therapeutic application of Achillea millefolium L. in female reproductive diseases from the viewpoint of Persian medicine and current medicine. J Med Plants. 2019;18(72):107-121. [Link]
33. Salomon L, Lorenz P, Bunse M, Spring O, Stintzing FC, Kammerer DR. Comparison of the phenolic compound profile and antioxidant potential of Achillea atrata L. and Achillea millefolium L. Molecules. 2021;26(6):1530. [doi: 10.3390/molecules26061530] [pmid: 33799635]
34. Radušienė J, Karpavičienė B, Raudone L, Vilkickyte G, Çırak C, Seyis F, et al. Trends in phenolic profiles of Achillea millefolium from different geographical gradients. Plants. 2023;12(4):746. [doi: 10.3390/plants12040746] [pmid: 36840094]
35. Ali SI, Gopalakrishnan B, Venkatesalu V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: a review. Phytother Res. 2017;31(8):1140-61. [doi: 10.1002/ptr.5840] [pmid: 28618131]
36. Akram M. Minireview on Achillea millefolium Linn. J Membr Biol. 2013;246(9):661-663. [doi: 10.1007/s00232-013-9588-x] [pmid: 23959026]
37. Barut EN, Barut B, Engin S, Yıldırım S, Yaşar A, Türkiş S, et al. Antioxidant capacity, anti-acetylcholinesterase activity and inhibitory effect on lipid peroxidation in mice brain homogenate of Achillea millefolium. Turkish J Biochem. 2017;42(4):493-502. [doi:10.1515/tjb-2017-0084]
38. Mohammadhosseini M, Sarker SD, Akbarzadeh A. Chemical composition of the essential oils and extracts of Achillea species and their biological activities: A review. J Ethnopharmacol. 2017;199:257-315. [doi: 10.1016/j.jep.2017.02.010] [pmid: 28179115]
39. Applequist WL, Moerman DE. Yarrow (Achillea millefolium L.): a neglected panacea? A review of ethnobotany, bioactivity, and biomedical research. Economic Botany. 2011;65(2):209-25. [doi:10.1007/s12231-011-9154-3]
40. Daniel PS, Lourenço ELB, Sete da Cruz RM, de Souza Goncalves CH, Marques Das Almas LR, Hoscheid J, et al. Composition and antimicrobial activity of essential oil of yarrow ('Achillea millefolium'L.). Australian J Crop Sci. 2020;14(3):545-50. [doi:10.21475/ajcs.20.14.03.p2325]
41. Farhadi N, Babaei K, Farsaraei S, Moghaddam M, Pirbalouti AG. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Industrial Crops Products. 2020;152:112570. [doi:10.1016/j.indcrop.2020.112570]
42. Villanueva-Bermejo D, Zahran F, Troconis D, Villalva M, Reglero G, Fornari T. Selective precipitation of phenolic compounds from Achillea millefolium L. extracts by supercritical anti-solvent technique. J Supercritical Fluids. 2017;120:52-8. [doi:10.1016/j.supflu.2016.10.011]
43. Becker L, Zaiter A, Petit J, Zimmer D, Karam M-C, Baudelaire E, et al. Improvement of antioxidant activity and polyphenol content of Hypericum perforatum and Achillea millefolium powders using successive grinding and sieving. Industrial Crops Products. 2016;87:116-123. [doi:10.1016/j.indcrop.2016.04.036]
44. Villalva M, Jaime L, Villanueva-Bermejo D, Lara B, Fornari T, Reglero G, et al. Supercritical anti-solvent fractionation for improving antioxidant and anti-inflammatory activities of an Achillea millefolium L. extract. Food Res Int. 2019;115:128-134. [doi: 10.1016/j.foodres.2018.08.027] [pmid: 30599924]
45. Vitalini S, Beretta G, Iriti M, Orsenigo S, Basilico N, Dall'Acqua S, et al. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim Pol. 2011;58(2):203-209. [pmid: 21503279]
46. Karwasra R, Kalra P, Gupta YK, Saini D, Kumar A, Singh S. Antioxidant and anti-inflammatory potential of pomegranate rind extract to ameliorate cisplatin-induced acute kidney injury. Food Funct. 2016;7(7):3091-3101. [doi: 10.1039/c6fo00188b] [pmid: 27273121]
47. Eslamifar Z, Sabbagh S. A histopathological study of cisplatin-induced acute vascular injuries in vital organs and protective effect of Achillea millefolium. J Pharm Res Int. 2020;32(10):56-69. [doi:10.9734/jpri/2020/v32i1030493]
48. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302-10. [doi: 10.1016/s0076-6879(78)52032-6][ pmid: 672633]
49. Eslamifar Z, Moridnia A, Sabbagh S, Ghaffaripour R, Jafaripour L, Behzadifard M. Ameliorative effects of gallic acid on cisplatin-induced nephrotoxicity in rat variations of biochemistry, histopathology, and gene expression. BioMed Res Int. 2021;2021:2195238. [doi: 10.1155/2021/2195238] [pmid: 34746299]
50. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70-77. [doi: 10.1016/0003-9861(59)90090-6] [pmid: 13650640]
51. Rostami R, Eslamifar Z, Nazemi S, Hosseini SZ, Jafaripour L. The effect of thyme essential oil on liver injuries caused by renal ischemia-reperfusion in rats. BioMed Res Int. 2022;2022:2988334. [doi: 10.1155/2022/2988334] [pmid: 36337844]
52. Rotruck JT, Pope AL, Ganther HE, Swanson A, Hafeman DG, Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588-90. [doi: 10.1126/science.179.4073.588] [pmid: 4686466]
53. Monfared SR, Valibeik A, Jafaripour L, Eslamifar Z, Veiskarami S, Ahmadvand H. Role of cineole in alleviation of acute kidney injury and renal function recovery following gentamicin administration in rats. Iranian J Basic Med Sci. 2023;26(5):504-510. [doi: 10.22038/IJBMS.2023.68430.14944] [pmid: 37051098]
54. Minami M, Yoshikawa H. A simplified assay method of superoxide dismutase activity for clinical use. Clinica chimica acta. 1979;92(3):337-342. [doi: 10.1016/0009-8981(79)90211-0] [pmid: 436274]
55. Naserzadeh R, Jafaripour L, Eslamifar Z, Alizamani E, Nouryazdan N, Ahmadvand H. The effect of receiving L-glutamine on the reduction of renal tissue damages and renal function recovery following gentamicin-induced nephrotoxicity in rats. Jo Babol Univ Med Sci. 2021;23. [doi: 10.22088/jbums.23.1.267]
56. Pourmohammadi Dezfouli S, Sabbagh S, Habibi Moghadam M, Eslamifar Z. Evaluation of the protective effect of propofol drug on liver tissue disorders caused by methotrexate administration in male Wistar rats. J Knowledge Health. 2022;17. [doi:10.22100/jkh.v17i3.2883]
57. Casanova AG, Hernández-Sánchez MT, López-Hernández FJ, Martínez-Salgado C, Prieto M, Vicente-Vicente L, et al. Systematic review and meta-analysis of the efficacy of clinically tested protectants of cisplatin nephrotoxicity. Eur J Clin Pharmacol. 2020;76:23-33. [doi: 10.1007/s00228-019-02771-5] [pmid: 31677116]
58. Zhang D, Luo G, Jin K, Bao X, Huang L, Ke J. The underlying mechanisms of cisplatin-induced nephrotoxicity and its therapeutic intervention using natural compounds. Naunyn-Schmiedeberg's Arch Pharmacol. 2023;396(11):2925-2941. [doi: 10.1007/s00210-023-02559-6] [pmid: 37289283]
59. Abbasnezhad A, Salami F, Mohebbati R. A review: Systematic research approach on toxicity model of liver and kidney in laboratory animals. Animal Model Exp Med. 2022;5(5):436-44. [doi: 10.1002/ame2.12230] [pmid: 35918879]
60. Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in toxicological research of the anticancer drug cisplatin. Chem Res Toxicol. 2019;32(8):1469-1486.[doi: 10.1021/acs.chemrestox.9b00204] [pmid: 31353895]
61. Ghassemi-Barghi N, Ehsanfar Z, Mohammadrezakhani O, Ashari S, Ghiabi S, Bayrami Z. Mechanistic approach for protective effect of ARA290, a specific ligand for the erythropoietin/CD131 heteroreceptor, against cisplatin-induced nephrotoxicity, the involvement of apoptosis and inflammation pathways. Inflammation. 2023;46(1):342-58. [doi: 10.1007/s10753-022-01737-7] [pmid: 36085231]
62. Wang W-t, Fan M-l, Hu J-n, Sha J-y, Zhang H, Wang Z, et al. Maltol, a naturally occurring flavor enhancer, ameliorates cisplatin-induced apoptosis by inhibiting NLRP3 inflammasome activation by modulating ROS-mediated oxidative stress. J Functional Foods. 2022;94:105127. [doi:10.1016/j.jff.2022.105127]
63. Barberino RS, Menezes VG, Ribeiro AE, Palheta Jr RC, Jiang X, Smitz JE, et al. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol Reprod. 2017;96(6):1244-55. [doi: 10.1093/biolre/iox053] [pmid: 28595266]
64. Al-Eitan LN, Alzoubi KH, Al-Smadi LI, Khabour OF. Vitamin E protects against cisplatin-induced genotoxicity in human lymphocytes. Toxicology In Vitro. 2020;62:104672. [doi: 10.1016/j.tiv.2019.104672] [pmid: 31629897]
65. Adefisayo MA, Adeyemi WJ, Alabi QK. Combined but not single administration of vitamin C and l-carnitine ameliorates cisplatin-induced gastric mucosa damage in male rats. Can J Physiol Pharmacol. 2018;96(8):830-838. [doi: 10.1139/cjpp-2017-0751] [pmid: 29677454]
66. Saif-Elnasr M, Abdel-Aziz N, El-Batal AI. Ameliorative effect of selenium nanoparticles and fish oil on cisplatin and gamma irradiation-induced nephrotoxicity in male albino rats. Drug Cheml Toxicol. 2019;42(1):94-103. [doi: 10.1080/01480545.2018.1497050] [pmid: 30203673]
67. Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 2006;44(8):1173-83. [doi: 10.1016/j.fct.2006.01.013] [pmid: 16530908]
68. Said RS, Mantawy EM, El-Demerdash E. Mechanistic perspective of protective effects of resveratrol against cisplatin-induced ovarian injury in rats: emphasis on anti-inflammatory and anti-apoptotic effects. Naunyn-Schmiedeberg's Arch Pharmacol. 2019;392:1225-1238. [doi: 10.1007/s00210-019-01662-x] [pmid: 31129703]
69. Habib SA, Abdelrahman RS, Abdel Rahim M, Suddek GM. Anti‐apoptotic effect of vinpocetine on cisplatin‐induced hepatotoxicity in mice: The role of Annexin‐V, Caspase‐3, and Bax. J Biochem Mol Toxicol. 2020;34(10):e22555. [doi: 10.1002/jbt.22555] [pmid: 32578916]
70. Abdelrahman RS. Sitagliptin exerts anti-apoptotic effect in nephrotoxicity induced by cisplatin in rats. Naunyn-Schmiedeberg's Arch Pharmacol. 2017;390:721-731. [doi: 10.1007/s00210-017-1367-2] [pmid: 28382499]
71. Esmat MA, Osman A, Hassan RE, Hagag SA, El-Maghraby TK. Hepatoprotective effect of ferulic acid and/or low doses of γ-irradiation against cisplatin-induced liver injury in rats. Human Exp Toxicol. 2022;41:09603271221136205. [doi: 10.1177/09603271221136205] [pmid: 36270770]
72. Bami E, Ozakpınar OB, Ozdemir-Kumral ZN, Köroglu K, Ercan F, Cirakli Z, et al. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ Toxicol Pharmacol. 2017;54:105-11. [doi: 10.1016/j.etap.2017.06.026] [pmid: 28704751]
73. Eltamany EE, Elhady SS, Nafie MS, Ahmed HA, Abo-Elmatty DM, Ahmed SA, et al. The antioxidant Carrichtera annua DC. ethanolic extract counteracts cisplatin triggered hepatic and renal toxicities. Antioxidants. 2021;10(6):825. [doi: 10.3390/antiox10060825] [pmid: 34064100]
74. Villalva M, Silvan JM, Alarcón-Cavero T, Villanueva-Bermejo D, Jaime L, Santoyo S, et al. Antioxidant, anti-inflammatory, and antibacterial properties of an Achillea millefolium L. extract and its fractions obtained by supercritical anti-solvent fractionation against Helicobacter pylori. Antioxidants. 2022;11(10):1849. [doi: 10.3390/antiox11101849] [pmid: 36290572]
75. Kazemi M. Chemical composition and antimicrobial, antioxidant activities and anti-inflammatory potential of Achillea millefolium L., Anethum graveolens L., and Carum copticum L. essential oils. J Herbal Med. 2015;5(4):217-22. [doi:10.1016/j.hermed.2015.09.001]
76. Okkay U, Ferah Okkay I, Cicek B, Aydin IC, Ertugrul MS, Bayram C, et al. Achillea millefolium alleviates testicular damage in paclitaxel‐intoxicated rats via attenuation of testicular oxido‐inflammatory stress and apoptotic responses. Andrologia. 2021;53(5):e14028. [doi: 10.1111/and.14028] [pmid: 33650701]
77. AL-Obaidi ZF, Yahya HN, Wannas AK, Hadi SA. Organs protective evaluation of Achillea mellifolium against methotrexate damaged albino male mice. Int J Pharm Res. 2020;12(3). [Link]
78. Jalali AS, Hasanzadeh S, Malekinejad H. Achillea millefolium inflorescence aqueous extract ameliorates cyclophosphamide-induced toxicity in rat testis: stereological evidences. Chinese J Natural Med. 2012;10(4):247-54. [doi:10.1016/S1875-5364(12)60050-8]
79. Okkay U, Ferah Okkay I, Aydin IC, Bayram C, Ertugrul MS, Gezer A, et al. Effects of Achillea millefolium on cisplatin induced ocular toxicity: an experimental study. Cutan Ocul Toxicol. 2021;40(3):214-220. [doi: 10.1080/15569527.2021.1919137] [pmid:34180746]
80. Almaghrabi OA. Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidney. Saudi J Biol Sci. 2015;22(2):227-231. [doi:10.1016/j.sjbs.2014.12.008] [pmid: 25737657]
81. Akomolafe SF, Akinyemi AJ, Anadozie SO. Phenolic acids (gallic and tannic acids) modulate antioxidant status and cisplatin induced nephrotoxicity in rats. Int Sch Res Notices. 2014;2014:984709. [doi: 10.1155/2014/984709] [pmid: 27382634]
82. Pınar N, Topaloğlu M, Secinti IE, Büyük E, Kaplan M. Protective effect of dexpanthenol on cisplatin induced nephrotoxicity in rats. Biotech Histoch. 2022;97(1):39-43. [doi: 10.1080/10520295.2021.1890215] [pmid: 33632031]
83. Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sökmen A, et al. Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan.(Asteraceae). J Ethnopharmacol. 2003;87(2-3):215-220. [doi: 10.1016/s0378-8741(03)00149-1] [pmid: 12860311]
84. Tadić V, Arsić I, Zvezdanović J, Zugić A, Cvetković D, Pavkov S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J Ethnopharmacol. 2017;199:138-148. [doi: 10.1016/j.jep.2017.02.002] [pmid: 28163113]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







